Themed collection Mechanistic, computational & physical organic chemistry in OBC

Stereogenic and conformational properties of medium-ring benzo-fused N-heterocycle atropisomers
By examining the various contributions to the conformational and stereogenic stability of medium-sized benzo-fused N-heterocyclic atropisomers, this review serves to aid the design, synthesis and study of these pharmaceutically relevant heterocycles.
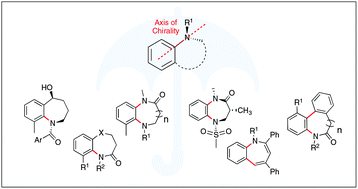
Org. Biomol. Chem., 2021,19, 7098-7115
https://doi.org/10.1039/D1OB00836F
The 1,3-dithiol-2-ide carbanion
This review covers the 1,3-dithiol-2-ide structural unit in relation to the dual electronic character of dithiafulvene and in relation to using it as a synthetic building block for constructing for example extended tetrathiafulvalenes.
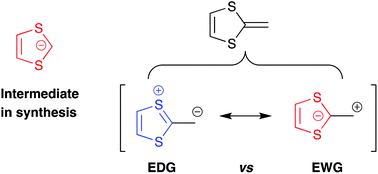
Org. Biomol. Chem., 2021,19, 5999-6006
https://doi.org/10.1039/D1OB00975C
Modelling biocompatible ionic liquids based on organic acids and amino acids: challenges for computational models and future perspectives
From isolated molecules to the bulk phase: building models of biocompatible ionic liquids.
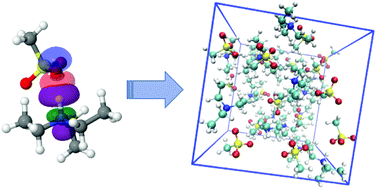
Org. Biomol. Chem., 2021,19, 4002-4013
https://doi.org/10.1039/D1OB00011J
Computational evidence of glycosyl cations
This review covers recent computational studies evidencing the presence of glycosyl cations as real intermediates in several glycosylation reactions.
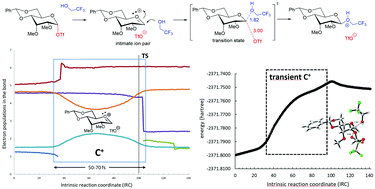
Org. Biomol. Chem., 2021,19, 2350-2365
https://doi.org/10.1039/D0OB02373F
The role of intermolecular forces in ionic reactions: the solvent effect, ion-pairing, aggregates and structured environment
A general view of the medium effects on ionic reactions involves the solvent effect, ion pairing, formation of aggregates and structured environment.
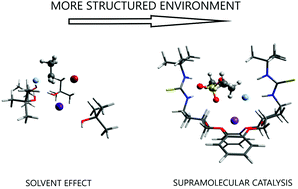
Org. Biomol. Chem., 2021,19, 1900-1914
https://doi.org/10.1039/D0OB02413A
A theoretical review for novel Lewis base amine/imine-catalyzed reactions
Recent advances in computational investigations of Lewis base amine/imine-catalyzed reactions have been systematically summarized and reviewed for the first time.
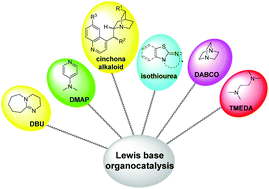
Org. Biomol. Chem., 2020,18, 6781-6800
https://doi.org/10.1039/D0OB01378A
Optical purity, enantiomeric excess and the Horeau effect
Optical purity (op) and enantiomeric excess (ee) become equal when either heterochiral dimerization constant is twice that of homochiral dimerization constant or specific rotations of monomer and dimer are equal.

Org. Biomol. Chem., 2020,18, 6801-6806
https://doi.org/10.1039/D0OB01497D
Synthetic and mechanistic aspects of sulfonyl migrations
Sulfonyl migrations, frequently described as ‘unusual’ or ‘unexpected’, from the last 20 years, including 1,2-, 1,3-, 1,4-, 1,5-, 1,6- and 1,7-sulfonyl shifts, through either radical or polar processes, either inter- or intramolecularly are reviewed.

Org. Biomol. Chem., 2020,18, 2549-2610
https://doi.org/10.1039/C9OB02587A
Mechanistic knowledge and noncovalent interactions as the key features for enantioselective catalysed multicomponent reactions: a critical review
This critical short review focuses on some key features which determine successful enantioselective catalysed multicomponent reactions (MCRs) and are typically underappreciated in the literature.
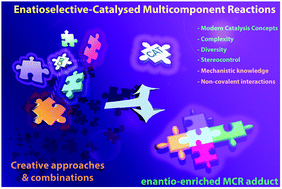
Org. Biomol. Chem., 2019,17, 7260-7269
https://doi.org/10.1039/C9OB01088B
The importance of N-heterocyclic carbene basicity in organocatalysis
This review highlights the importance of N-heterocyclic carbene (NHC) basicity for transformations in which NHCs are used as catalysts.

Org. Biomol. Chem., 2018,16, 8230-8244
https://doi.org/10.1039/C8OB01667D
Diazaborines oxidize slowly with H2O2 but rapidly with peroxynitrite in aqueous buffer
Diazaborines react slowly with H2O2 but rapidly with ONOO−, offering a new platform for discriminating between these two cellular ROS.
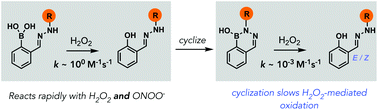
Org. Biomol. Chem., 2022,20, 995-999
https://doi.org/10.1039/D1OB01668G
Hydrogen bonding interactions can decrease clar sextet character in acridone pigments
Magnetic evaluations of aromaticity suggest that hydrogen bonding interactions can perturb the Clar sextet characters of acridones, which have implications for their electronic properties.
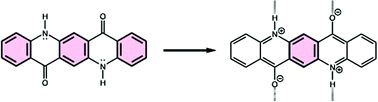
Org. Biomol. Chem., 2021,19, 9619-9623
https://doi.org/10.1039/D1OB01720A
QM/MM modeling of class A β-lactamases reveals distinct acylation pathways for ampicillin and cefalexin
QM/MM chain-of-states calculations of CTX-M-44 show distinct acylation profiles for ampicillin and cefalexin, the acylation resistance observed for cefalexin attributes to decreased proton affinity induced by the delocalized π-conjugation.
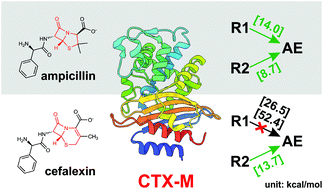
Org. Biomol. Chem., 2021,19, 9182-9189
https://doi.org/10.1039/D1OB01593A
Glutamate carboxypeptidase II as a model system for designing host–guest units: a theoretical approach
Through a combined crystallographic and computational analysis, we designed and investigated two novel host units for the recognition of neutral and charged guests inspired by a glutamate carboxypeptidase II system and its inhibitors.
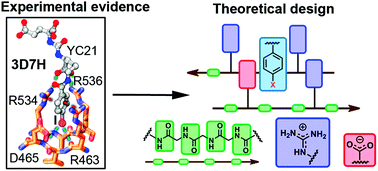
Org. Biomol. Chem., 2021,19, 7816-7821
https://doi.org/10.1039/D1OB01209F
Thermal decomposition of hexamethylenetetramine: mechanistic study and identification of reaction intermediates via a computational and NMR approach
In a joint DFT and chemometrics study applied to NMR spectra, we disclose the structure of the main decomposition products of hexamethylenetetramine at thermal conditions. The mechanism involves a protonation of HMTA followed by a ring opening and finally a Sigmatropic rearragnment [1,5-H].
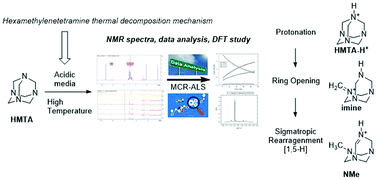
Org. Biomol. Chem., 2021,19, 7374-7378
https://doi.org/10.1039/D1OB01522B
Water clusters as bifunctional catalysts in organic chemistry: the hydrolysis of oxirane and its methyl derivatives
This contribution explores the bifunctional catalytic activity of water clusters ((H2O)n with n = 1–5) in organic chemistry similar to that observed in the formation of H2SO4 in acid rain.
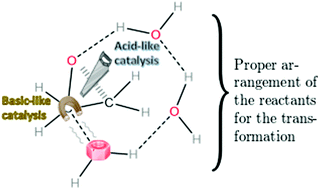
Org. Biomol. Chem., 2021,19, 6776-6780
https://doi.org/10.1039/D1OB01026C
Mechanism of Pd-catalysed C(sp3)–H arylation of thioethers with Ag(I) additives
The mechanism of Pd-catalysed γ-C(sp3)–H arylation of thioethers has been disclosed, wherein all steps proceed through the heterodimeric Pd–Ag pathway.

Org. Biomol. Chem., 2021,19, 6766-6770
https://doi.org/10.1039/D1OB00704A
Understanding the sigmatropic shifts of cyclopenta-2,4-dien-1-yltrimethylsilane in its Diels–Alder addition
Investigation on the dynamic processes of 1-(C5H5)Si(Me)3 in its Diels–Alder addition showed that Si(Me)3 migration is the dominant factor and hydrogen shifts are negligible.

Org. Biomol. Chem., 2021,19, 1732-1737
https://doi.org/10.1039/D0OB02386H
On the reciprocal relationship between σ-hole bonding and (anti)aromaticity gain in ketocyclopolyenes
σ-Hole bonding interactions (e.g., tetrel, pnictogen, chalcogen, and halogen bonding) can polarize π-electrons to enhance cyclic [4n] π-electron delocalization (i.e., antiaromaticity gain) or cyclic [4n + 2] π-electron delocalization (i.e., aromaticity gain).

Org. Biomol. Chem., 2020,18, 5125-5129
https://doi.org/10.1039/D0OB01076F
Divergent pathway and reactivity control of intramolecular arene C–H vinylation by vinyl cations
A computational study revealed the mechanistic details and controlling factors of reactivity in intramolecular C–H vinylation through a vinyl cation intermediate.
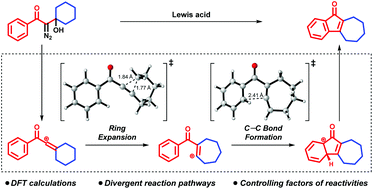
Org. Biomol. Chem., 2019,17, 9135-9139
https://doi.org/10.1039/C9OB01916B
The optimal DFT approach in DP4 NMR structure analysis – pushing the limits of relative configuration elucidation
What computational methods should be used to achieve the most reliable result in computational structure elucidation? A study on the effect of quality and quantity of geometries on computational NMR structure elucidation performance is reported.

Org. Biomol. Chem., 2019,17, 5886-5890
https://doi.org/10.1039/C9OB00840C
Mechanism of nitrones and allenoates cascade reactions for the synthesis of dihydro[1,2-a]indoles
Quantum mechanical calculations (DLPNO-CCSD(T) and M06-2X) are employed to gain insights into the mechanism and selectivity in the catalytic synthesis of dihydropyrido[1,2-a]indoles from the cascade reaction between nitrones and allenes.
![Graphical abstract: Mechanism of nitrones and allenoates cascade reactions for the synthesis of dihydro[1,2-a]indoles](/ta/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8OB02346H&imageInfo.ImageIdentifier.Year=2019)
Org. Biomol. Chem., 2019,17, 1767-1772
https://doi.org/10.1039/C8OB02346H
A Viedma ripening route to an enantiopure building block for Levetiracetam and Brivaracetam
A simple route to enantiomerically pure (S)-2-aminobutyramide – the chiral component of the anti-epileptic drugs Levetiracetam and Brivaracetam has been developed. This approach is based on the rational design and application of a Viedma ripening process. The practical potential of the process is demonstrated on a large scale.

Org. Biomol. Chem., 2019,17, 35-38
https://doi.org/10.1039/C8OB02660B
Superelectrophilic activation of 1-nitronaphthalene in the presence of aluminum chloride. Reactions with benzene and cyclohexane
Aluminum chloride was successfully used instead of liquid superacids to mediate selective reactions of 1-nitronaphthalene with benzene and cyclohexane.
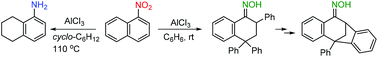
Org. Biomol. Chem., 2018,16, 9129-9132
https://doi.org/10.1039/C8OB02653J
Experimental evidence for the formation of cationic intermediates during iodine(III)-mediated oxidative dearomatization of phenols
Hammett analysis reveals iodine(III)-mediated oxidative dearomatization reactions proceed through cationic intermediates and not direct nucleophilic attack on an activated phenol.
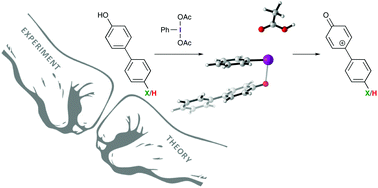
Org. Biomol. Chem., 2018,16, 8249-8252
https://doi.org/10.1039/C8OB01652F
On the dual reactivity of a Janus-type mesoionic dipole: experiments and theoretical validation
A mesoionic bicycle, easily synthesized from a proteinogenic amino acid, L-leucine, behaves as both thiazolium-olate and diazolium-olate dipoles, as unveiled by its dipolar cycloadditions.
Org. Biomol. Chem., 2018,16, 4778-4783
https://doi.org/10.1039/C8OB01195H
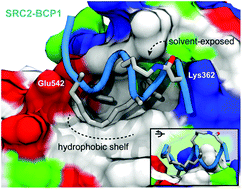
A “cross-stitched” peptide with improved helicity and proteolytic stability
Peptide “cross-stitching” maintains binding affinity and can enhance helical and proteolytic stability.
Org. Biomol. Chem., 2018,16, 3702-3706
https://doi.org/10.1039/C8OB00790J
Cross-conjugation controls the stabilities and photophysical properties of heteroazoarene photoswitches
Azoarene photoswitches are versatile molecules that interconvert from their E-isomer to their Z-isomer with light.

Org. Biomol. Chem., 2022,20, 5989-5998
https://doi.org/10.1039/D1OB02026A
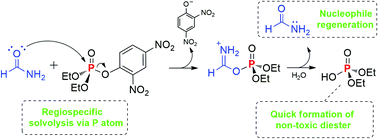
Mechanistic insights into the amidolysis of a phosphate triester: the antagonistic role of water
Aqueous formamide solutions can promote catalytic amidolysis of an activated organophosphate in a regiospecific process.
Org. Biomol. Chem., 2022,20, 2462-2466
https://doi.org/10.1039/D2OB00180B
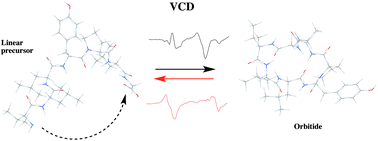
Conformational preferences induced by cyclization in orbitides: a vibrational CD study
Vibrational CD and DFT calculations reveal solution-state conformational preferences of orbitides following cyclization.
Org. Biomol. Chem., 2022,20, 1306-1314
https://doi.org/10.1039/D1OB02170B
The role of acetylated cyclooxygenase-2 in the biosynthesis of resolvin precursors derived from eicosapentaenoic acid
Aspirin-acetylated COX-2, but not COX-2, triggers the biosynthesis of anti-inflammatory E-series resolvins. The role of aspirin in the molecular mechanism of resolvin formation from EPA is revealed by MD simulations and QM/MM calculations.

Org. Biomol. Chem., 2022,20, 1260-1274
https://doi.org/10.1039/D1OB01932E
On the mechanism of the dyotropic expansion of hydrindanes into decalins
A combined computational/experimental approach has revealed key mechanistic aspects in a recently reported dyotropic expansion of hydrindanes into decalins.
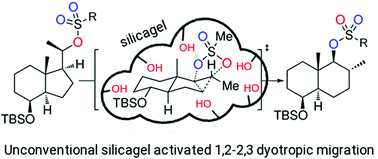
Org. Biomol. Chem., 2022,20, 1073-1079
https://doi.org/10.1039/D1OB02150H
Deuterium labelling to extract local stereochemical information by VCD spectroscopy in the C–D stretching region: a case study of sugars
Methoxy-d3 group installed at the C-1 position of a series of epimeric pairs of sugars generated mirror-image VCD patterns in the 2300–1900 cm−1 region depending on the C-1 stereochemistry irrespective of the configurations at other positions.
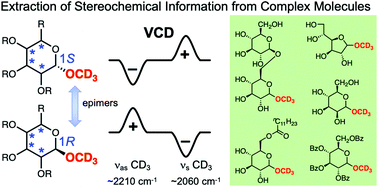
Org. Biomol. Chem., 2022,20, 1067-1072
https://doi.org/10.1039/D1OB02317A
A DFT examination of the role of proximal boron functionalities in the S-alkylation of sulfenic acid anions
DFT modelling predicts proximal boron groups can accelerate sulfenate alkylation reactions, depending on boron substituents and boron distance from the reaction site.
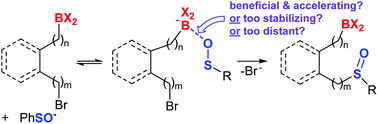
Org. Biomol. Chem., 2022,20, 649-657
https://doi.org/10.1039/D1OB02083H
Conformation-dependent antioxidant properties of β-carotene
The antioxidant capacity of β-carotene has been studied in terms of H-atom abstraction reactions using quantum chemical methods.

Org. Biomol. Chem., 2022,20, 152-162
https://doi.org/10.1039/D1OB01723C
Visible light mediated synthesis of 6H-benzo[c]chromenes: transition-metal-free intramolecular direct C–H arylation
A synthetic approach towards the 6H-benzo[c]chromene ring under visible light and transition-metal-free conditions has been developed. A theoretical analysis was performed using DFT methods in order to study the ET mechanism.
![Graphical abstract: Visible light mediated synthesis of 6H-benzo[c]chromenes: transition-metal-free intramolecular direct C–H arylation](/ta/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1OB01673C&imageInfo.ImageIdentifier.Year=2022)
Org. Biomol. Chem., 2022,20, 228-239
https://doi.org/10.1039/D1OB01673C
Simulations reveal the key role of Arg15 in the promiscuous activity in the HisA enzyme
The dynamics of the loop controls the promiscuous activity in the HisA enzyme.
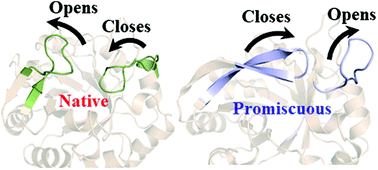
Org. Biomol. Chem., 2021,19, 10652-10661
https://doi.org/10.1039/D1OB02029C
Characterization of the low energy conformations and differential reactivities of D-glucose and D-mannose based oxepines
Low energy conformations of two carbohydrate-based oxepines were characterized by computational methods and 3JH,H coupling constant analysis. Oxepine reactivity in Ferrier reactions was correlated with percentage vinylogous anomeric effect (VAE).
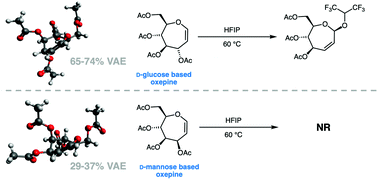
Org. Biomol. Chem., 2021,19, 10635-10646
https://doi.org/10.1039/D1OB01900G
Computational studies on the sterol-like cyclization of a monodomain class II terpene cyclase
Delineation of the mechanism with DFT calculations.
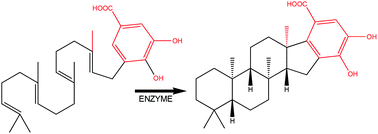
Org. Biomol. Chem., 2021,19, 10647-10651
https://doi.org/10.1039/D1OB02018H
Mechanistic investigations of alcohol silylation with isothiourea catalysts
The surprising mechanistic results for the silylation of alcohols revealed multiple intermediates and a higher order of silyl chloride leading up to the rate-determining step.
Org. Biomol. Chem., 2021,19, 10181-10188
https://doi.org/10.1039/D1OB01732B
Mechanistic insights into lysine-targeting covalent inhibition through a theoretical study of ester aminolysis
Activated phenolic esters are promising as lysine-targeted covalent inhibitors of the PI3Kδ enzyme. Quantum chemical calculations on model reactions provide insights into the reaction mechanisms and factors determining inhibitor efficiency.

Org. Biomol. Chem., 2021,19, 9996-10004
https://doi.org/10.1039/D1OB01963E
A computational study of the reaction mechanism of 2,2-azobis(isobutyronitrile)-initiated oxidative cleavage of geminal alkenes
A computational study of 2,2-azobis(isobutyronitrile) (AIBN)-initiated aerobic oxidative cleavage of alkenes is carried out employing density functional theory (DFT) and high-level coupled-cluster methods.
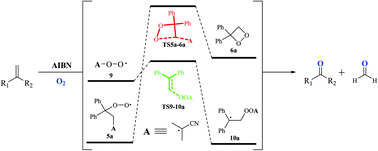
Org. Biomol. Chem., 2021,19, 9483-9490
https://doi.org/10.1039/D1OB01607E
On the rearrangements of biologically-relevant vinyl allene oxides to cis-cyclopentenones, ketols, and Favorskii-type carboxylic acids
The intriguing rearrangement of naturally occurring vinyl allene oxides to ketols, cyclopropylcarbinols and Favorskii-type carboxylic acids has been computational justified with model systems.
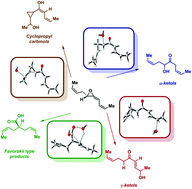
Org. Biomol. Chem., 2021,19, 9460-9469
https://doi.org/10.1039/D1OB01847G
Correlations between the ECD spectra and absolute configuration of bridged-ring lactones: revisiting Beecham's rule
We present the theoretical verification, revision and enrichment of Beecham's rule for bridged-ring lactones. This provides an alternative way to correlate experimental ECD data with stereochemistry in addition to quantum-chemical calculation.
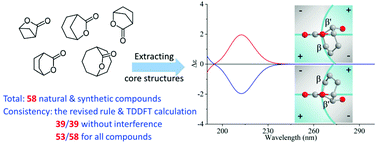
Org. Biomol. Chem., 2021,19, 9266-9275
https://doi.org/10.1039/D1OB01557E
Water biocatalytic effect attenuates cytochrome P450-mediated carcinogenicity of diethylnitrosamine: A computational insight
The mechanism-based mutagenicity and carcinogenicity of diethylnitrosamine (DEN) are believed to act through interactions with cytochrome P450 (P450) enzymes.

Org. Biomol. Chem., 2021,19, 9031-9042
https://doi.org/10.1039/D1OB01439K
Computational study on N-triflylphosphoramide-catalyzed enantioselective hydroamination of alkenyl thiourea
The mechanism of the enantioselective intramolecular hydroamination of alkenyl thiourea catalyzed by chiral phosphoramide was revealed using density functional theory calculations.
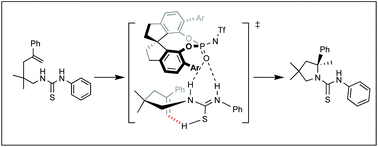
Org. Biomol. Chem., 2021,19, 8806-8811
https://doi.org/10.1039/D1OB01672E
Predicting the catalytic activity of azolium-based halogen bond donors: an experimentally-verified theoretical study
The most promising types of iodoazolium compounds exhibiting high catalytic activity toward halogen abstraction and carbonyl activation are highlighted.

Org. Biomol. Chem., 2021,19, 7611-7620
https://doi.org/10.1039/D1OB01158H
Nucleophilic catalysis of p-substituted aniline derivatives in acylhydrazone formation and exchange
Mechanistic study and superior performance of electron-rich p-substituted aniline derivatives as catalysts for efficient hydrazone formation and exchange in both protic and aprotic solvents.

Org. Biomol. Chem., 2021,19, 7202-7210
https://doi.org/10.1039/D1OB00871D
Tautomers of N-acetyl-D-allosamine: an NMR and computational chemistry study
D-AllNAc shows in water solution a significant presence of four tautomers arising from pyranoid and furanoid ring forms and anomeric configurations.

Org. Biomol. Chem., 2021,19, 7190-7201
https://doi.org/10.1039/D1OB01139A
Experimental lipophilicity scale for coded and noncoded amino acid residues
Log P values for amino acid derivatives were integrated on one scale allowing comparison between common amino acids and their chemical analogues.
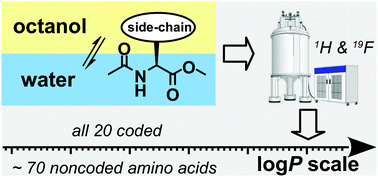
Org. Biomol. Chem., 2021,19, 7031-7040
https://doi.org/10.1039/D1OB01213D
In silico characterization and prediction of thiourea-like neutral bidentate halogen bond catalysts
Through DFT calculations, thiourea-like halogen bond (XB)-based catalysts, with XB donor moieties connected via covalent bonds, have been designed and applied to Diels–Alder reaction and sulfa-Michael addition.
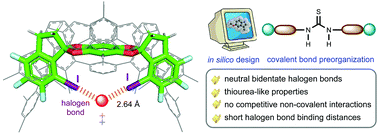
Org. Biomol. Chem., 2021,19, 7051-7060
https://doi.org/10.1039/D1OB01092A
Mannosylated adamantane-containing desmuramyl peptide recognition by the NOD2 receptor: a molecular dynamics study
The dipeptide isoGln moiety of ManAdDMP significantly contributes to the binding to the NOD2 receptor. Loops 2 and 7 are important for ligand recognition and could be useful for further investigation of NOD2 activation/inhibition.
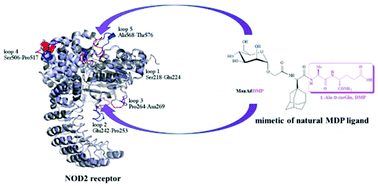
Org. Biomol. Chem., 2021,19, 7001-7012
https://doi.org/10.1039/D1OB00679G
Biological halogen bonds in protein–ligand complexes: a combined QTAIM and NCIPlot study in four representative cases
In this study, the PDB has been inspected for the analysis of HaBs in biological systems, highlighting their importance in four different protein–ligand systems.

Org. Biomol. Chem., 2021,19, 6858-6864
https://doi.org/10.1039/D1OB01212F
Structural and mechanistic insight into DNA bending by antitumour calicheamicins
Atomistic molecular dynamics simulations shed structural light on the curvature inflicted on DNA upon binding of calicheamicin ε to high-affinity and low-affinity target sites.
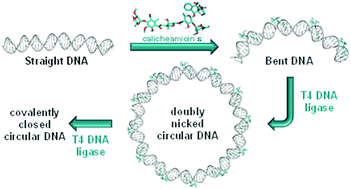
Org. Biomol. Chem., 2021,19, 6707-6717
https://doi.org/10.1039/D1OB01077H
An examination of the relationship between molecular dipole moment and blood-gas partition for common anaesthetic gases
The overall molecular dipole moments calculated for some common fluorinated anaesthetics correlate with the respective blood-gas partition. This finding may aid the design of novel last-generation anaesthetics.
Org. Biomol. Chem., 2021,19, 6665-6670
https://doi.org/10.1039/D1OB01067K
Synthesis of homochiral sulfanyl- and sulfoxide-substituted naphthyltriazoles and study of the conformational stability
Novel homochiral sulfanyl-substituted naphthyltriazoles are synthesized via the triazolization reaction, and their rotational stability is studied in detail.

Org. Biomol. Chem., 2021,19, 6521-6526
https://doi.org/10.1039/D1OB00784J
The mechanism of conversion of substituted glycals to chiral acenes via Diels–Alder reaction: a computational study
Transformation of substituted glycals to chiral fused aromatic cores using DFT calculations reveal that the base catalyzed reaction is favorable over sigmatropic [1,5] hydrogen shift and the product formation is governed by aromaticity.

Org. Biomol. Chem., 2021,19, 6353-6367
https://doi.org/10.1039/D1OB00408E
Accelerating the optimization of enzyme-catalyzed synthesis conditions via machine learning and reactivity descriptors
Enzyme-catalyzed synthesis reactions are of crucial importance for a wide range of applications.
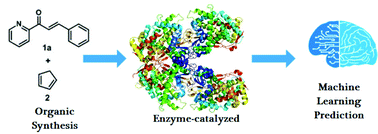
Org. Biomol. Chem., 2021,19, 6267-6273
https://doi.org/10.1039/D1OB01066B
The inverting mechanism of the metal ion-independent LanGT2: the first step to understand the glycosylation of natural product antibiotic precursors through QM/MM simulations
Glycosyltransferases (GTs) from the GT1 family are responsible for the glycosylation of various important organic structures such as terpenes, steroids and peptide antibiotics, making it one of the most intensely studied families of GTs.
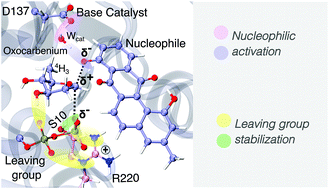
Org. Biomol. Chem., 2021,19, 5888-5898
https://doi.org/10.1039/D1OB00544H
Structural variation of protein–ligand complexes of the first bromodomain of BRD4
The importance of the water network in BRD4-BD1 complexes is illustrated using molecular docking and absolute free energy perturbation simulations. 82% of the ligand poses were better predicted when including water molecules as part of the receptor.
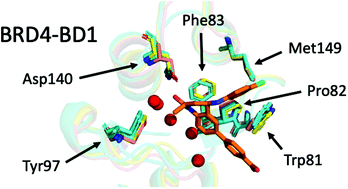
Org. Biomol. Chem., 2021,19, 5632-5641
https://doi.org/10.1039/D1OB00658D
Tuning the exchange dynamics of boronic acid hydrazones and oximes with pH and redox control
Hydrazones and oximes with proximal boronic acids undergo facile exchange, which can be sped up or slowed down with pH control or abolished with oxidation.
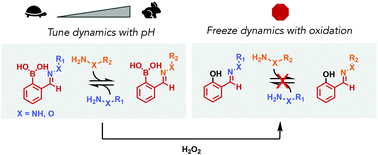
Org. Biomol. Chem., 2021,19, 4986-4991
https://doi.org/10.1039/D1OB00191D
Wavelength dependent photochemistry of BODIPY–phenols and their applications in the fluorescent labeling of proteins
The molecules undergo wavelength dependent photochemistry, since photodeamination to QMs takes place only upon excitation to higher excited singlet states, showing unusual anti-Kasha photochemical reactivity.

Org. Biomol. Chem., 2021,19, 4891-4903
https://doi.org/10.1039/D1OB00278C
A computational study of site-selective hydrogen abstraction by sulfate radical anion
Ab initio and DFT calculations revealed that β-hydrogen abstraction would be favourable, which supports experimental findings (i.e. β-selective abstraction).

Org. Biomol. Chem., 2021,19, 4775-4782
https://doi.org/10.1039/D1OB00587A
How is vitamin B1 oxidized to thiochrome? Elementary processes revealed by a DFT study
The oxidation mechanism of thiamine (vitamin B1) to thiochrome was investigated by DFT calculations for reaction models, thiamine + oxidant + (H2O)8. A key thiazolone intermediate (Int2) was commonly found to intervene during the oxidation.
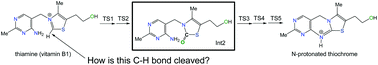
Org. Biomol. Chem., 2021,19, 4529-4536
https://doi.org/10.1039/D1OB00677K
VRAI-selectivity: calculation of selectivity beyond transition state theory
We present the VRAI-selectivity program which predicts the major product and selectivity not only for transition-state controlled reactions but also for processes controlled by reaction dynamics.

Org. Biomol. Chem., 2021,19, 3940-3947
https://doi.org/10.1039/D1OB00234A
Mechanistic studies of Cp*Ir(III)/Cp*Rh(III)-catalyzed branch-selective allylic C–H amidation: why is Cp*Ir(III) superior to Cp*Rh(III)?
DFT mechanistic investigations uncovered the origins of the reactivity and regioselectivity of Cp*Ir(III)/Cp*Rh(III)-catalyzed allylic C–H amidation reactions.
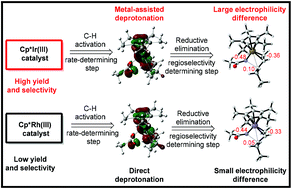
Org. Biomol. Chem., 2021,19, 3850-3858
https://doi.org/10.1039/D1OB00446H
Studies on chemoselective synthesis of 1,4- and 1,2-dihydropyridine derivatives by a Hantzsch-like reaction: a combined experimental and DFT study
In the experimental process of preparing 1,4-DHP by a Hantzsch-like reaction, it was found that a by-product named 1,2-DHP was produced.
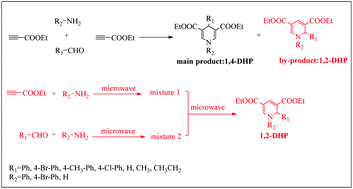
Org. Biomol. Chem., 2021,19, 3882-3892
https://doi.org/10.1039/D0OB02289F
Chemisorption of CO2 by diamine-tetraamido macrocyclic motifs: a theoretical study
A series of diamine-tetraamido macrocyclic molecules with different organic linkers are explored as potential CO2 capturing agents using quantum chemical calculations.
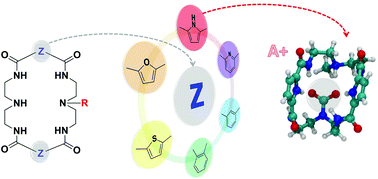
Org. Biomol. Chem., 2021,19, 3873-3881
https://doi.org/10.1039/D1OB00180A
Understanding the mechanism of the chiral phosphoric acid-catalyzed aza-Cope rearrangement
Using Density Functional Theory, the mechanism of the enantioselective phosphoric acid-catalyzed aza-Cope rearrangement was investigated. Stabilization of the preferred reaction pathway was rationalized by studying the non-bonding interactions between substrate and catalyst.
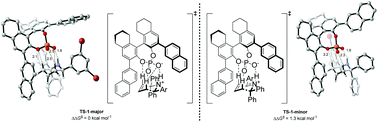
Org. Biomol. Chem., 2021,19, 3656-3664
https://doi.org/10.1039/D0OB02458A
Catalytic and non-catalytic hydroboration of carbonyls: quantum-chemical studies
The present paper examines both catalyst-free and KF-mediated hydroboration of carbonyl compounds with the use of quantum-chemical methods.
Org. Biomol. Chem., 2021,19, 3004-3015
https://doi.org/10.1039/D1OB00037C
About this collection
The latest articles published in Organic and Biomolecular Chemistry related to mechanistic, computational & physical organic chemistry research.