Computational studies on the sterol-like cyclization of a monodomain class II terpene cyclase†
Abstract
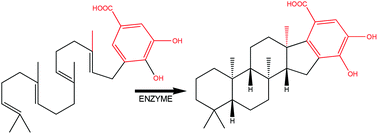
Recently the first example of a class II terpene cyclase comprised of only a single domain was reported. Class II synthases are a diverse group of enzymes that catalyze exceptionally complex reactions, including the remarkable cyclization of steroids. This discovery of a single-domain enzyme being able to catalyze a steroid-like product contradicted the long-held tenet that complex class II cyclizations required double-domain enzymes. The proposed mechanism for the sterol-like cyclization of a monodomain class II terpene cyclase was studied computationally by using density functional theory (DFT). The complete pathway for the conversion of 5-geranyl-3,4-dihydroxybenzoate to the steroid-like pentacyclic product merosterolic acid A was elucidated. The formation of a tricyclic carbocation intermediate with three cyclohexane rings was found to be a concerted, but asynchronous, cyclization. The formation of the fourth ring proceeds with a low energy activation Friedel–Crafts reaction. Subsequent deprotonation of this pentacyclic system gave as the final product merosterolic acid. The overall conversion was found to be highly exothermic due to the conversion of three C–C double bonds to C–C single bonds.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...