Universal synthesis of rare earth-doped FeP nanorod arrays for the hydrogen evolution reaction†
Minnan
Chen
,
Zijing
Lin
,
Yi
Ren
,
Xuan
Wang
,
Meng
Li
,
Dongmei
Sun
 *,
Yawen
Tang
*,
Yawen
Tang
 * and
Gengtao
Fu
* and
Gengtao
Fu
 *
*
Jiangsu Key Laboratory of New Power Batteries, Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing 210023, China. E-mail: sundongmei@njnu.edu.cn; tangyawen@njnu.edu.cn; gengtaofu@njnu.edu.cn
First published on 6th July 2023
Abstract
A universal plasma-assisted strategy is proposed for the fabrication of rare earth (RE)-doped FeP nanorod arrays (RE-FeP) as a kind of potential electrocatalyst for the hydrogen evolution reaction (HER). The energetic Ar plasma can induce the vacancy-enriched feature of the Fe-precursor, which assists in the anchoring of RE ions. As a typical model, Sm-FeP affords a low overpotential of 71 mV at 10 mA cm−2 for the HER, which is 63 mV smaller than that of FeP and superior to most reported Fe-based catalysts. The robust long-term stability of Sm-FeP is also demonstrated. Furthermore, the as-assembled Sm-FeP‖RuO2 water-splitting electrolyzer also displays a low cell voltage of 1.59 V at 10 mA cm−2. Sm-induced electronic configuration modulation at the Fe site mainly contributes to the improved HER performance of Sm-FeP relative to FeP. The combination between the Sm site and *OH produces labile O 2p states below the Fermi level, thus weakening the co-adsorption of *OH and *H derived from the splitting of H2O for the facilitated formation of *H. Moreover, the other RE-FeP catalysts (e.g., Yb, Eu, La, and Er) extended by such a plasma-induced strategy also exhibit various improved degrees in the HER, implying that RE-FeP is a promising class of electrocatalyst towards the HER.
Introduction
Hydrogen (H2) has attracted great attention as a clean and renewable alternative energy source for traditional fossil fuels due to its zero-carbon emission and high gravimetric energy density (142 MJ kg−1).1,2 The electrocatalytic water splitting is deemed as an environment-friendly strategy to meet the rising demands of high-purity hydrogen production.3,4 However, the efficiency of water splitting is mainly decided by the electrocatalytic performance of catalysts to lower the energy barrier of the involved reactions such as the hydrogen evolution reaction (HER).5–8 To date, Pt-based materials are still considered the benchmark HER electrocatalyst,9–12 but high-cost and limited reserves hinder their large-scale application. Therefore, it is highly desirable to develop earth-abundant, cost-effective, and efficient non-Pt catalysts for the HER.13,14Recently, a myriad of 3d-transition metal (TM)-based catalysts, such as alloys,15,16 hydroxides,17,18 nitrides,19,20 oxides,21,22 sulfides,23,24 and phosphides,25,26 have been developed to accelerate the HER process. Thereinto, FeP has been identified as one of the prospective HER catalysts owing to its unique proton attractivity.27,28 Meanwhile, P element with slightly high electronegativity compared with Fe can regulate the surface charge state of Fe and also increase the stability of FeP through a covalent Fe–P bond.29 However, there is still a huge performance gap between FeP and Pt-based catalysts and bulk FeP cannot provide sufficient exposed active sites and mass transfer channels for the HER, which is far from the demand of renewable energy systems. To address the above-mentioned problems, incorporation of FeP with heteroatoms seems to be a reliable strategy to improve the HER activity. Owing to the extensibility of the electron wave function of d orbitals, the electronic structure of Fe can be finely adjusted by foreign elements, which affects the adsorption behaviors of the reaction intermediates in the HER.30–32
More recently, rare earth (RE) elements such as La, Ce, Nb, Eu, Gd, and Yb have been proven as efficient promoters for a number of electrocatalytic reactions.33–40 The unique 4f-subshell electron configuration, flexible valence states, and strong spin–orbital effect of RE elements can offer great potential for tuning the electronic structures and improving the electrocatalytic performance of various TM-based catalysts.41–44 For example, Sun et al. found that the combination of Yb2O3 and Ni can reduce the energy barrier of water dissociation and enlarge the active-site density, which greatly enhances the HER performance of Ni.45 Li et al. demonstrated that the co-doping of Y and Ce into Ni-MOF can finely tailor the electronic structure of NiYCe-MOF/NF by a bimetallic synergistic effect, resulting in greatly enhanced HER and OER performance.46 However, compared with Fe, RE elements have a larger atomic radius, more negative reduction potential, and stronger oxygen affinity, which increase the difficulty of controllable synthesis of RE-FeP hybrid catalysts. According to defect theory, the unsaturated properties of atoms around defect sites tend to possess high binding energy with metal ions, which can act as anchoring sites for foreign atoms or clusters. This vacancy-assisted anchoring method can effectively avoid self-nucleation of heterogeneous elements due to crystal nucleation and differences in growth factors of different elements (e.g., atomic radius, charge, and solubility). For example, Gong et al. reported that O-doped MoS2 could generate rich sulfur vacancies that provide specific anchoring sites for Pt and facilitate the formation of homogeneous and monodispersed Pt nanoparticles.47 Wang et al. also reported that Au atoms were captured by oxygen vacancy-enriched TiO2 with the local atomic configuration of Ti–Au–Ti by a coprecipitation method.48 Therefore, the hybridization of FeP and RE species through the vacancy-assisted method may be a viable strategy to improve the HER activity of FeP.
Herein, we elaborate on a universal plasma-assisted strategy for the synthesis of RE-FeP nanorod arrays on carbon cloth (RE-FeP@CC) as highly efficient HER catalysts. The Ar-plasma beam sheds the surface light atoms of the FeO(OH)@CC precursor to form vacancies, so as to anchor glycine-stabilized RE ions during the subsequent hydrothermal process. Taking Sm-FeP@CC as an example, Sm-FeP@CC exhibits outstanding electrocatalytic activity with a low overpotential of 71 mV at 10 mA cm−2, a small Tafel slope of 85.9 mV dec−1, and robust stability, outperforming those of Sm-free FeP@CC and most reported Fe-based catalysts. When utilized in overall water splitting, Sm-FeP@CC‖RuO2 only requires a voltage of 1.59 V at 10 mA cm−2. The improved HER activity of Sm-FeP@CC is highly correlated with the Sm-induced electronic modulation of Fe and the unique nanorod arrayed feature. Theoretical calculations demonstrate that the increased HER performance on Sm-FeP is due to the weakened co-adsorption of *OH and *H that is contributed by the splitting of H2O, where the labile O 2p state in *OH denotes the weak bonding at the Sm site, which is favored for the consecutive H2 release.
Results and discussion
Synthesis and characterization
Sm-FeP@CC was synthesized by the combination of an Ar-plasma-assisted treatment and subsequent topological transformation with FeO(OH) as the precursor, as schematically illustrated in Fig. 1(a). Firstly, the FeO(OH) nanorod arrays were uniformly grown on the carbon cloth (CC) substrate through a simple hydrothermal process. Then, the FeO(OH)@CC was treated with Ar plasma for obtaining anionic vacancies on its surface, which was further soaked in SmCl3 glycine solution. Glycine not only can be adsorbed by anionic vacancies through electrostatic interactions but also can coordinate with the Sm3+ (Fig. 1(b)). UV-vis spectra illustrated that Sm3+–glycine solution has a distinct red shift compared to SmCl3 solution (Fig. 1(c)), indicating that the Sm3+ ion was coordinated with glycine. Finally, Sm-modified FeO(OH)@CC was topologically transferred into Sm-FeP@CC by the phosphating process.The crystalline structure of Sm-FeP@CC was analyzed by X-ray powder diffraction (XRD), as shown in Fig. 2(a). The characteristic diffraction peaks for FeP were observed at 32.7°, 37.1° and 48.3°, which are well indexed to (011), (111), and (211) facets of orthorhombic-phase FeP (JCPDF no. 65-2595), demonstrating that the introduction of Sm element does not change the host crystalline structure of FeP. Two obvious diffraction peaks of about 26.5° and 44.0° correspond to the lattice planes of carbon cloth. The magnified (211) facet peak of Sm-FeP shows an obvious shift to a lower angle region compared with FeP (Fig. S1, ESI†), which can be ascribed to the lattice expansion due to the lager atomic radius of Sm. Sm-FeP@CC was converted from the orthorhombic FeO(OH) phase (Fig. S2, ESI†). The existence of Sm in Sm-FeP@CC was also confirmed by the energy dispersive spectroscopy (EDS) spectrum (Fig. S3, ESI†). The molar content of Sm in Sm-FeP was calculated to be about 1.37% by inductively coupled plasma mass spectrometry (ICP-MS). The surface composition and valence information of Sm-FeP@CC were further investigated by X-ray photoelectron spectroscopy (XPS). The coexistence of Fe, P, Sm and C was clearly observed in the survey scan spectrum for Sm-FeP@CC (Fig. S4, ESI†), consistent with the analysis of EDS. The Sm 3d peaks of Sm 3d5/2 and Sm 3d3/2 were found at two distinct binding energies of 1083.3 and 1110.5 eV (Fig. 2(b)). The binding energy difference between the two spin–orbit peaks is about 27.2 eV, indicating that Sm presents the +3 oxidation state in the host.49 For the high-resolution Fe 2p spectrum (Fig. 2(c)), the deconvoluted peaks located at 711.1 and 724.3 eV are ascribed to Fe2+ species, while the deconvoluted peaks at 713.7 and 727.1 eV are assigned to Fe3+ species with two satellite peaks.50,51 Besides, a pair of sharp peaks represented at 720.2 and 707.6 eV are ascribed to FeP.52–54 The binding energy of the Fe 2p3/2 peak for Sm-FeP@CC shifts to a lower position by about 0.3 eV compared with FeP@CC (Fig. 2(c)), while a significant positive shift of about 0.50 eV of Sm 3d5/2 was also observed in Sm-FeP@CC compared to Sm2O3 (Fig. S5, ESI†). This indicates that the introduction of Sm optimizes the electronic structure of FeP owing to the different electronegativities of Sm and Fe.53,55 Such spontaneous electron migration can equilibrate the adsorption energies of intermediates and improve the hydrogen evolution performance.56–58 The high-resolution P 2p spectrum (Fig. 2(d)) of Sm-FeP@CC is disassembled into one peak of P–O at 133.9 eV originating from surface oxidation of the phosphides and other peaks centered at 129.5 eV and 130.3 eV belong to P 2p3/2 and P 2p1/2 peaks of the P-metal bond.59,60 The strong P–O bond can be ascribed to both surface oxidation caused by exposure to air and the deposition of PO33− species due to the decomposition of sodium hypophosphite.61 After Sm doping, the Sm-FeP@CC exhibits a higher electron spin response (ESR) signal intensity at a g value of 2.004 than FeP@CC (Fig. 2(e)), which implies the presence of high concentration P vacancies in Sm-FeP@CC. Sm with a flexible 4f-subshell electron configuration and the strong spin–orbital effect can significantly decrease the formation energy of vacancies, which may result in the vacancy-rich feature of Sm-FeP@CC.62,63
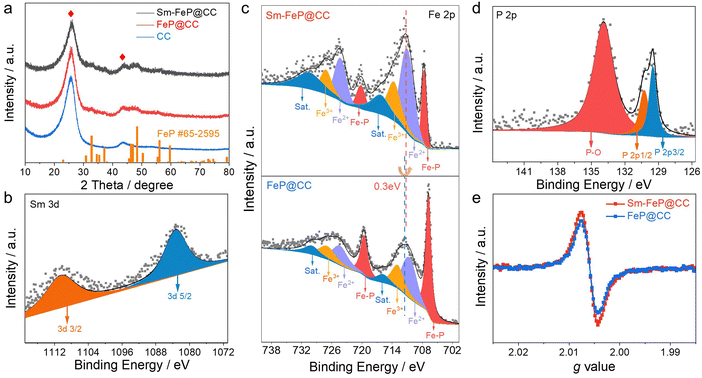 | ||
| Fig. 2 (a) XRD pattern of Sm-FeP@CC; (b) Sm 3d XPS spectrum of Sm-FeP@CC; (c) Fe 2p XPS spectra of Sm-FeP@CC and FeP@CC; (d) P 2p XPS spectrum of Sm-FeP@CC; (e) ESR spectra of Sm-FeP@CC and FeP@CC. | ||
The morphological characteristics of the samples were characterized by scanning electron microscopy (SEM). The precursor of FeO(OH)@CC presents an array structure consisting of abundant one-dimensional (1D) nanorods on the surface of carbon cloth (Fig. S6, ESI†). After the plasma treatment and topological transformation, the Sm-FeP@CC still maintains a good rod-like array structure (Fig. 3(a)). The average length of Sm-FeP nanorods is about 160 nm and the thickness is about 50 nm (Fig. 3(b)). Likewise, FeP@CC displays a similar structure characteristic of Sm-FeP@CC (Fig. S7, ESI†), implying that Sm doping did not affect the morphology of Sm-FeP@CC. The Sm-FeP nanorods present a porous feature, as verified by transmission electron microscopy (TEM) in Fig. 3(c) and (d), which favors the exposure of more active sites. As shown in Fig. 3(f), the integrated pixel intensity derived from Fig. 3(e) shows two lattice fringe values of 0.280 nm and 0.322 nm, which are slightly larger than those of the FeP (103) and FeP (113) planes (0.276 nm and 0.320 nm). This can be attributed to the lattice expansion of FeP caused by Sm-doping. The nanoarray structure generally increases the active surface area and facilitates contact with the electrolyte,64,65 which leads to good hydrophilicity of Sm-FeP@CC and FeP@CC with small contact angles of about 23° and 36° (Fig. 3(g)). The high-angle annular dark-field scanning TEM (HAADF-STEM) image (Fig. 3(h)) and the line scanning profiles (Fig. 3(i)) demonstrate the uniform distribution of Sm, Fe and P elements in Sm-FeP, in accordance with elemental mapping images (Fig. 3(j)).
Electrocatalytic performance for the HER
The HER performance of Sm-FeP@CC was explored by using a typical three-electrode cell in an Ar-saturated 1 M KOH solution and compared to those of FeO(OH)@CC, FeP@CC, and commercial Pt/C. Fig. 4(a) shows the linear sweep voltammetry (LSV) curves of all catalysts at a scan rate of 5 mV s−1. As observed, the FeO(OH)@CC shows almost no HER activity. The Sm-FeP@CC requires an overpotential of merely 71 mV to yield the current density of 10 mA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−2, which is 63 mV more negative than that of FeP@CC (Fig. 4(b)). The overpotential of Sm-FeP@CC at a high current density of 100 mA cm−2 is about 175 mV, much smaller than that of FeP@CC (281 mV) and commercial Pt/C (237 mV), indicating that Sm-FeP@CC is expected to be developed for practical industrial application. The good HER performance of Sm-FeP at a high current density may have contributed to the nanorod arrayed structure and the heteroatom Sm doping, which facilitates the mass transfer process and charge-transfer rate between the electrolyte and electrode.66–68 Tafel slope is a good indicator of reaction kinetics and the rate-determining step of electrocatalytic reactions. The Sm-FeP@CC exhibits a much lower Tafel slope of 85.9 mV dec−1 than that of FeP@CC (184.3 mV dec−1), which is close to commercial Pt/C (63.9 mV dec−1), demonstrating the favorable HER kinetics via the Volmer–Heyrovsky route (Fig. 4(c)). Besides, such remarkable HER performance of Sm-FeP@CC surpasses that of most recently reported Fe-based catalysts in alkaline electrolyte, as listed in Fig. 4(d) and Table S1 (ESI†). The HER selectivity of Sm-FeP@CC was further studied by a sealed drainage gas collector system (Fig. S8, ESI†). As displayed in Fig. 4(e), the experimental H2 yield of Sm-FeP@CC is close to the theoretical value and the faradaic efficiency is close to 100%, indicating the high HER selectivity. The Sm-FeP@CC also displays good electrocatalytic stability for the HER. As indicated in Fig. 4(f), the polarization curve after 3000 cyclic voltammetry (CV) cycles overlaps the initial one; while the current density barely changes after more than 40 hours of the i–t chronoamperometric test, which is better than that of FeP@CC (Fig. S9, ESI†). After the i–t test, the nanorod array structure and composition of Sm-FeP@CC are well maintained (Fig. S10 and S11, ESI†), which is probably the main reason for the excellent stability. The above electrocatalytic results confirm that Sm-FeP@CC is a highly active and stable HER electrocatalyst. To elucidate the superior HER activity of Sm-FeP@CC, the reactive active sites were studied through the electrochemical surface area (ECSA), which was calculated by the double-layer capacitance (Cdl). The CV curves at different scan rates in the non-faradaic region were provided in Fig. S12 (ESI†). In Fig. 4(g), the Sm-FeP@CC has a much larger Cdl value of 31.2 mF cm−2 than that of FeP@CC (22.7 mF cm−2) and FeOOH@CC (4.78 mF cm−2). Based on the obtained Cdl values (Fig. 4(g)), the ECSA of Sm-FeP@CC was calculated to be about 780 cm−2, which is much higher than that of FeP@CC (567.5 cm−2) and FeO(OH)@CC (119.5 cm−2), demonstrating the significant improvement of ECSA after the phosphating and the Sm doping. The fast charge transport and high electron conductivity also contribute to the excellent HER performance of Sm-FeP@CC. The electrochemical impedance spectroscopy (EIS) Nyquist plots show that the charge transfer resistance (Rct) for Sm-FeP@CC is smaller than that for FeP@CC and FeO(OH)@CC (Fig. 4(h)).
cm−2, which is 63 mV more negative than that of FeP@CC (Fig. 4(b)). The overpotential of Sm-FeP@CC at a high current density of 100 mA cm−2 is about 175 mV, much smaller than that of FeP@CC (281 mV) and commercial Pt/C (237 mV), indicating that Sm-FeP@CC is expected to be developed for practical industrial application. The good HER performance of Sm-FeP at a high current density may have contributed to the nanorod arrayed structure and the heteroatom Sm doping, which facilitates the mass transfer process and charge-transfer rate between the electrolyte and electrode.66–68 Tafel slope is a good indicator of reaction kinetics and the rate-determining step of electrocatalytic reactions. The Sm-FeP@CC exhibits a much lower Tafel slope of 85.9 mV dec−1 than that of FeP@CC (184.3 mV dec−1), which is close to commercial Pt/C (63.9 mV dec−1), demonstrating the favorable HER kinetics via the Volmer–Heyrovsky route (Fig. 4(c)). Besides, such remarkable HER performance of Sm-FeP@CC surpasses that of most recently reported Fe-based catalysts in alkaline electrolyte, as listed in Fig. 4(d) and Table S1 (ESI†). The HER selectivity of Sm-FeP@CC was further studied by a sealed drainage gas collector system (Fig. S8, ESI†). As displayed in Fig. 4(e), the experimental H2 yield of Sm-FeP@CC is close to the theoretical value and the faradaic efficiency is close to 100%, indicating the high HER selectivity. The Sm-FeP@CC also displays good electrocatalytic stability for the HER. As indicated in Fig. 4(f), the polarization curve after 3000 cyclic voltammetry (CV) cycles overlaps the initial one; while the current density barely changes after more than 40 hours of the i–t chronoamperometric test, which is better than that of FeP@CC (Fig. S9, ESI†). After the i–t test, the nanorod array structure and composition of Sm-FeP@CC are well maintained (Fig. S10 and S11, ESI†), which is probably the main reason for the excellent stability. The above electrocatalytic results confirm that Sm-FeP@CC is a highly active and stable HER electrocatalyst. To elucidate the superior HER activity of Sm-FeP@CC, the reactive active sites were studied through the electrochemical surface area (ECSA), which was calculated by the double-layer capacitance (Cdl). The CV curves at different scan rates in the non-faradaic region were provided in Fig. S12 (ESI†). In Fig. 4(g), the Sm-FeP@CC has a much larger Cdl value of 31.2 mF cm−2 than that of FeP@CC (22.7 mF cm−2) and FeOOH@CC (4.78 mF cm−2). Based on the obtained Cdl values (Fig. 4(g)), the ECSA of Sm-FeP@CC was calculated to be about 780 cm−2, which is much higher than that of FeP@CC (567.5 cm−2) and FeO(OH)@CC (119.5 cm−2), demonstrating the significant improvement of ECSA after the phosphating and the Sm doping. The fast charge transport and high electron conductivity also contribute to the excellent HER performance of Sm-FeP@CC. The electrochemical impedance spectroscopy (EIS) Nyquist plots show that the charge transfer resistance (Rct) for Sm-FeP@CC is smaller than that for FeP@CC and FeO(OH)@CC (Fig. 4(h)).
Theoretical analysis
Density functional theory (DFT) calculations were performed to measure the enhanced HER activity after the Sm modulation over FeP. For the slab model, we compared the surface formation energy of (010), (011), and (101). Afterward, the (011) surface was used for mechanism investigation due to its lowest surface formation energy according to our tested results. After the cleavage of FeP bulk into the (011) slab model, the projected density of states (PDOS) for Fe-3d donates a certain occupancy around the Fermi level (EF), indicating the metallic behavior of FeP for electronic conductivity (Fig. 5(a)). After the introduction of Sm into FeP, the metallic behavior is still conserved and accordingly, the induced Sm exhibits the large occupancy of the valence 4f states below the EF (Fig. 5(b)). However, such Sm-4f states exhibit the orphaned property, which can be attributed to the localized nature that is hard to participate in the chemical bonding, also noted as low covalency in the Sm–P bond. For the HER performance, we completely consider the splitting of the H–OH bond to produce the co-adsorption of *H and *OH and the consecutive H2 formation (Fig. 5(d) and (e)). For the co-adsorption state on FeP, both the *H and *OH are bonded with surface Fe sites, where the adsorption of *OH leads to the obvious bonding states contributed by Fe-3d and O-2p (Fig. S13, ESI†), while for Sm-FeP, the lower contribution of Sm-4f for covalency leads to the limited orbital overlap between the Sm site and *OH, which can be confirmed by the promotion of O-2p states (around 2 eV in PDOS) from bonding states, equipping O-2p states as the labile property (Fig. 5(c)). Such a limited orbital overlap between the Sm site and *OH can decrease the adsorption of *OH, which is also confirmed by free energy calculation in Fig. 5(f). For FeP, the desorption of *OH step is a non-spontaneous process with a great step uphill, which is assigned as the potential-determining step (PDS) in the HER. When introducing Sm into FeP, the limited orbital overlap between Sm and *OH can greatly decrease the step downhill for the formation of *H + *OH, resulting in a thermodynamic spontaneous process for the desorption of *OH step for Sm-FeP. As a result, the PDS process for Sm-FeP was switched to the formation process of H2. As a result, with the modulation effect of Sm into FeP for decreased co-adsorption of *H and *OH, the Sm-FeP delivers the facilitated formation of *H from the water splitting. For the consideration of hydrophilicity, the free energy of H2O adsorption for Sm-FeP was calculated to be −3.05 eV (Fig. S14, ESI†), indicating the spontaneous coverage of H2O molecules for good hydrophilicity of Sm-FeP.Application exploration and universal adaptation
Based on the excellent HER catalytic activity of Sm-FeP@CC, we carried out the explorations of practical application and universal adaptation. A two-electrode OER‖HER electrolyzer was assembled to investigate a feasible catalyst candidate of Sm-FeP@CC for overall water splitting. The Sm-FeP@CC‖RuO2 requires a cell voltage of 1.59 V to achieve a current density of 10 mA cm−2 (Fig. 6(a)), which exceeds FeP@CC. As the cell voltage increases, large amounts of hydrogen and oxygen escape from the surface of the carbon cloth, respectively (Fig. 6(a), inset). Besides, the current density increases sharply following the increase of the cell voltage for the Sm-FeP@CC‖RuO2 (Fig. 6(b)), which leads to a low cell voltage of 1.74 and 1.78 V at 100 and 200 mA cm−2 respectively. The stability of overall water splitting on Sm-FeP@CC‖RuO2 was evaluated by the i–t curve (Fig. S15, ESI†). As observed, the operating current density of Sm-FeP@CC‖RuO2 shows a small attenuation up to 25 h, which is better than that of the FeP@CC‖RuO2 electrolyzer, verifying that the HER activity of Sm-FeP@CC was well retained. To explore the universality of rare-earth-doped FeP for improving the HER, the FeP samples doped with different rare earths (such as Yb, Eu, La and Er) were prepared by a similar method as with Sm-FeP@CC. The successful doping of rare earths on carbon cloth-loaded FeP was confirmed by elemental mapping images (Fig. S16, ESI†), suggesting a universal synthesis strategy. Fig. 6(c) and (d) show that the HER activity of the catalysts after rare earth doping has been improved to varying degrees, implying that rare-earth-doped FeP is a promising class of HER catalyst.Conclusions
In summary, a series of RE-doped FeP catalysts was synthesized through a universal plasma-assisted strategy for improving the HER performance. An Ar-plasma beam with high energy can shed the surface light atoms to form vacancies, which facilitates the doping of RE elements. Taking Sm-FeP as an example, it exhibits high electrocatalytic performance for the HER including a low overpotential, a small Tafel slope and good long-term stability, which exceeds the pure FeP catalyst. The as-assembled Sm-FeP@CC‖RuO2 electrolyzer performs well in water splitting (1.59 V at 10 mA cm−2). Theoretical calculations reveal that the increased HER performance on Sm-FeP is due to the weakened co-adsorption of *OH and *H that are contributed by the splitting of H2O, where the labile O 2p state in *OH denotes the weak bonding at the Sm site. As a result, the formation of *H by removing the *OH is favored for the consecutive H2 release. Finally, the plasma-assisted strategy proved to be useful for the synthesis of other RE-FeP (RE = Yb, Eu, La, and Er) catalysts in general, which also exhibited varying degrees of performance enhancement towards the HER.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22109073, 22072067), the Natural Science Foundation of Jiangsu Province (BK20221321), Jiangsu Specially Appointed Professor Plan, and Science and Technology Innovation Project for Overseas Researchers in Nanjing. The authors are also grateful for support from the National and Local Joint Engineering Research Center of Biomedical Functional Materials and a project sponsored by the Priority Academic Program Development of Jiangsu Higher Education Institutions.References
- G. Glenk and S. Reichelstein, Economics of converting renewable power to hydrogen, Nat. Energy, 2019, 4, 216–222 CrossRef CAS.
- Q. Zhou, Z. Wang, H. Yuan, J. Wang and H. Hu, Rapid hydrogen adsorption–desorption at sulfur sites via an interstitial carbon strategy for efficient HER on MoS2, Appl. Catal., B, 2023, 332, 122750 CrossRef CAS.
- J. Zhang, R. Cui, C. Gao, L. Bian, Y. Pu, X. Zhu, X. A. Li and W. Huang, Cation-modulated HER and OER activities of hierarchical VOOH hollow architectures for high-efficiency and stable overall water splitting, Small, 2019, 15, 1904688 CrossRef CAS.
- Y. Huang, M. Li, F. Pan, Z. Zhu, H. Sun, Y. Tang and G. Fu, Plasma-induced Mo-doped Co3O4 with enriched oxygen vacancies for electrocatalytic oxygen evolution in water splitting, Carbon Energy, 2022, 5, e279 Search PubMed.
- D. Wang, X. Jiang, Z. Lin, X. Zeng, Y. Zhu, Y. Wang, M. Gong, Y. Tang and G. Fu, Ethanol-induced hydrogen insertion in ultrafine IrPdH boosts pH-universal hydrogen evolution, Small, 2022, 18, 2204063 CrossRef CAS.
- S. Li, P. Ren, C. Yang, X. Liu, Z. Yin, W. Li, H. Yang, J. Li, X. Wang, Y. Wang, R. Cao, L. Lin, S. Yao, X. Wen and D. Ma, Fe5C2 nanoparticles as low-cost HER electrocatalyst: the importance of Co substitution, Sci. Bull., 2018, 63, 1358–1363 CrossRef CAS.
- W. Sheng, Z. Zhuang, M. Gao, J. Zheng, J. G. Chen and Y. Yan, Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy, Nat. Commun., 2015, 6, 5848 CrossRef CAS PubMed.
- B. Ren, J. Cao, H. Zhang, C. Han and W. Xu, Recent progress in the development of single-atom electrocatalysts for highly efficient hydrogen evolution reactions, Mater. Chem. Front., 2023 10.1039/D3QM00157A.
- Q. Dang, Y. Sun, X. Wang, W. Zhu, Y. Chen, F. Liao, H. Huang and M. Shao, Carbon dots-Pt modified polyaniline nanosheet grown on carbon cloth as stable and high-efficient electrocatalyst for hydrogen evolution in pH-universal electrolyte, Appl. Catal., B, 2019, 257, 117905 CrossRef CAS.
- S. Anantharaj and S. Noda, Layered 2D PtX2 (X = S, Se, Te) for the electrocatalytic HER in comparison with Mo/WX2 and Pt/C: are we missing the bigger picture?, Energy Environ. Sci., 2022, 15, 1461–1478 RSC.
- S. Anantharaj, P. E. Karthik, B. Subramanian and S. Kundu, Pt nanoparticle anchored molecular self-assemblies of DNA: an extremely stable and efficient HER electrocatalyst with ultralow Pt content, ACS Catal., 2016, 6, 4660–4672 CrossRef CAS.
- Y. Guo, B. Hou, X. Cui, X. Liu, X. Tong and N. Yang, Pt Atomic layers loosted hydrogen evolution reaction in nonacidic media, Adv. Energy Mater., 2022, 12, 2201548 CrossRef CAS.
- X. Lu, X. Tan, Q. Zhang, R. Daiyan, J. Pan, R. Chen, H. A. Tahini, D.-W. Wang, S. C. Smith and R. Amal, Versatile electrocatalytic processes realized by Ni, Co and Fe alloyed core coordinated carbon shells, J. Mater. Chem. A, 2019, 7, 12154–12165 RSC.
- Y. Gu, B. Wei, D. Legut, Z. Fu, S. Du, H. Zhang, J. S. Francisco and R. Zhang, Single atom-modified hybrid transition metal carbides as efficient hydrogen evolution reaction catalysts, Adv. Funct. Mater., 2021, 31, 2104285 CrossRef CAS.
- D. Jin, F. Qiao, H. Chu and Y. Xie, Progress in electrocatalytic hydrogen evolution of transition metal alloys: synthesis, structure, and mechanism analysis, Nanoscale, 2023, 15, 7202 RSC.
- W. Du, Y. Shi, W. Zhou, Y. Yu and B. Zhang, Unveiling the in situ dissolution and polymerization of Mo in Ni4Mo alloy for promoting the hydrogen evolution reaction, Angew. Chem., Int. Ed., 2021, 60, 7051–7055 CrossRef CAS PubMed.
- Z. Zhu, H. Yin, C.-T. He, M. Al-Mamun, P. Liu, L. Jiang, Y. Zhao, Y. Wang, H.-G. Yang, Z. Tang, D. Wang, X.-M. Chen and H. Zhao, Ultrathin transition metal dichalcogenide/3d metal hydroxide hybridized nanosheets toenhance hydrogen evolution activity, Adv. Mater., 2018, 30, 1801171 CrossRef PubMed.
- Y. Zhu, X. Wang, X. Zhu, Z. Wu, D. Zhao, F. Wang, D. Sun, Y. Tang, H. Li and G. Fu, Improving the oxygen evolution activity of layered double-hydroxide via erbium-induced electronic engineering, Small, 2023, 19, 2206531 CrossRef CAS.
- D. Tian, S. R. Denny, K. Li, H. Wang, S. Kattel and J. G. Chen, Density functional theory studies of transition metal carbides and nitrides as electrocatalysts, Chem. Soc. Rev., 2021, 50, 12338–12376 RSC.
- J. Li, M. Song, Y. Hu, Y. Zhu, J. Zhang and D. Wang, Hybrid heterostructure Ni3N|NiFeP/FF self-supporting electrode for high-current-density alkaline water electrolysis, Small Methods, 2023, 7, 2201616 CrossRef CAS PubMed.
- Y. Zhang, J. Yang, Z. Yu, Y. Hou, R. Jiang, J. Huang, F. Yang, S. Yao, L. Gao and W. Tang, Modulating carbon-supported transition metal oxide by electron-giving and electron-absorbing functional groups towards efficient overall water splitting, Chem. Eng. J., 2021, 416, 129124 CrossRef CAS.
- X.-F. Lu, L.-F. Gu, J.-W. Wang, J.-X. Wu, P.-Q. Liao and G.-R. Li, Bimetal-organic framework derived CoFe2O4/C porous hybrid nanorod arrays as high-performance electrocatalysts for oxygen evolution reaction, Adv. Mater., 2017, 29, 1604437 CrossRef.
- K. Liu, Z. Zhu, M. Jiang, L. Li, L. Ding, M. Li, D. Sun, G. Yang, G. Fu and Y. Tang, Boosting electrocatalytic oxygen evolution over Ce-Co9S8 core-shell nanoneedle arrays by electronic and architectural dual engineering, Chem. – Eur. J., 2022, 28, e202200664 CAS.
- Y. Guo, T. Park, J. W. Yi, J. Henzie, J. Kim, Z. Wang, B. Jiang, Y. Bando, Y. Sugahara, J. Tang and Y. Yamauchi, Nanoarchitectonics for transition-metal-sulfide-based electrocatalysts for water splitting, Adv. Mater., 2019, 31, 1807134 CrossRef PubMed.
- J. Su, J. Zhou, L. Wang, C. Liu and Y. Chen, Synthesis and application of transition metal phosphides as electrocatalyst for water splitting, Sci. Bull., 2017, 62, 633–644 CrossRef CAS.
- A. Parra-Puerto, K. L. Ng, K. Fahy, A. E. Goode, M. P. Ryan and A. Kucernak, Supported transition metal phosphides: activity survey for HER, ORR, OER, and corrosion resistance in acid and alkaline electrolytes, ACS Catal., 2019, 9, 11515–11529 CrossRef CAS.
- H. J. Pei, L. M. Zhang, G. Zhi, D. Z. Kong, Y. Wang, S. Z. Huang, J. H. Zang, T. T. Xu, H. Wang and X. J. Li, Rational construction of hierarchical porous FeP nanorod arrays encapsulated in polypyrrole for efficient and durable hydrogen evolution reaction, Chem. Eng. J., 2022, 433, 133643 CrossRef CAS.
- P. Jiang, Q. Liu, Y. H. Liang, J. Q. Tian, A. M. Asiri and X. P. Sun, A cost-effective 3D hydrogen evolution cathode with high catalytic activity: FeP nanowire array as the active phase, Angew. Chem., Int. Ed., 2014, 53, 12855–12859 CrossRef CAS.
- Y. Shi and B. Zhang, Recent advances in transition metal phosphide nanomaterials: synthesis and applications in hydrogen evolution reaction, Chem. Soc. Rev., 2016, 45, 1529–1541 RSC.
- J. Q. Zhang, X. Shang, H. Ren, J. Q. Chi, H. Fu, B. Dong, C. G. Liu and Y. M. Chai, Modulation of inverse spinel Fe3O4 by phosphorus doping as an industrially promising electrocatalyst for hydrogen evolution, Adv. Mater., 2020, 32, 1907792 CrossRef CAS.
- Y. D. Yao, N. Mahmood, L. Pan, G. Q. Shen, R. R. Zhang, R. J. Gao, F. E. Aleem, X. Y. Yuan, X. W. Zhang and J. J. Zou, Iron phosphide encapsulated in P-doped graphitic carbon as efficient and stable electrocatalyst for hydrogen and oxygen evolution reactions, Nanoscale, 2018, 10, 21327–21334 RSC.
- X. J. Zhai, Q. P. Yu, G. S. Liu, J. L. Bi, Y. Zhang, J. Q. Chi, J. P. Lai, B. Yang and L. Wang, Hierarchical microsphere MOF arrays with ultralow Ir doping for efficient hydrogen evolution coupled with hydrazine oxidation in seawater, J. Mater. Chem. A, 2021, 9, 27424–27433 RSC.
- S. Zhu, L. Yang, J. Bai, Y. Chu, J. Liu, Z. Jin, C. Liu, J. Ge and W. Xing, Ultra-stable Pt5La intermetallic compound towards highly efficient oxygen reduction reaction, Nano Res., 2023, 16, 2035–2040 CrossRef CAS.
- X. Wei, Y. Liu, X. Zhu, S. Bo, L. Xiao, C. Chen, T. T. T. Nga, Y. He, M. Qiu, C. Xie, D. Wang, Q. Liu, F. Dong, C.-L. Dong, X.-Z. Fu and S. Wang, Dynamic reconstitution between copper single atoms and clusters for electrocatalytic urea synthesis, Adv. Mater., 2023, 2300020 CrossRef CAS PubMed.
- X. Peng, Y. Mi, H. Bao, Y. Liu, D. Qi, Y. Qiu, L. Zhuo, S. Zhao, J. Sun, X. Tang, J. Luo and X. Liu, Ambient electrosynthesis of ammonia with efficient denitration, Nano Energy, 2020, 78, 105321 CrossRef CAS.
- X. Wang, J. Wang, P. Wang, L. Li, X. Zhang, D. Sun, Y. Li, Y. Tang, Y. Wang and G. Fu, Engineering 3d–2p–4f gradient orbital coupling to enhance electrocatalytic oxygen reduction, Adv. Mater., 2022, 34, 2206540 CrossRef CAS.
- M. Li, Y. Wang, Y. Zheng, G. Fu, D. Sun, Y. Li, Y. Tang and T. Ma, Gadolinium-induced valence structure engineering for enhanced oxygen electrocatalysis, Adv. Energy Mater., 2020, 10, 1903833 CrossRef CAS.
- W. Peng, X. G. Li, Z. M. He, Z. S. Li, X. Y. Zhang, X. P. Sun, Q. Li, H. Yang, J. T. Han and Y. H. Huang, Electron density modulation of MoP by rare earth metal as highly efficient electrocatalysts for pH-universal hydrogen evolution reaction, Appl. Catal., B, 2021, 299, 120657 CrossRef.
- M. Li, X. Wang, K. Liu, H. Sun, D. Sun, K. Huang, Y. Tang, W. Xing, H. Li and G. Fu, Reinforce the Co–O covalency via Ce(4f)–O(2p)–Co(3d) gradient orbital coupling for high-efficiency oxygen evolution, Adv. Mater., 2023 DOI:10.1002/adma.202302462.
- L. Yin, S. Zhang, M. Sun, S. Wang, B. Huang and Y. Du, Heteroatom-driven coordination fields altering single cerium atom sites for efficient oxygen reduction reaction, Adv. Mater., 2023 DOI:10.1002/adma.202302485.
- Z. Liang, L. Yin, H. Yin, Z. Yin and Y. Du, Rare earth element based single-atom catalysts: synthesis, characterization and applications in photo/electro-catalytic reactions, Nanoscale Horiz., 2022, 7, 31–40 RSC.
- S. Meng, G. Li, P. Wang, M. He, X. Sun and Z. Li, Rare earth-based MOFs for photo/electrocatalysis, Mater. Chem. Front., 2023, 7, 806–827 RSC.
- X. Wang, Y. Zhu, H. Li, J.-M. Lee, Y. Tang and G. Fu, Rare-earth single-atom catalysts: a new frontier in photo/electrocatalysis, Small Methods, 2022, 6, 2200413 CrossRef CAS PubMed.
- X. Wang, Y. Tang, J.-M. Lee and G. Fu, Recent advances in rare-earth-based materials for electrocatalysis, Chem. Catal., 2022, 2, 967–1008 CrossRef CAS.
- H. Sun, Z. Yan, C. Tian, C. Li, X. Feng, R. Huang, Y. Lan, J. Chen, C.-P. Li, Z. Zhang and M. Du, Bixbyite-type Ln2O3 as promoters of metallic Ni for alkaline electrocatalytic hydrogen evolution, Nat. Commun., 2022, 13, 3857 CrossRef CAS PubMed.
- F. Li, M. Jiang, C. Lai, H. Xu, K. Zhang and Z. Jin, Yttrium- and cerium-codoped ultrathin metal–organic framework nanosheet arrays for high-efficiency electrocatalytic overall water splitting, Nano Lett., 2022, 22, 7238–7245 CrossRef CAS PubMed.
- F. Gong, S. Ye, M. Liu, J. Zhang, L. Gong, G. Zeng, E. Meng, P. Su, K. Xie, Y. Zhang and J. Liu, Boosting electrochemical oxygen evolution over yolk-shell structured O–MoS2 nanoreactors with sulfur vacancy and decorated Pt nanoparticles, Nano Energy, 2020, 78, 105284 CrossRef CAS.
- J. Wan, W. Chen, C. Jia, L. Zheng, J. Dong, X. Zheng, Y. Wang, W. Yan, C. Chen, Q. Peng, D. Wang and Y. Li, Defect effects on TiO2 nanosheets: stabilizing single atomic site au and promoting catalytic properties, Adv. Mater., 2018, 30, 1705369 CrossRef PubMed.
- I. Kumar and A. K. Gathania, Photoluminescence and quenching study of the Sm3+-doped LiBaPO4 phosphor, J. Mater. Sci.: Mater. Electron., 2022, 33, 328–341 CrossRef CAS.
- Z. Li, X. Wu, X. Jiang, B. Shen, Z. Teng, D. Sun, G. Fu and Y. Tang, Surface carbon layer controllable Ni3Fe particles confined in hierarchical N-doped carbon framework boosting oxygen evolution reaction, Adv. Powder Mater., 2022, 1, 100020 CrossRef.
- T. Li, Y. Hu, K. Liu, J. Yin, Y. Li, G. Fu, Y. Zhang and Y. Tang, Hollow yolk-shell nanoboxes assembled by Fe-doped Mn3O4 nanosheets for high-efficiency electrocatalytic oxygen reduction in Zn–Air battery, Chem. Eng. J., 2022, 427, 131992 CrossRef CAS.
- Y. Deng, X. Xi, Y. Xia, Y. Cao, S. Xue, S. Wan, A. Dong and D. Yang, 2D FeP nanoframe superlattices via space-confined topochemical transformation, Adv. Mater., 2022, 34, 2109145 CrossRef CAS.
- M. Wang, Y. Tuo, X. Li, Q. Hua, F. Du and L. Jiang, Mesoporous Mn-doped FeP: facile synthesis and enhanced electrocatalytic activity for hydrogen evolution in a wide ph range, ACS Sustainable Chem. Eng., 2019, 7, 12419–12427 CAS.
- X. Zhao, Z. Zhang, X. Cao, J. Hu, X. Wu, A. Y. R. Ng, G.-P. Lu and Z. Chen, Elucidating the sources of activity and stability of FeP electrocatalyst for hydrogen evolution reactions in acidic and alkaline media, Appl. Catal., B, 2020, 260, 118156 CrossRef CAS.
- Y. X. Luo, W. B. Qiu, R. P. Liang, X. H. Xia and J. D. Qiu, Mo-doped FeP nanospheres for artificial nitrogen fixation, ACS Appl. Mater. Interfaces, 2020, 12, 17452–17458 CrossRef CAS.
- W. Zhong, C. Yang, J. Wu, W. Xu, R. Zhao, H. Xiang, K. Shen and X. Li, Adsorption site engineering: Cu–Ni(OH)2 sheets for efficient hydrogen evolution, J. Mater. Chem. A, 2021, 9, 17521–17527 RSC.
- G. Zhou, S. Zhang, Y. Zhu, J. Li, K. Sun, H. Pang, M. Zhang, Y. Tang and L. Xu, Manipulating the rectifying contact between ultrafine Ru nanoclusters and N-Doped carbon nanofibers for high-efficiency ph-universal electrocatalytic hydrogen evolution, Small, 2023, 19, 2206781 CrossRef CAS PubMed.
- Y. Liu, Q. Feng, W. Liu, Q. Li, Y. Wang, B. Liu, L. Zheng, W. Wang, L. Huang, L. Chen, X. Xiong and Y. Lei, Boosting interfacial charge transfer for alkaline hydrogen evolution via rational interior Se modification, Nano Energy, 2021, 81, 105641 CrossRef CAS.
- Z. Wu, D. Nie, M. Song, T. Jiao, G. Fu and X. Liu, Facile synthesis of Co–Fe–B–P nanochains as an efficient bifunctional electrocatalysts for overall water-splitting, Nanoscale, 2019, 11, 7506–7512 RSC.
- M. Song, Z. Zhang, Q. Li, W. Jin, Z. Wu, G. Fu and X. Liu, Ni-foam supported Co(OH)F and Co–P nanoarrays for energy-efficient hydrogen production via urea electrolysis, J. Mater. Chem. A, 2019, 7, 3697–3703 RSC.
- Y. Zhang, L. Gao, E. J. M. Hensen and J. P. Hofmann, Evaluating the stability of Co2P electrocatalysts in the hydrogen evolution reaction for both acidic and alkaline electrolytes, ACS Energy Lett., 2018, 3, 1360–1365 CrossRef CAS PubMed.
- S. Liu, J. Zhu, M. Sun, Z. Ma, K. Hu, T. Nakajima, X. Liu, P. Schmuki and L. Wang, Promoting the hydrogen evolution reaction through oxygen vacancies and phase transformation engineering on layered double hydroxide nanosheets, J. Mater. Chem. A, 2020, 8, 2490–2497 RSC.
- Y. Ren, X. Lei, H. Wang, J. Xiao and Z. Qu, Enhanced Catalytic Performance of La-doped CoMn2O4 catalysts by regulating oxygen species activity for VOCs oxidation, ACS Catal., 2023, 13, 8293–8306 CrossRef CAS.
- Y. Li, J. Liu, X. Chen, J. Wu, N. Li, W. He and Y. Feng, T Tailoring surface properties of electrodes for synchronous enhanced extracellular electron transfer and enriched exoelectrogens in microbial fuel cells, ACS Appl. Mater. Interfaces, 2021, 13, 58508–58521 CrossRef CAS PubMed.
- Z. Zhao, W. Jin, L. Xu, C. Wang, Y. Zhang and Z. Wu, Ultrafine Ir nanoparticles decorated on FeP/FeOOH with abundant interfaces via a facile corrosive approach for alkaline water-splitting, J. Mater. Chem. A, 2021, 9, 12074–12079 RSC.
- L. Zhang, J. Zhang, J. Fang, X.-Y. Wang, L. Yin, W. Zhu and Z. Zhuang, Cr-doped CoP nanorod arrays as high-performance hydrogen evolution reaction catalysts at high current density, Small, 2021, 17, 2100832 CrossRef CAS PubMed.
- Y. Tan, R. Xie, S. Zhao, X. Lu, L. Liu, F. Zhao, C. Li, H. Jiang, G. Chai, D. J. L. Brett, P. R. Shearing, G. He and I. P. Parkin, Facile fabrication of robust hydrogen evolution electrodes under high current densities via Pt@Cu interactions, Adv. Funct. Mater., 2021, 31, 2105579 CrossRef CAS.
- B. Geng, F. Yan, L. Liu, C. Zhu, B. Li and Y. Chen, Ni/MoC heteronanoparticles encapsulated within nitrogen-doped carbon nanotube arrays as highly efficient self-supported electrodes for overall water splitting, Chem. Eng. J., 2021, 406, 126815 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3qm00516j |
| This journal is © the Partner Organisations 2023 |





