Open Access Article
Open Access ArticleVariable-valence element doping mediated photogenerated electron trapping for selective oxidation reactions†
Xia
Zhong‡
,
Yan
Zhao‡
,
Lei
Li
 ,
Xin
He
,
Hui
Wang
*,
Xiaodong
Zhang
,
Xin
He
,
Hui
Wang
*,
Xiaodong
Zhang
 * and
Yi
Xie
* and
Yi
Xie
 *
*
Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, Anhui 230026, China. E-mail: wanghuig@ustc.edu.cn; zhxid@ustc.edu.cn; yxie@ustc.edu.cn
First published on 24th April 2024
Abstract
Photocatalytic selective oxidation provides a green and mild way of producing high-value added chemicals, whose conversion and selectivity are limited by complex oxidation pathways mediated by various reactive radical species. Thus, using photogenerated holes as an oxidant to directly drive these oxidation reactions could overcome the above problems, whereas the simultaneously formed electrons would cause the quenching of holes or the formation of other unfavorable reactive oxygen species that would affect the reaction efficiency. Herein, a variable-valence element doping method was proposed to realize hole-mediated photocatalytic selective oxidation. By taking Cu-doped Bi2WO6 as a typical prototype, we show that the doped Cu element with monovalent and divalent character can effectively trap photogenerated electrons, thereby boosting hole accumulation for selective oxidation reactions. As expected, Cu-doped Bi2WO6 exhibited excellent catalytic performances in oxidative coupling of benzylamines. This study provides a perspective on optimizing selective oxidation by hole regulation.
Broader contextPhotocatalytic selective oxidation is considered a green route for the synthesis of high value-added organic chemicals. However, the complex reaction pathways caused by a variety of free radicals limit the conversion and selectivity of photocatalysis. Using holes as a single oxidant is a promising strategy for photocatalytic selective oxidation. Herein, variable-valence element Cu is introduced into the semiconductor-based photocatalyst Bi2WO6 as electron-trapping sites to regulate the hole behavior. Both experimental and theoretical investigations show that the impurity level dominated by Cu states could effectively capture photogenerated electrons, thus allowing more holes to participate in selective oxidation reactions. In addition, Cu-doping-induced reconstruction of the electronic structure of Bi2WO6 not only promotes the dissociation of excitons in the confined layered structure to free carriers, but also optimizes the transport properties of the sample. Owing to the above advantages, Cu-doped Bi2WO6 shows excellent conversion and selectivity in photocatalytic oxidative coupling of amines. |
Introduction
Selective oxidation is one of the largest industrial processes in producing modern chemicals, and plenty of important intermediates such as imines, epoxides and aldehydes are synthesized by using suitable oxidants.1–3 In general, these organic reactions are usually carried out under harsh conditions such as high temperature and pressure, accompanied by high energy consumption and environmental pollution. Photocatalytic selective oxidation triggered by semiconductor-based photocatalysts provides a green and mild way of preparing high value-added organic compounds.4 Although some progress has been made, photocatalytic oxidation reactions still face the problem of low selectivity, due to the continuous and complex character of reaction processes.5,6 For example, the parallel oxidation pathways dominated by different photoinduced reactive radical species would result in a wide product distribution.7 In that case, controlling the generation of certain oxidants is considered to be the key scientific problem in photocatalytic selective oxidation reactions. As is known, reactive oxygen species (ROS) and photogenerated holes are the dominant oxidants for photocatalytic aerobic oxidation reactions.8 Generally, ROS including superoxide, singlet oxygen, and hydroxyl radicals with various lifetimes and oxidizability may trigger uncontrolled oxidation processes or even destruction of substrates and products.9,10 In contrast, directly using holes as a single oxidant to initiate photocatalytic organic oxidation would be a promising pathway in avoiding overoxidation reactions.As for the semiconductor-based photocatalysts, electrons and holes are photogenerated simultaneously, and thus sacrificial agents to quench electrons are widely used to boost the accumulation of holes during the reaction. However, these sacrificial agents, such as potassium persulfate and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, are difficult to separate from the reaction system, resulting in the pollution of the products. Alternatively, constructing an electron trap state in the semiconductor-based photocatalysts would be a practical way of offering selective hole accumulation and avoiding the use of external agents.11 Besides, in order to preserve the trapped photogenerated electrons, the trap state should store electrons at the same time. Bearing this in mind, we pay our attention to element doping, which has attracted extensive attention due to its advantages in regulating the electronic band structure, promoting charge separation, expanding light absorption, and so on.12–14 Meanwhile, the introduction of variable-valence elements, such as Cu with monovalent and divalent character, could not only generate an impurity state, but also offer a redox pair based on Cu(II)/Cu(I), as schematically illustrated in Scheme 1.15–18
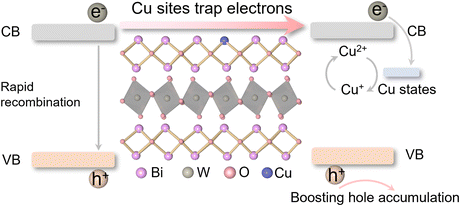 | ||
| Scheme 1 A scheme of variable-valence Cu doping in Bi2WO6 for capturing photogenerated electrons and boosting hole accumulation. | ||
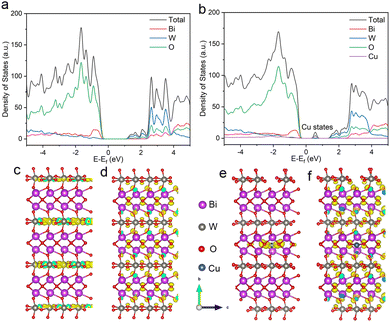
Herein, a typical layered semiconductor, bismuth tungstate (Bi2WO6), with ideal visible light absorption was selected as a model, and we interrogated the feasibility of variable-valence Cu doping in regulating the involved photogenerated hole behaviors. Density functional theory (DFT) calculations show that Cu doping forms a deep impurity level and reconstructs the electronic structure of Bi2WO6 (Fig. 1a and b). Compared with shallow states, these deep states could effectively trap photogenerated electrons and prolong the lifetime of holes, which are favorable to hole-mediated photocatalytic selective oxidation.19,20 In addition, the homogeneities of the valence and conduction band-edge states are broken in Cu–Bi2WO6, which could facilitate the dissociation of excitons in the confined layered structure into free carriers.21 Band-edge charge density isosurfaces show that the charge of the conduction band in Cu–Bi2WO6 is mainly localized at Cu sites, while that of the pristine Bi2WO6 is mainly distributed in the [WO4]2− layers (Fig. 1c–f). Electron trapping at Cu sites could effectively restrain the recombination of photogenerated electrons and holes, and promote the encounter of the holes and substrates. Therefore, Cu–Bi2WO6 is expected to exhibit excellent performance in photocatalytic selective oxidation reactions.
Results and discussion
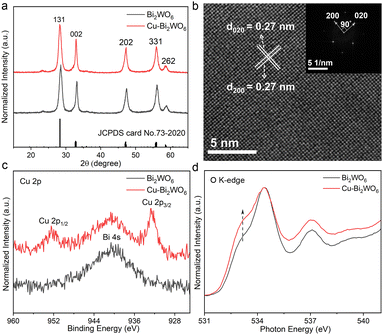
In this work, Cu-doped Bi2WO6 (Cu–Bi2WO6) and pristine Bi2WO6 were synthesized through a hydrothermal method. The powder X-ray diffraction patterns (XRD) of the as prepared samples shown in Fig. 2a suggested similar structures of them, which could be well indexed to an orthorhombic phase of Bi2WO6 (JCPDS No. 73-2020). As shown in Fig. S1 (ESI†), the transmission electron microscopy (TEM) images of Cu–Bi2WO6 and pristine Bi2WO6 indicated their 2D sheet-like morphologies. The high-resolution TEM (HRTEM) image of the Cu–Bi2WO6 nanosheets in Fig. 2b displays distinct mutually perpendicular lattice fringes with an interplanar spacing of 0.27 nm, and the fast Fourier transition (FFT) pattern of the top facet obviously determines the two crystal plane orientations, and the angle labelled is 90° which is consistent with the theoretical value between the (020) and (200) planes.22 Bi2WO6 exhibits similar lattice fringes (Fig. S2, ESI†). The energy-dispersive X-ray spectroscopy (EDS) (Fig. S3, ESI†) elemental mapping and electron probe microanalysis (EPMA) (Fig. S4, ESI†) confirm the existence of homogeneously distributed Cu in elemental doped Bi2WO6. The content of doped Cu is estimated to be about 0.2 wt% in Cu–Bi2WO6 by inductively coupled plasma atomic emission spectroscopy (ICP-AES). Furthermore, X-ray photoelectron spectroscopy (XPS) was used to analyse the chemical compositions and surface chemical states of the related elements. The peaks observed at around 952.5 eV and 932.7 eV can be attributed to the Cu 2p1/2 and Cu 2p3/2 levels, respectively, providing direct evidence for the existence of surface copper (Fig. 2c). Note that the peak around 940 eV is indexed to Bi 4s. Meanwhile, both Bi 4f spectra and W 4f spectra (Fig. S5, ESI†) are almost comparable for the two samples, indicating that their chemical states are hardly affected by the copper. As shown in Fig. S6 (ESI†), the peak at 720![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−1 of the Raman spectra was interpreted as an antisymmetric bridging mode, associated with the tungstate chains. The characteristic bands near 795 cm−1 and 830 cm−1 could be assigned to the asymmetric and symmetric vibrations of O–W–O, respectively.23 For Cu–Bi2WO6, the symmetric stretching vibration of the O–W–O bond is weakened, indicating that copper doping would effectively modify the O–W–O vibration scenarios. X-ray absorption near-edge structure (XANES), a strong technique to study the electronic structure of materials, was carried out to reveal the effect of Cu doping on Bi2WO6. The O K-edge XANES spectra in Fig. 2d reveal a main peak located at the X-ray energy of 534.4 eV with a shoulder peak at 533.2 eV for both samples. Compared with Bi2WO6, the intensity of the shoulder peak in Cu–Bi2WO6 showed a sharp increase, indicating the rehybridization of O 2p and Bi 6p due to local structural distortion associated with Cu doping.24 And the electron occupied state of the oxygen atom in Cu–Bi2WO6 decreased in comparison to the pristine sample, which is possibly related to electron trapping at the Cu sites.25 Besides, both samples exhibit similar Brunner–Emmett–Teller (BET) surface area (Fig. S7, ESI†). According to the above characterization and analysis, we could confirm that Cu-doped Bi2WO6 with local structural distortion was successfully prepared.
cm−1 of the Raman spectra was interpreted as an antisymmetric bridging mode, associated with the tungstate chains. The characteristic bands near 795 cm−1 and 830 cm−1 could be assigned to the asymmetric and symmetric vibrations of O–W–O, respectively.23 For Cu–Bi2WO6, the symmetric stretching vibration of the O–W–O bond is weakened, indicating that copper doping would effectively modify the O–W–O vibration scenarios. X-ray absorption near-edge structure (XANES), a strong technique to study the electronic structure of materials, was carried out to reveal the effect of Cu doping on Bi2WO6. The O K-edge XANES spectra in Fig. 2d reveal a main peak located at the X-ray energy of 534.4 eV with a shoulder peak at 533.2 eV for both samples. Compared with Bi2WO6, the intensity of the shoulder peak in Cu–Bi2WO6 showed a sharp increase, indicating the rehybridization of O 2p and Bi 6p due to local structural distortion associated with Cu doping.24 And the electron occupied state of the oxygen atom in Cu–Bi2WO6 decreased in comparison to the pristine sample, which is possibly related to electron trapping at the Cu sites.25 Besides, both samples exhibit similar Brunner–Emmett–Teller (BET) surface area (Fig. S7, ESI†). According to the above characterization and analysis, we could confirm that Cu-doped Bi2WO6 with local structural distortion was successfully prepared.
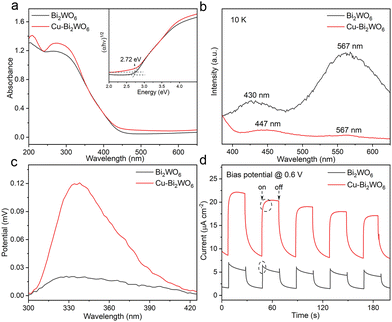
Elemental doping can greatly influence the optical properties and band structures of photocatalysts. As shown in Fig. 3a, there is an additional absorption tail in the UV-vis spectra of Cu–Bi2WO6 as compared to the undoped sample, which might be related to plenty of defects formed by Cu doping.26 Meanwhile, the valence band maximum (VBM) shifted toward lower energy in Cu–Bi2WO6, closer to the Fermi level, indicating that the hole concentration increased after Cu doping (Fig. S8, ESI†).27 Such broad band-offset in the VBM suggests the interplay between the different types of valence bands, which would contribute to the transport properties of the hole.28 In addition to the band structures and transport properties, Cu doping also has a dramatic impact on photogenerated species. As seen in the low temperature fluorescence spectra (PL), compared with pristine Bi2WO6, the emission peak of the exciton (∼567 nm) in Cu–Bi2WO6 is negligible, implying that excitons dissociate into free electrons and holes (Fig. 3b).29 Moreover, the PL spectra of Cu–Bi2WO6 reveal a remarkable lower intensity as compared to the pristine sample, indicating its suppressed recombination of photoexcited electrons and holes. Time-resolved phosphorescence (PH) spectra (Fig. S9, ESI†) of the two samples were measured to investigate the dynamics of the charge carrier, where Cu–Bi2WO6 shows a shorter PH life compared with Bi2WO6, indicating its fast electron transfer. Weaker PL emission intensity and shorter PH lifetime manifest that Cu doping might effectively promote the photogenerated electron extraction to the Cu sites.30 The surface photovoltage (SPV) (Fig. 3c) and the transient photocurrent response (Fig. 3d) were also used to investigate the carrier separation efficiency. The drastically improved intensity of the SPV signal and the transient photocurrent response had demonstrated improved photogenerated carrier concentration after photoexcitation in Cu–Bi2WO6. In detail, Bi2WO6 shows spikes in the photocurrent, which is generally interpreted as rapid recombination of carriers in Bi2WO6. For Cu–Bi2WO6, as light cuts on, the photocurrent did not rise directly to the highest value, but had a slow rising process. This phenomenon can be understood as that part of the charge carriers are gradually captured by the Cu sites and cannot transmit charge, inhibiting the increase in photocurrent. The photocurrent shows a trailing curve as the light cuts off, which is because of the release of previously trapped charge in Cu sites.31 Electrochemical impedance spectroscopy (EIS) was carried out to detect charge transfer characteristics of the samples (Fig. S10, ESI†), where the Cu–Bi2WO6 possesses smaller charge-transfer resistance (Rct) than that of Bi2WO6, revealing the enhanced charge-transfer process and conductivity. Thus, the results clearly demonstrate that electron capture at Cu sites improves the carrier separation efficiency and optimizes the transport properties of the sample.
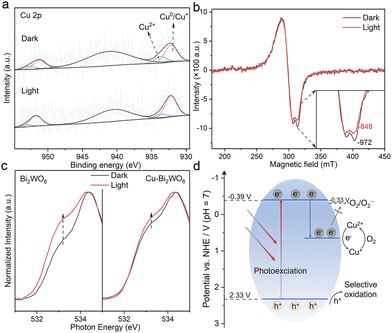
In order to reveal the electron capture at Cu sites of Cu–Bi2WO6, in situ XPS experiments were performed to study the chemical state of Cu–Bi2WO6 in photocatalysis. Fig. 4a shows XPS spectra of Cu 2p in the dark and under irradiation conditions. The peak observed at around 933.7 eV can be attributed to Cu2+, and the peak observed at around 932.2 eV is assigned to Cu0/Cu+.32 It is interesting that the concentration of Cu0/Cu+ in Cu–Bi2WO6 increased from 73.3% to 83.7% after light irradiation, which could be understood as the photogenerated electron accumulation on the Cu2+ species, beneficial for the hole generation. Besides, the Bi 4f and W 4f in situ XPS spectra of Cu–Bi2WO6 exhibit tailing in the low binding energy direction under light irradiation (Fig. S11, ESI†), indicating the appearance of lower chemical states of Bi and W species. In situ ESR spectra under different conditions were also used to unravel the chemical state of the copper species, where Cu–Bi2WO6 exhibited a strong signal of Cu2+ in dark conditions, and hyperfine structure could be observed through the ESR spectra since I = 3/2 of Cu2+ (Fig. 4b).33 Upon illumination, the intensity of Cu2+ decreased, indicating that Cu2+ could accept electrons to form ESR-silent Cu+, confirming the presence of electron capture in the Cu sites. In situ ESR spectra of Bi2WO6 (Fig. S12, ESI†) turned out to possess a typical weak ESR signal centered at g = 2.003 owing to electron trapped oxygen vacancies. Furthermore, XANES measurements under dark and light conditions were carried out to study the influence of carrier trapping by Cu doping. As shown in Fig. 4c, the shoulder peak of Cu–Bi2WO6 changed insignificantly under light conditions compared with the pristine Bi2WO6, indicating that the electron occupied state of the O element had no evident changes in Cu–Bi2WO6 after illumination. Combined with XPS analysis, it can be inferred that owing to the strong electron trapping ability of Cu sites, photogenerated electrons are more easily captured by Cu sites rather than O atoms in Cu–Bi2WO6, which is conducive to improving the tolerance for illumination and stability of the sample. The above in situ tests have proved that Cu doping can trap photogenerated electrons and extend the lifetime of the holes, which can be demonstrated by the oxygen activation behavior. Generally, the hydroxyl radical (˙OH) is generated by the oxidation of adsorbed water, whose generation was dependent on the hole concentration of the system. As shown in Fig. S13 (ESI†), Cu–Bi2WO6 has a strong ESR signal, while Bi2WO6 generates almost no ˙OH, indicating that Cu–Bi2WO6 has a strong ability to generate holes. Due to the electron capture at the Cu sites, Cu–Bi2WO6 has a weak ability to activate oxygen into a superoxide anion radical (˙O2−) (Fig. S14a and b, ESI†). Complementary evolutions of ˙OH and ˙O2− in Cu–Bi2WO6 further confirmed the positive role of Cu doping on the electron capture and hole accumulation. According to all the analysis above and Mott–Schottky plots (Fig. S15, ESI†), we drew the band structure diagram (Fig. 4d), and proposed the catalytic mechanism of Cu–Bi2WO6. In detail, electrons and holes are generated by photoexcitation in Cu–Bi2WO6, and electrons in the conduction band are captured by the Cu states, resulting in the reduction of Cu(II) to Cu(I), which could be reversed via electron-mediated molecular oxygen activation. According to the DFT calculation (Fig. 1b), the Cu-impurity state in Cu–Bi2WO6 is far lying above the conduction band, that is, electrons trapped by these states have insufficient reduction potential to trigger O2 to ˙O2− conversion, which could be confirmed by weaker generation of ˙O2− in Cu–Bi2WO6. The selective removal of photogenerated electrons suppresses hole consumption and hence facilitates the accumulation of holes in the system, which directly leads to the utilization efficiency of photogenerated holes being significantly elevated for selective oxidation reactions.
In view of its boosting hole accumulation and optimizing transport properties, Cu–Bi2WO6 is expected to be an ideal photocatalyst for selective oxidation, such as oxidation of amines to the corresponding imines. Imines and their derivatives are important multifunctional organic intermediates, which are widely used in biology, pharmaceuticals and fine chemical areas.34 Here, selective oxidative coupling of benzylamines was carried out to evaluate the photocatalytic performance of Cu–Bi2WO6. As shown in Table 1, Cu–Bi2WO6 exhibited better oxidizability and selectivity than that of pristine Bi2WO6, where oxygen plays an important role in the reaction process (entry 2). Since O2 to ˙O2− conversion is inhibited in Cu–Bi2WO6 (Fig. S14a and b, ESI†), the oxygen in this system would mainly oxidize low-valence Cu(I) to Cu(II) to realize the cycle between the redox pair of Cu, thereby promoting the continuous formation of holes for oxidative coupling of benzylamine. In detail, entry 1 indicates that the conversion rate of Cu-Bi2WO6 (99%) is three times that of Bi2WO6 (28%). The introduction of methanol as the sacrificial agent for holes in the reaction could suppress the oxidative coupling of benzylamine, indicating the critical role of holes for the selective oxidation (entry 3). Based on the band structure of Cu–Bi2WO6 (Fig. 4d), the valence band maximum of Cu–Bi2WO6 is 2.33 V, which is located at a more positive position than the oxidation potential of benzylamine (0.76 V vs. NHE).35 Thus, the hole in the valence band contributed to oxidation of benzylamine to N-centered free-radical C6H5CH2NH2˙+ in the coupling process (Fig. S16 and S17, ESI†). Besides, we further select a series of benzylamine derivatives to explore the scope of this oxidative coupling of benzylamines reaction. It could be seen that Cu–Bi2WO6 exhibits better catalytic performance than that of Bi2WO6, whether with electron-withdrawing (entries 4–7) or electron-donating (entries 8–10) groups. The excellent catalytic performance of Cu–Bi2WO6 has contributions from the comprehensive regulation of hole behavior and transport properties by copper doping, which renders Cu–Bi2WO6 to be a promising catalyst in selective oxidation reactions.
| Entry | R | Time (h) | Bi2WO6 | Cu-Bi2WO6 | ||
|---|---|---|---|---|---|---|
| Conv.b (%) | Sel.c (%) | Conv.b (%) | Sel.c (%) | |||
| a Reaction conditions: substrate (0.2 mmol), catalyst (10 mg), trifluorotoluene (2 mL), O2 (1 atm), 298 K, xenon lamp (CEL-PF300-T10, Beijing China Education Au-light Technology Co. Ltd) with a cutoff filter (λ ≥ 420 nm). b Determined by NMR analyses, using 1,1,2,2-tetrachloroethane as the internal standard, mol%. c Selectivity = yieldimine × 2/conversionamine, mol%. d Argon atmosphere. e Additional 300 μL of methanol. | ||||||
| 1 | H | 16 | 28 | 84 | 99 | 99 |
| 2d | H | 16 | Trace | Trace | Trace | Trace |
| 3e | H | 16 | 12 | 12 | 48 | 48 |
| 4 | m-F | 16 | 27 | 73 | 78 | 97 |
| 5 | p-Cl | 12 | 19 | 99 | 84 | 99 |
| 6 | m-Cl | 16 | 27 | 99 | 76 | 99 |
| 7 | p-Br | 16 | 28 | 65 | 88 | 93 |
| 8 | m-Me | 16 | 34 | 95 | 95 | 97 |
| 9 | p-Me | 16 | 30 | 70 | 91 | 91 |
| 10 | p-OMe | 16 | 43 | 91 | 77 | 93 |
Conclusions
In summary, a variable-valence element method was proposed to regulate the behavior of photogenerated holes in semiconductor-based photocatalysts for highly efficient selective oxidation reactions. By taking Cu-doped Bi2WO6 as a proof-of-concept model, theoretical and experimental results have proved that Cu element doping would cause energetic disorder to promote exciton dissociation to carriers in the Bi2WO6 system. What is more, the deep impurity level formed by Cu doping can effectively trap photogenerated electrons, obviously improving the concentration and mobility of hole. Besides, the electron-trapping induced reduction of Cu(II) to Cu(I) contributes to improving the transport properties of Bi2WO6. Owing to these advantages, Cu–Bi2WO6 exhibits excellent photocatalytic performance in selective oxidation of amines to imines. This study provides a feasible method for hole-behavior regulation in semiconductor-based photocatalysts for achieving excellent performance in selective oxidation reactions.Experimental
Preparation of samples
Cu-Bi2WO6 was synthesized by a simple hydrothermal method. In detail, 2 mmol bismuth nitrate pentahydrate and 0.15 mmol copper(II) nitrate trihydrate were added to 20 mL acetic acid solution, and 1 mmol sodium tungsten oxide dihydrate was added to 10 mL water, denoted as solutions A and B, respectively. After the solution is fully dissolved, solution B was added to solution A drop by drop. After stirring for 30 min, the mixture was transferred to a 50 mL Teflon-lined autoclave and heated at 180 °C for 12 hours. After natural cooling to room temperature, the precipitation was collected and washed several times with ethanol and distilled water, and then dried under vacuum overnight. For Bi2WO6, except for the fact that copper(II) nitrate trihydrate is not added during the synthetic process, the other synthetic steps are consistent with the above method.Material characterizations
The transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM) and energy dispersive spectrometer (EDS) measurements were performed on a Talos F200X field emission electron microscope with an acceleration voltage of 200 kV. The NMR experiments were conducted with a 400-MHz Bruker AVANCE AV III NMR spectrometer. The X-ray diffraction (XRD) spectra were acquired by a Philips X’Pert Pro Super diffractometer with Cu-Kα radiation (λ = 1.54178 Å). The X-ray photoelectron spectra (XPS) were obtained on an ESCALAB MKII based on C 1s (284.6 eV) and Mg Kα as the excitation source. The ESR trapping experiments were performed on a Jeol JES-FA200 X-band ESR spectrometer. The electrochemical measurements were delivered by an electrochemical workstation (CHI760E, Shanghai Chenhua Limited, China). The X-ray absorption near-edge structure (XANES) spectra were tested at the Beamlines MCD-A and MCD-B (Soochow Beamline for Energy Materials) at the National Synchrotron Radiation Laboratory (NSRL). UV-vis spectra were acquired on a PerkinElmer Lambda 950 UV-vis-NIR spectrophotometer. The steady-state fluorescence and the time-resolved phosphorescence spectra were obtained on a FLUOROLOG-3-TAU fluorescence spectrometer.Electron spin resonance (ESR)-trapping measurements
ESR-trapping tests in water can detect ˙OH by utilizing the tapping reagent DMPO (5,5-dimethyl-1-pyrroline N-oxide). 500 μL of distilled water, 50 μL of DMPO (10 mM) solution and 50 μL of aqueous suspension of the samples (10 mg mL−1) were mixed. After irradiation for 1 min, the mixture was characterized by using a JEOL JES-FA200 electron spin resonance spectrometer (298 K, 9.097 GHz). For the test of amine free-radicals, using DMPO as the radical trapping reagent, 500 μL of acetonitrile, 50 μL of DMPO (10 mM) solution, 20 μL benzylamine and 50 μL of acetonitrile suspension of the sample (10 mg mL−1) were mixed. The signals were collected under dark and light irradiation.Calculation method
The present first principle DFT calculations are performed by the Vienna Ab initio Simulation Package (VASP)36 with the projector augmented wave (PAW) method.37 The self-consistent calculations apply a convergence energy threshold of 10−5 eV. The exchange-functional is treated using the generalized gradient approximation (GGA) of the Perdew–Burke–Ernzerhof (PBE)38 functional. The Brillouin zone integration was performed using 3 × 2 × 3 k-point sampling for both structure optimization and electron self-consistent calculation, respectively. The energy cutoff for the plane wave basis expansion was set to 450 eV and the force on each atom of less than 0.02 eV Å−1 was set for the convergence criterion of geometry relaxation. A 2 × 1 × 2 supercell of Bi2WO6 was adopted for further calculations, while one Bi atom was replaced by a Cu atom in the Cu doping model.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2022YFA1502903), the National Natural Science Foundation of China (T2122004, 92163105, U2032212, 22275179), the Youth Innovation Promotion Association of CAS (Y2021123), the Postdoctoral Fellowship Program of CPSF (GZB20230706), and the Fundamental Research Funds for the Central Universities (WK2060000039, WK2060000068). The numerical calculations in this paper have been done on the supercomputing system in the Supercomputing Center of the University of Science and Technology of China. We also thank the Beamlines MCD-A and MCD-B (Soochow Beamline for Energy Materials) at the National Synchrotron Radiation Laboratory (NSRL) for characterization help. This work was partially carried out at the Instruments Center for Physical Science, University of Science and Technology of China.Notes and references
- L. Xiong and J. Tang, Adv. Energy Mater., 2021, 11, 2003216 CrossRef CAS.
- X. Liu, R. Qi, S. Li, W. Liu, Y. Yu, J. Wang, S. Wu, K. Ding and Y. Yu, J. Am. Chem. Soc., 2022, 144, 23396–23404 CrossRef CAS.
- H. Wang, C. Cao, D. Li, Y. Ge, R. Chen, R. Song, W. Gao, X. Wang, X. Deng, H. Zhang, B. Ye, Z. Li and C. Li, J. Am. Chem. Soc., 2023, 145, 16852–16861 CrossRef CAS PubMed.
- G. Ciamician, Science, 1912, 36, 385–394 CrossRef CAS PubMed.
- X. Xiao, Z. Ruan, Q. Li, L. Zhang, H. Meng, Q. Zhang, H. Bao, B. Jiang, J. Zhou, C. Guo, X. Wang and H. Fu, Adv. Mater., 2022, 34, 2200612 CrossRef CAS PubMed.
- G. Palmisano, V. Augugliaro, M. Pagliaro and L. Palmisano, Chem. Commun., 2007, 3425–3437 RSC.
- J. Tripathy, K. Lee and P. Schmuki, Angew. Chem., Int. Ed., 2014, 53, 12605–12608 CrossRef CAS PubMed.
- Y. Nosaka and A. Y. Nosaka, Chem. Rev., 2017, 117, 11302–11336 CrossRef CAS PubMed.
- W. Liu, Y. Wang, H. Huang, J. Wang, G. He, J. Feng, T. Yu, Z. Li and Z. Zou, J. Am. Chem. Soc., 2023, 145, 7181–7189 CrossRef CAS PubMed.
- Z. Tian, C. Han, Y. Zhao, W. Dai, X. Lian, Y. Wang, Y. Zheng, Y. Shi, X. Pan, Z. Huang, H. Li and W. Chen, Nat. Commun., 2021, 12, 2039 CrossRef CAS PubMed.
- A. Yamakata, J. J. M. Vequizo and H. Matsunaga, J. Phys. Chem. C, 2015, 119, 24538–24545 CrossRef CAS.
- W. Shao, H. Wang and X. Zhang, Dalton Trans., 2018, 47, 12642–12646 RSC.
- C. Liu, D. Hao, J. Ye, S. Ye, F. Zhou, H. Xie, G. Qin, J. Xu, J. Liu, S. Li and C. Sun, Adv. Energy Mater., 2023, 13, 2204126 CrossRef CAS.
- Y. Shi, Z. Yang, L. Shi, H. Li, X. Liu, X. Zhang, J. Cheng, C. Liang, S. Cao, F. Guo, X. Liu, Z. Ai and L. Zhang, Environ. Sci. Technol., 2022, 56, 14478–14486 CrossRef CAS PubMed.
- X. Chen, S. Xiao, H. Wang, W. Wang, Y. Cai, G. Li, M. Qiao, J. Zhu, H. Li, D. Zhang and Y. Lu, Angew. Chem., Int. Ed., 2020, 59, 17182–17186 CrossRef CAS PubMed.
- W. Xie, Y. Liu, X. Zhang, H. Yan, X.-H. Liu, X. Zhang, Q. Zhao and H. Huang, Angew. Chem., Int. Ed., 2024, 63, e202314384 CrossRef CAS PubMed.
- J. Yang, W. Liu, M. Xu, X. Liu, H. Qi, L. Zhang, X. Yang, S. Niu, D. Zhou, Y. Liu, Y. Su, J.-F. Li, Z.-Q. Tian, W. Zhou, A. Wang and T. Zhang, J. Am. Chem. Soc., 2021, 143, 14530–14539 CrossRef CAS PubMed.
- M. Zheng, P. Wang, X. Zhi, K. Yang, Y. Jiao, J. Duan, Y. Zheng and S.-Z. Qiao, J. Am. Chem. Soc., 2022, 144, 14936–14944 CrossRef CAS PubMed.
- J. J. M. Vequizo, H. Matsunaga, T. Ishiku, S. Kamimura, T. Ohno and A. Yamakata, ACS Catal., 2017, 7, 2644–2651 CrossRef CAS.
- E. Pastor, M. Sachs, S. Selim, J. R. Durrant, A. A. Bakulin and A. Walsh, Nat. Rev. Mater., 2022, 7, 503–521 CrossRef CAS.
- Y. Shi, G. Zhan, H. Li, X. Wang, X. Liu, L. Shi, K. Wei, C. Ling, Z. Li, H. Wang, C. Mao, X. Liu and L. Zhang, Adv. Mater., 2021, 33, 2100143 CrossRef CAS PubMed.
- Y. Zhou, Y. Zhang, M. Lin, J. Long, Z. Zhang, H. Lin, J. C.-S. Wu and X. Wang, Nat. Commun., 2015, 6, 8340 CrossRef PubMed.
- H. Huang, C. Zhou, X. Jiao, H. Yuan, J. Zhao, C. He, J. Hofkens, M. B. J. Roeffaers, J. Long and J. A. Steele, ACS Catal., 2020, 10, 1439–1443 CrossRef CAS.
- C.-H. Chen, B.-J. Hwang, C.-Y. Chen, S.-K. Hu, J.-M. Chen, H.-S. Sheu and J.-F. Lee, J. Power Sources, 2007, 174, 938–943 CrossRef CAS.
- F. Frati, M. O. J. Y. Hunault and F. M. F. de Groot, Chem. Rev., 2020, 120, 4056–4110 CrossRef CAS PubMed.
- H. Li, J. Li, Z. Ai, F. Jia and L. Zhang, Angew. Chem., Int. Ed., 2018, 57, 122–138 CrossRef CAS PubMed.
- H. Kang, J. Li, Y. Liu, E. Guo, Z. Chen, D. Liu, G. Fan, Y. Zhang, X. Jiang and T. Wang, J. Mater. Chem. C, 2018, 6, 8479–8487 RSC.
- J. Androulakis, I. Todorov, D.-Y. Chung, S. Ballikaya, G. Wang, C. Uher and M. Kanatzidis, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 115209 CrossRef.
- H. Wang, D. Yong, S. Chen, S. Jiang, X. Zhang, W. Shao, Q. Zhang, W. Yan, B. Pan and Y. Xie, J. Am. Chem. Soc., 2018, 140, 1760–1766 CrossRef CAS PubMed.
- Y. Zhang, J. Zhao, H. Wang, B. Xiao, W. Zhang, X. Zhao, T. Lv, M. Thangamuthu, J. Zhang, Y. Guo, J. Ma, L. Lin, J. Tang, R. Huang and Q. Liu, Nat. Commun., 2022, 13, 58–67 CrossRef CAS PubMed.
- S. Wang, J. Zhan, K. Chen, A. Ali, L. Zeng, H. Zhao, W. Hu, L. Zhu and X. Xu, ACS Sustainable Chem. Eng., 2020, 8, 8214–8222 CrossRef CAS.
- Q. Yang, X. Li, L. Chen, X. Han, F. R. Wang and J. Tang, Angew. Chem., Int. Ed., 2023, 62, e202307907 CrossRef CAS PubMed.
- G. Li, N. M. Dimitrijevic, L. Chen, T. Rajh and K. A. Gray, J. Phys. Chem. C, 2008, 112, 19040–19044 CrossRef CAS.
- X. Lang, H. Ji, C. Chen, W. Ma and J. Zhao, Angew. Chem., Int. Ed., 2011, 50, 3934–3937 CrossRef CAS PubMed.
- H. Liu, C. Xu, D. Li and H. Jiang, Angew. Chem., Int. Ed., 2018, 57, 5379–5383 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- J. P. Perdew, J. A. Chevary, S. H. Vosko, K. A. Jackson, M. R. Pederson, D. J. Singh and C. Fiolhais, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 6671–6687 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ey00024b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |