Open Access Article
Open Access ArticleA tetracyclic-bislactone-based copolymer donor for efficient semitransparent organic photovoltaics†
Mingjie
Li‡
ab,
Tai
An‡
bc,
Zongliang
Ou
bd,
Ke
Jin
bc,
Zhiwen
Jin
 e,
Keyou
Yan
e,
Keyou
Yan
 f,
He
Tian
f,
He
Tian
 g,
Wentao
Wang
h,
Shangfeng
Yang
g,
Wentao
Wang
h,
Shangfeng
Yang
 i,
Guan-Wu
Wang
i,
Guan-Wu
Wang
 *a,
Qiuling
Song
*d,
Zuo
Xiao
*a,
Qiuling
Song
*d,
Zuo
Xiao
 *bc and
Liming
Ding
*bc and
Liming
Ding
 bc
bc
aHefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei 230026, China. E-mail: gwang@ustc.edu.cn
bCenter for Excellence in Nanoscience, Key Laboratory of Nanosystem and Hierarchical Fabrication (CAS), National Center for Nanoscience and Technology, Beijing 100190, China. E-mail: xiaoz@nanoctr.cn
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
dInstitute of Next Generation Matter Transformation, College of Materials Science & Engineering, Huaqiao University, Xiamen 361021, China. E-mail: qsong@hqu.edu.cn
eSchool of Physical Science and Technology, Lanzhou University, Lanzhou 730000, China
fSchool of Environment and Energy, South China University of Technology, Guangzhou 510000, China
gSchool of Integrated Circuits, Tsinghua University, Beijing 100084, China
hSchool of Materials Science and Engineering, Zhejiang Sci-Tech University, Hangzhou 310018, China
iDepartment of Materials Science and Engineering, University of Science and Technology of China, Hefei 230026, China
First published on 13th June 2023
Abstract
A copolymer donor PBDTTPTP based on a tetracyclic bislactone unit, thieno[2′,3′:5,6]pyrano[3,4-d]thieno[3,2-b]pyran-4,9-dione (TPTP), was developed. The single crystal structure of a TPTP derivative indicates the π-extended planar geometry of TPTP and its good coplanarity with the adjacent thiophene units. Thanks to the strong electron-withdrawing properties and good coplanarity of TPTP, the copolymer PBDTTPTP shows a deep HOMO level of −5.60 eV, a small optical bandgap of 1.65 eV and a good hole mobility of 6.57 × 10−4 cm2 V−1 s−1. These features make PBDTTPTP an efficient donor material for semitransparent organic photovoltaics (STOPVs). STOPVs based on PBDTTPTP and N3 gave a high light utilization efficiency of 4.38%, with a power conversion efficiency of 12.26% and an average visible light transmittance of 35.7%.
Introduction
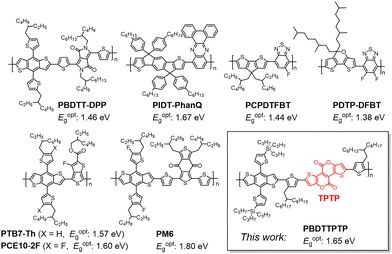
Semitransparent photovoltaics (STPVs) with balanced energy-harvesting functionality and aesthetics show promise for architecture applications, such as power generating windows, curtains, and rooftops.1–5 Power conversion efficiency (PCE) is no longer the only performance criterion. An ideal STPV should allow as much visible photons to pass through as possible to achieve a high average visible light transmittance (AVT), and should effectively convert non-visible photons into electricity to achieve a high PCE.6–9 In this regard, the light utilization efficiency (LUE) (LUE = PCE × AVT) has been suggested as a figure of merit for STPVs.10,11 As the human eyes are mainly sensitive to light at 400–700 nm,12 it is wise to develop STPVs that selectively harvest the near-infrared (NIR) photons.13–16 Organic photovoltaics (OPVs) can meet such requirements since organic semiconductors generally show a band-like absorption spectrum and it is easy to adjust their absorption bands in the NIR region.17–20 In recent years, the development of low-bandgap small molecule acceptors (SMAs) has greatly accelerated the advancement of OPVs. Single-junction OPVs based on a wide-bandgap polymer donor and a low-bandgap SMA can afford >19% PCEs.21–30 However, for semitransparent OPVs (STOPVs) the use of wide-bandgap donors is not a good choice since the donors absorb considerable visible light and reduce the AVT.15,31–33 The development of efficient low-bandgap or mid-bandgap donors is crucial for high-performance STOPVs.In 2012, Yang et al. first reported the diketopyrrolopyrrole based low-bandgap copolymer PBDTT-DPP for STOPVs (Fig. 1).34,35 PBDTT-DPP shows an optical bandgap (Eoptg) of 1.46 eV. STOPVs based on PBDTT-DPP and PC71BM gave a PCE of 4.0% and an AVT of 61%, yielding a LUE of 2.44%. Later, Jen et al. developed a fused-quinoxaline based copolymer PIDT-PhanQ (Eoptg of 1.67 eV) for STOPVs.36 The semitransparent devices showed a LUE of 1.34%, with a PCE of 4.2% and an AVT of 32%. By using a benzothiadiazole based copolymer PCPDTFBT with a smaller Eoptg of 1.44 eV, Jen et al. achieved a higher LUE of 2.37%, with a PCE of 5.0% and an AVT of 47.3%.37 The benzothiadiazole copolymer PDTP-DFBT developed by Yang et al. shows a very small Eoptg of 1.38 eV.38 By blending PDTP-DFBT with a low-bandgap acceptor FOIC, Sun et al. made STOPVs with a LUE of 2.15%, a PCE of 3.5% and an AVT of 61.5%.39 PTB7-Th is a classic low-bandgap copolymer with an Eoptg of 1.57 eV.40,41 By combining PTB7-Th with two low-bandgap acceptors COi8DFIC and IEICO-4F, Zhang et al. achieved a LUE of 1.71% for semitransparent solar cells.42 Zhu et al. improved the LUE to 3.33% by blending PTB7-Th with another acceptor ATT-9.43 By using an outcoupling layer, an antireflection layer and a low-bandgap acceptor A078, Forrest et al. achieved a remarkable LUE of 5.0% for PTB7-Th-based STOPVs.44 Very recently, Chen et al. achieved a high LUE of 5.01% by using PCE10-2F (a congener of PTB7-Th) as the donor and Y6 as the acceptor via a sequential deposition technique.45 PM6 is a highly efficient mid-bandgap copolymer donor for both opaque and semitransparent OPVs.46 By introducing a small molecule 2PACz into the PM6:Y6-BO blend, Huang et al. realized a LUE of 3.39% for semitransparent devices.47 Chen et al. demonstrated high-performance STOPVs with a LUE of 5.0% based on PM6 and two Y-series acceptors, BTP-eC9 and L8-BO.48 By integrating an aperiodic band-pass filter (ABPF), Li et al. achieved a LUE of 5.35%, with a PCE of 11.44% and an AVT of 46.79%.49 The 5.35% LUE is the highest value for STOPVs to date.
Despite the progress mentioned above, there is still plenty of room for designing new and efficient low-bandgap or mid-bandgap copolymers for STOPVs. Previously, our group demonstrated that fused-ring aromatic lactones (FRAL) are efficient building blocks for high-performance copolymer donors.50–57 In this work, we report the synthesis of a copolymer donor PBDTTPTP by using a novel tetracyclic bislactone unit, thieno[2′,3′:5,6]pyrano[3,4-d]thieno[3,2-b]pyran-4,9-dione (TPTP). Thanks to the strong electron-withdrawing properties and good coplanarity of TPTP, PBDTTPTP shows a relatively small Eoptg of 1.65 eV, a deep HOMO level and good hole mobility, and exhibits good performance in STOPVs. An impressive LUE of 4.38% was achieved by the STOPVs based on PBDTTPTP and a low-bandgap acceptor N3.58
Results and discussion
The synthesis of PBDTTPTP is shown in Fig. 2a. Synthetic details are provided in the ESI.† 3-Thiophene boric acid was oxidized by hydrogen peroxide to give the intermediate 3-hydroxythiophene. Then, the hydroxyl group of 3-hydroxythiophene was quickly protected by the methoxymethyl (MOM) group, affording compound 1 in 65% yield. Compound 1 was transformed into the organotin compound 2 in 43% yield. Stille coupling of 2 and dimethyl 2,3-dibromobut-2-enedicarboxylate gave compound 3 in 72% yield. Two stages of bromination transformed compound 3 to compound 4 in 74% yield. Treating 4 with p-toluenesulfonic acid gave the brominated tetracyclic bislactone TPTP-Br in 77% yield. It should be noted that compounds 1–4 with MOM groups are not stable, especially in solution. Therefore, they should be quickly used for the next reaction step. TPTP-Br further underwent Stille coupling with tributyl(4-(2-butyloctyl)thiophen-2-yl)stannane or tributyl(4-(2-hexyldecyl)thiophen-2-yl)stannane to give compound 5 or 6 in 86% or 85% yield, respectively. Treating compound 5 or 6 with N-bromosuccinimide afforded compound 7 or 8 in 98% yield. Finally, Stille copolymerization of 8 and (2,6-bis(trimethylstannyl)benzo[1,2-b:4,5-b′]dithiophene-4,8-diyl)bis(thiophene-5,2-diyl))bis(tripropylsilane) (BDT-Si-Sn) gave the copolymer donor PBDTTPTP in 67% yield. The number-average molecular weight (Mn) and polydispersity index (PDI) for PBDTTPTP are 51.6 kDa and 1.80, respectively. PBDTTPTP shows good solubility in chloroform and chlorobenzene. We tried the copolymerization of 7 and BDT-Si-Sn (Scheme S1, ESI†). However, the resulting copolymer is insoluble, probably due to the shorter 2-butyloctyl side chains on the thiophene bridges. We also tried the copolymerization of 8 with other BDT donor units, (4,8-bis(5-(2-ethylhexyl)-4-fluorothiophen-2-yl)benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl)bis(trimethylstannane) (BDT-F-Sn) and (4,8-bis(5-(2-ethylhexyl)-4-chlorothiophen-2-yl)benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl)bis(trimethylstannane) (BDT-Cl-Sn). But these copolymers are also insoluble in common organic solvents (Scheme S1, ESI†). These results indicate that the bulky tripropylsilyl side chains on BDT units and the long 2-hexyldecyl side chains on the thiophene bridges are crucial for providing sufficient solubility for the copolymer. The synthetic complexity (SC)59,60 of PBDTTPTP was analysed (Table S1, ESI†). Compared with other reported polymers shown in Fig. 1, PBDTTPTP shows a moderate SC of 66.91%. All new compounds were characterized by spectroscopic methods like NMR and mass spectroscopy. The characterization data are consistent with the molecular structures shown in Fig. 2. The structure of compound 7 was unambiguously determined by single crystal X-ray diffraction (XRD) analysis (Fig. 2b). The top-view confirms the linear and π-extended molecular geometry of the TPTP unit. The side-view indicates the good coplanarity between the thiophene bridge and TPTP. Interestingly, the thiophene bridge and the thiophene ring of TPTP adopt a cis arrangement. The molecular packing indicates the efficient π–π stacking of 7 in the crystal. Due to the extended molecular plane of TPTP and the good coplanarity, compound 7 shows a short average π–π stacking distance of 3.54 Å. Efficient π–π stacking would enhance the crystallinity and charge carrier mobility of the copolymer PBDTTPTP. We measured the hole mobility (μh) of PBDTTPTP by using the space charge limited current (SCLC) method (Fig. S15, ESI†).61–63 The copolymer shows a good μh of 6.57 × 10−4 cm2 V−1 s−1.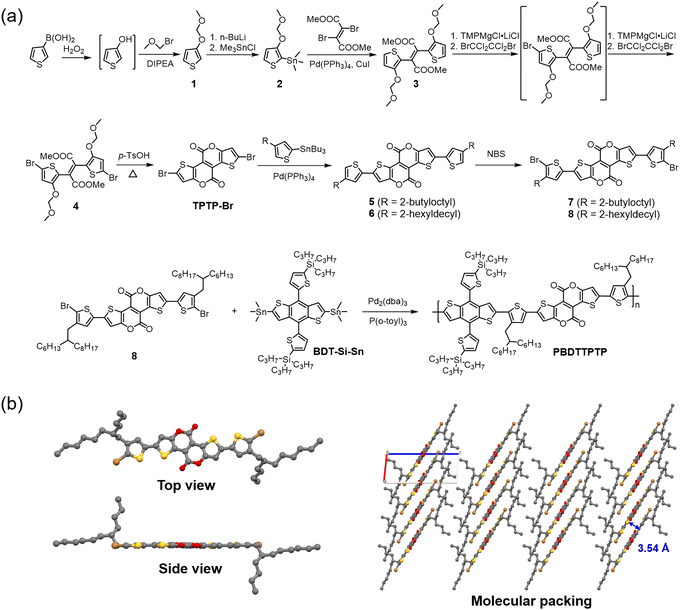 | ||
| Fig. 2 (a) Synthetic route for PBDTTPTP; (b) the top view, side view and molecular packing (viewing along the b-axis) of the single crystal structure of compound 7. | ||
The optical and electrochemical properties of PBDTTPTP were investigated (Fig. 3). In solution, PBDTTPTP presents a major absorption band in the range of 520–750 nm, with a peak at 683 nm and a shoulder peak at 654 nm. In the film state, these two peaks shift to 688 nm and 636 nm, respectively. The absorption onset of the PBDTTPTP film is at 752 nm, corresponding to a small Eoptg of 1.65 eV. The HOMO and the lowest unoccupied molecular orbital (LUMO) energy levels were estimated from cyclic voltammetry (CV) measurements (Fig. S16, ESI†).64 The PBDTTPTP film shows an onset oxidation potential of 0.80 V and an onset reduction potential of −1.69 V, respectively, corresponding to the HOMO level of −5.60 eV and LUMO level of −3.11 eV, respectively. Such a deep HOMO level of PBDTTPTP is favorable for high open-circuit voltages (Voc) in solar cells.
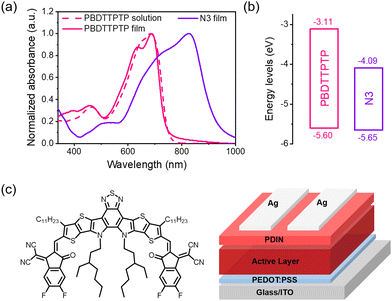 | ||
| Fig. 3 (a) Absorption spectra of PBDTTPTP in solution and film states and N3 film. (b) Energy level diagram. (c) The chemical structure of N3 and the conventional device structure. | ||
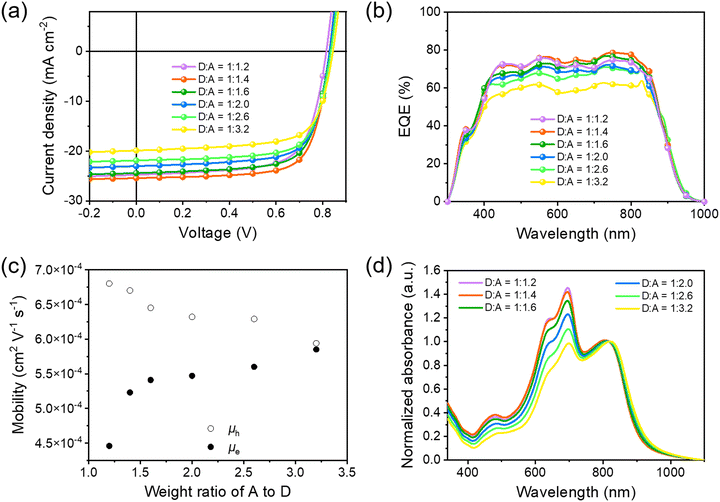
Before application in STOPVs, we evaluated the performance of PBDTTPTP in opaque solar cells with a conventional device structure of glass/ITO/PEDOT:PSS/PBDTTPTP:N3/PDIN/Ag (80 nm). The active layer thickness (Table S2, ESI†) and the D/A ratio (Fig. 4a and Table 1) were optimized. It was found that when the active layer thickness is 128 nm and the D/A ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4, the opaque devices gave the best PCE of 15.28%, with a Voc of 0.829 V, a short-circuit current density (Jsc) of 25.43 mA cm−2 and a fill factor (FF) of 72.5%. The cells showed over 70% external quantum efficiency (EQE) in the 430–842 nm region, with a maximum EQE of 78.5% at 750 nm (Fig. 4b). For STOPV development, a common strategy is reducing the donor content in the active layer to enhance the visible transmittance.19,65,66 Therefore, efficient low-donor-content cells are favorable for STOPVs. We found that the PBDTTPTP:N3 cells can maintain decent PCEs even with a very low donor content. When the D/A ratio is 1
1.4, the opaque devices gave the best PCE of 15.28%, with a Voc of 0.829 V, a short-circuit current density (Jsc) of 25.43 mA cm−2 and a fill factor (FF) of 72.5%. The cells showed over 70% external quantum efficiency (EQE) in the 430–842 nm region, with a maximum EQE of 78.5% at 750 nm (Fig. 4b). For STOPV development, a common strategy is reducing the donor content in the active layer to enhance the visible transmittance.19,65,66 Therefore, efficient low-donor-content cells are favorable for STOPVs. We found that the PBDTTPTP:N3 cells can maintain decent PCEs even with a very low donor content. When the D/A ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 or 1
2.6 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.2, the cells still afforded good PCEs of 13.11% and 11.76%, respectively (Table 1). For these low-donor-content cells, decent FFs of over 70% were achieved, suggesting that the charge carrier transport is still efficient in these cells. SCLC measurements indicate that the D/A ratio variation has insignificant effects on charge carrier mobilities (Fig. 4c). When the D/A ratio changed from 1
3.2, the cells still afforded good PCEs of 13.11% and 11.76%, respectively (Table 1). For these low-donor-content cells, decent FFs of over 70% were achieved, suggesting that the charge carrier transport is still efficient in these cells. SCLC measurements indicate that the D/A ratio variation has insignificant effects on charge carrier mobilities (Fig. 4c). When the D/A ratio changed from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 to 1
1.2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.2, μh decreased from 6.80 × 10−4 to 5.94 × 10−4 cm2 V−1 s−1 and the electron mobility (μe) increased from 4.46 × 10−4 to 5.85 × 10−4 cm2 V−1 s−1 (Fig. S19, S20 and Table S3, ESI†). In low-donor-content cells, the active layer kept its efficient and balanced hole and electron transport capability, thus showing high FF. To understand the robust charge transport capability of the PBDTTPTP:N3 layer, we studied the film morphology at different D/A ratios (1
3.2, μh decreased from 6.80 × 10−4 to 5.94 × 10−4 cm2 V−1 s−1 and the electron mobility (μe) increased from 4.46 × 10−4 to 5.85 × 10−4 cm2 V−1 s−1 (Fig. S19, S20 and Table S3, ESI†). In low-donor-content cells, the active layer kept its efficient and balanced hole and electron transport capability, thus showing high FF. To understand the robust charge transport capability of the PBDTTPTP:N3 layer, we studied the film morphology at different D/A ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4, 1
1.4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 and 1
2.0 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6) by employing an atomic force microscope (AFM).67 All films show similar morphology with a nanofibrillar texture (Fig. S21, ESI†), suggesting that the donor–acceptor phase segregation is less affected by the D/A ratio in PBDTTPTP:N3 cells. The Voc increases along with the N3 content. It could be due to the decrease in the energy loss (Eloss) of the cells as the nonfullerene acceptor content increases.68–71 The absorption spectra (normalized at 817 nm) of the active layer with different D/A ratios are shown in Fig. 4d. The films with a lower donor content show much reduced absorbance in the visible region, which might be favorable for STOPV development. It should be noted that the shape of the EQE spectra of the low-donor-content cells did not change much as compared to those of the high-donor-content cells. This suggests that the photon-to-electron conversion in the visible region could be more efficient than that in the NIR region for the low-donor-content cells.
2.6) by employing an atomic force microscope (AFM).67 All films show similar morphology with a nanofibrillar texture (Fig. S21, ESI†), suggesting that the donor–acceptor phase segregation is less affected by the D/A ratio in PBDTTPTP:N3 cells. The Voc increases along with the N3 content. It could be due to the decrease in the energy loss (Eloss) of the cells as the nonfullerene acceptor content increases.68–71 The absorption spectra (normalized at 817 nm) of the active layer with different D/A ratios are shown in Fig. 4d. The films with a lower donor content show much reduced absorbance in the visible region, which might be favorable for STOPV development. It should be noted that the shape of the EQE spectra of the low-donor-content cells did not change much as compared to those of the high-donor-content cells. This suggests that the photon-to-electron conversion in the visible region could be more efficient than that in the NIR region for the low-donor-content cells.
D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A [w A [w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) w] w] |
V oc [V] | J sc [mA cm−2] | FF [%] | PCE [%] |
|---|---|---|---|---|
| a Device structure: glass/ITO/PEDOT:PSS/PBDTTPTP:N3/PDIN/Ag (80 nm). b Data in parentheses stand for the average PCEs for 10 cells. | ||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
0.816 | 24.77 | 69.1 | 13.95 (13.71)b |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
0.829 | 25.43 | 72.5 | 15.28 (15.17) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
0.829 | 24.41 | 71.0 | 14.38 (14.22) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0 |
0.830 | 23.03 | 71.0 | 13.56 (13.41) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 2.6 |
0.835 | 21.88 | 71.8 | 13.11 (13.00) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.2 3.2 |
0.844 | 19.90 | 70.0 | 11.76 (11.43) |
The charge recombination and collection in PBDTTPTP:N3 cells were investigated. Charge recombination was studied by plotting Voc against light intensity (Plight) (Fig. S22, ESI†). The slope can be expressed as nkBT/q, where kB is the Boltzmann constant, T is the absolute temperature and q is the elemental charge. When n is close to 1, bimolecular recombination is dominant. When n is close to 2, trap-assisted charge recombination is dominant. We found that the slopes for varied D/A ratios are always closer to 1, suggesting that bimolecular recombination is the dominant charge recombination pathway for these systems. Then, the bimolecular recombination was studied by plotting Jsc against light intensity (Plight) (Fig. S23, ESI†). The exponent α reflects the degree of bimolecular recombination (α = 1 manifests no bimolecular recombination). Among the solar cells, the devices with the D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 show an α of 0.982 (the closest value to 1), suggesting the minimum bimolecular recombination in these cells. We further analysed the exciton dissociation probabilities (Pdiss) and charge collection probabilities (Pcoll) by plotting the photocurrent density (Jph) against effective voltage (Veff) for the solar cells with different D/A ratios (Fig. S24, ESI†). It was found that the cells with the ratio of 1
1.4 show an α of 0.982 (the closest value to 1), suggesting the minimum bimolecular recombination in these cells. We further analysed the exciton dissociation probabilities (Pdiss) and charge collection probabilities (Pcoll) by plotting the photocurrent density (Jph) against effective voltage (Veff) for the solar cells with different D/A ratios (Fig. S24, ESI†). It was found that the cells with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 gave highest Pdiss of 97.1% and highest Pcoll of 87.9%, suggesting the most efficient charge generation and collection. The above results are consistent with the highest Jsc of the PBDTTPTP:N3 (1
1.4 gave highest Pdiss of 97.1% and highest Pcoll of 87.9%, suggesting the most efficient charge generation and collection. The above results are consistent with the highest Jsc of the PBDTTPTP:N3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4) solar cells.
1.4) solar cells.
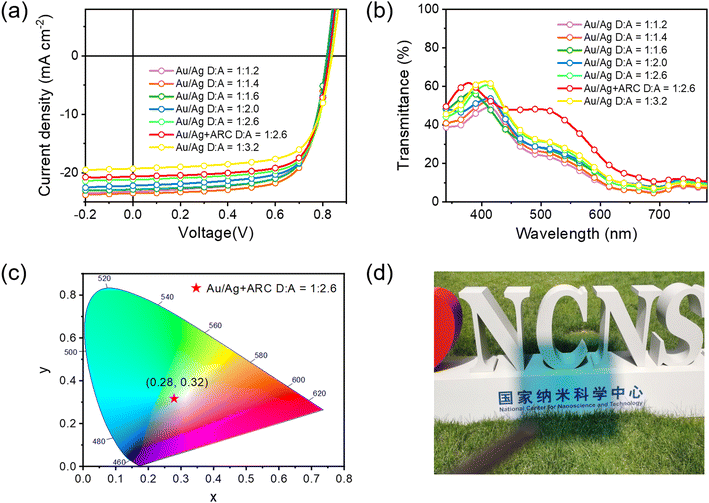
Next, we fabricated STOPVs by replacing the opaque thick Ag electrode (80 nm) with a semitransparent thin Au/Ag electrode.68,72 The thicknesses of Au and Ag were optimized. When using 1 nm-thick Au and 15 nm-thick Ag as the electrode, the STOPV gave the most balanced PCE and AVT (Tables S4, S5 and Fig. S25, S26, ESI†). According to the literature, the Au (1 nm) seed layer could improve the conductivity thus enhancing the Jsc of semitransparent devices.73,74 The J–V curves and device performance data for semitransparent cells with different D/A ratios are shown in Fig. 5a and Table 2, respectively. The EQE spectra are shown in Fig. S28 (ESI†). The variation trend of PCE along with D/A ratios was similar to that in opaque cells. Thus, the cells with a D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 afforded the highest PCE of 13.61%. However, these cells showed a relatively low AVT of 18.4%, thus giving a moderate LUE of 2.50%. As the donor content in the active layer gradually decreases, the PCE slightly decreases while the AVT continuously increases, leading to the increment in LUE. When the D/A ratio is 1
1.4 afforded the highest PCE of 13.61%. However, these cells showed a relatively low AVT of 18.4%, thus giving a moderate LUE of 2.50%. As the donor content in the active layer gradually decreases, the PCE slightly decreases while the AVT continuously increases, leading to the increment in LUE. When the D/A ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6, the semitransparent cells gave the highest LUE of 2.88%, with a PCE of 12.61% and an AVT of 22.8%. We also tried to combine PBDTTPTP with other low-bandgap acceptors like Y6 and BTP-eC9 (Table S7 and Fig. S29, ESI†). Under the same conditions, although PBDTTPTP:Y6 and PBDTTPTP:BTP-eC9 semitransparent cells achieved higher Voc, their Jsc, FF and AVT are lower than those of PBDTTPTP:N3 semitransparent cells, leading to reduced PCEs and LUEs. The light-soaking stability of the opaque and semitransparent PBDTTPTP:N3 cells was investigated (Fig. S30, ESI†). Continuously illuminating opaque and semitransparent cells (with a D/A ratio of 1
2.6, the semitransparent cells gave the highest LUE of 2.88%, with a PCE of 12.61% and an AVT of 22.8%. We also tried to combine PBDTTPTP with other low-bandgap acceptors like Y6 and BTP-eC9 (Table S7 and Fig. S29, ESI†). Under the same conditions, although PBDTTPTP:Y6 and PBDTTPTP:BTP-eC9 semitransparent cells achieved higher Voc, their Jsc, FF and AVT are lower than those of PBDTTPTP:N3 semitransparent cells, leading to reduced PCEs and LUEs. The light-soaking stability of the opaque and semitransparent PBDTTPTP:N3 cells was investigated (Fig. S30, ESI†). Continuously illuminating opaque and semitransparent cells (with a D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6) with a Xenon lamp (AM 1.5G, 1 sun irradiation) for 20 hours, the PCEs dropped to 27% and 31% of the initial values for opaque and semitransparent cells, respectively. It can be concluded that these cells are not so stable under continuous illumination.
2.6) with a Xenon lamp (AM 1.5G, 1 sun irradiation) for 20 hours, the PCEs dropped to 27% and 31% of the initial values for opaque and semitransparent cells, respectively. It can be concluded that these cells are not so stable under continuous illumination.
D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A [w A [w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) w] w] |
V oc [V] | J sc [mA cm−2] | FF [%] | PCE [%] | AVT [%] | LUE [%] |
|---|---|---|---|---|---|---|
| a Device structure: glass/ITO/PEDOT:PSS/PBDTTPTP:N3/PDIN/Au (1 nm)/Ag (15 nm). b Data in parentheses stand for the average PCEs for 10 cells. c Device structure: MgF2 (100 nm)/glass/ITO/PEDOT:PSS/PBDTTPTP:N3/PDIN/Au (1 nm)/Ag (15 nm)/MoO3 (35 nm). | ||||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
0.817 | 23.21 | 68.9 | 13.05 (13.00)b | 16.9 | 2.21 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
0.821 | 23.47 | 70.6 | 13.61 (13.46) | 18.4 | 2.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
0.818 | 22.83 | 70.5 | 13.17 (12.61) | 20.8 | 2.74 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0 |
0.824 | 22.20 | 70.5 | 12.91 (12.48) | 21.6 | 2.79 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 2.6 |
0.831 | 21.14 | 71.8 | 12.61 (12.21) | 22.8 | 2.88 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6c 2.6c |
0.828 | 20.65 | 71.7 | 12.26 (12.17) | 35.7 | 4.38 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.2 3.2 |
0.838 | 19.28 | 69.6 | 11.25 (10.83) | 24.0 | 2.70 |
To further improve the LUE of PBDTTPTP:N3 semitransparent cells, we used antireflective coating (ARC) to enhance the AVT of the whole device. Thus, MgF2 (100 nm) and MoO3 (35 nm) were employed as ARC to reduce the reflection at the ITO side and the Ag side, respectively.75 With MgF2 as the sole ARC, the AVT increased from 22.8% to 26.7%, leading to an enhanced LUE of 3.33% (Fig. S27 and Table S6, ESI†). With both MgF2 and MoO3 ARC, the transmittance at 450–700 nm was largely enhanced (Fig. 5b). The AVT increased to 35.7% and the LUE increased to 4.38% for the best semitransparent cells. To our knowledge, the 4.38% LUE is among the highest values for STOPVs to date. The aesthetic aspect is also an important criterion for STOPVs. The transmitted light should have a chromaticity close to that of the natural white light source for a harmonious visual environment and comfort. According to the chromaticity diagram released by Commission Internationale de l'Eclairage (CIE) in 1931, chromaticity coordinates of white light are (0.33, 0.33). We measured the color coordinates of our best-LUE cells. As shown in Fig. 5c, the coordinates are (0.28, 0.32), which are close to that of the white light and suggest the good color rendering properties of these devices. The measured color rendering index (CRI) of the best-LUE cells is 91.76–78 The high LUE together with the good color rendering properties of the semitransparent cells suggest their potential in power generating windows. Fig. 5d shows the photograph of a 1 cm2 semitransparent cell made under optimal conditions. The background can be seen clearly. The 1 cm2 semitransparent cell gave a slightly reduced PCE of 11.40% (Table S8 and Fig. S31, ESI†).
Conclusions
To conclude, by using a tetracyclic bislactone unit TPTP, we developed an efficient copolymer donor PBDTTPTP for STOPVs. The strong electron-withdrawing properties and good coplanarity of TPTP endow PBDTTPTP with a small Eoptg of 1.65 eV, a deep HOMO level and good hole mobility. PBDTTPTP not only delivered a decent PCE of 15.28% in opaque solar cells, but also achieved a high LUE of 4.38% (a PCE of 12.26% and an AVT of 35.7%) in STOPVs. This work suggests that FRAL units are promising building blocks for low-bandgap copolymer donors for STOPVs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the open research fund of Songshan Lake Materials Laboratory (2021SLABFK02), the National Key Research and Development Program of China (2022YFB3803300), the Strategic Priority Research Program of Chinese Academy of Sciences (XDB36000000) and the National Natural Science Foundation of China (21961160720) for financial support.References
- Y. Li, G. Xu, C. Cui and Y. Li, Adv. Energy Mater., 2018, 8, 1701791 CrossRef.
- Y. Liu, P. Cheng, T. Li, R. Wang, Y. Li, S.-Y. Chang, Y. Zhu, H.-W. Cheng, K.-H. Wei, X. Zhan, B. Sun and Y. Yang, ACS Nano, 2019, 13, 1071–1077 CrossRef CAS PubMed.
- D. Wang, H. Liu, Y. Li, G. Zhou, L. Zhan, H. Zhu, X. Lu, H. Chen and C.-Z. Li, Joule, 2021, 5, 945–957 CrossRef CAS.
- W. Li, C. Lin, G. Huang, J. Hur, B. Huang and S. Yao, Adv. Sci., 2022, 9, 2201738 CrossRef CAS PubMed.
- D. Wang, Y. Li, G. Zhou, E. Gu, R. Xia, B. Yan, J. Yao, H. Zhu, X. Lu, H.-L. Yip, H. Chen and C.-Z. Li, Energy Environ. Sci., 2022, 15, 2629–2637 RSC.
- V. V. Brus, J. Lee, B. R. Luginbuhl, S.-J. Ko, G. C. Bazan and T.-Q. Nguyen, Adv. Mater., 2019, 31, 1900904 CrossRef PubMed.
- T. Jiang, G. Zhang, R. Xia, J. Huang, X. Li, M. Wang, H.-L. Yip and Y. Cao, Mater. Today Energy, 2021, 21, 100807 CrossRef CAS.
- C. Xu, K. Jin, Z. Xiao, Z. Zhao, Y. Yan, X. Zhu, X. Li, Z. Zhou, S. Y. Jeong, L. Ding, H. Y. Woo, G. Yuan and F. Zhang, Sol. RRL, 2022, 6, 2200308 CrossRef CAS.
- T. Xu, Y. Luo, S. Wu, B. Deng, S. Chen, Y. Zhong, S. Wang, G. Lévêque, R. Bachelot and F. Zhu, Adv. Sci., 2022, 9, 2202150 CrossRef CAS PubMed.
- C. J. Traverse, R. Pandey, M. C. Barr and R. R. Lunt, Nat. Energy, 2017, 2, 849–860 CrossRef.
- C. Yang, D. Liu, M. Bates, M. C. Barr and R. R. Lunt, Joule, 2019, 3, 1803–1809 CrossRef.
- Q. Xue, R. Xia, C. J. Brabec and H.-L. Yip, Energy Environ. Sci., 2018, 11, 1688–1709 RSC.
- R. R. Lunt and V. Bulovic, Appl. Phys. Lett., 2011, 98, 113305 CrossRef.
- Y. Li, J.-D. Lin, X. Che, Y. Qu, F. Liu, L.-S. Liao and S. R. Forrest, J. Am. Chem. Soc., 2017, 139, 17114–17119 CrossRef CAS PubMed.
- X. Huang, J. Oh, Y. Cheng, B. Huang, S. Ding, Q. He, F. Wu, C. Yang, L. Chen and Y. Chen, J. Mater. Chem. A, 2021, 9, 5711–5719 RSC.
- W. Liu, S. Sun, L. Zhou, Y. Cui, W. Zhang, J. Hou, F. Liu, S. Xu and X. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202116111 CAS.
- A. Polman, M. Knight, E. C. Garnett, B. Ehrler and W. C. Sinke, Science, 2016, 352, aad4424 CrossRef PubMed.
- W. Wang, C. Yan, T.-K. Lau, J. Wang, K. Liu, Y. Fan, X. Lu and X. Zhan, Adv. Mater., 2017, 29, 1701308 CrossRef PubMed.
- Y. Xie, Y. Cai, L. Zhu, R. Xia, L. Ye, X. Feng, H.-L. Yip, F. Liu, G. Lu, S. Tan and Y. Sun, Adv. Funct. Mater., 2020, 30, 2002181 CrossRef CAS.
- N. Schopp and V. V. Brus, Energies, 2022, 15, 4639 CrossRef CAS.
- K. Jin, Z. Xiao and L. Ding, J. Semicond., 2021, 42, 060502 CrossRef.
- X. Meng, K. Jin, Z. Xiao and L. Ding, J. Semicond., 2021, 42, 100501 CrossRef.
- K. Chong, X. Xu, H. Meng, J. Xue, L. Yu, W. Ma and Q. Peng, Adv. Mater., 2022, 34, 2109516 CrossRef CAS PubMed.
- W. Gao, F. Qi, Z. Peng, F. R. Lin, K. Jiang, C. Zhong, W. Kaminsky, Z. Guan, C.-S. Lee, T. J. Marks, H. Ade and A. K.-Y. Jen, Adv. Mater., 2022, 34, 2202089 CrossRef CAS PubMed.
- R. Sun, Y. Wu, X. Yang, Y. Gao, Z. Chen, K. Li, J. Qiao, T. Wang, J. Guo, C. Liu, X. Hao, H. Zhu and J. Min, Adv. Mater., 2022, 34, 2110147 CrossRef CAS PubMed.
- Y. Wei, Z. Chen, G. Lu, N. Yu, C. Li, J. Gao, X. Gu, X. Hao, G. Lu, Z. Tang, J. Zhang, Z. Wei, X. Zhang and H. Huang, Adv. Mater., 2022, 34, 2204718 CrossRef CAS PubMed.
- L. Zhu, M. Zhang, J. Xu, C. Li, J. Yan, G. Zhou, W. Zhong, T. Hao, J. Song, X. Xue, Z. Zhou, R. Zeng, H. Zhu, C.-C. Chen, R. C. I. MacKenzie, Y. Zou, J. Nelson, Y. Zhang, Y. Sun and F. Liu, Nat. Mater., 2022, 21, 656–663 CrossRef CAS PubMed.
- Q. Liu, Y. Jiang, K. Jin, J. Qin, J. Xu, W. Li, J. Xiong, J. Liu, Z. Xiao, K. Sun, S. Yang, X. Zhang and L. Ding, Sci. Bull., 2020, 65, 272–275 CrossRef CAS PubMed.
- J. Qin, L. Zhang, C. Zuo, Z. Xiao, Y. Yuan, S. Yang, F. Hao, M. Cheng, K. Sun, Q. Bao, Z. Bin, Z. Jin and L. Ding, J. Semicond., 2021, 42, 010501 CrossRef CAS.
- P. Li, X. Meng, K. Jin, Z. Xu, J. Zhang, L. Zhang, C. Niu, F. Tan, C. Yi, Z. Xiao, Y. Feng, G.-W. Wang and L. Ding, Carbon Energy, 2023, 5, e250 CAS.
- D. H. Shin and S.-H. Choi, Coatings, 2018, 8, 329 CrossRef.
- P. Yin, Z. Yin, Y. Ma and Q. Zheng, Energy Environ. Sci., 2020, 13, 5177–5185 RSC.
- H.-W. Cheng, Y. Zhao and Y. Yang, Adv. Energy Mater., 2022, 12, 2102908 CrossRef CAS.
- C.-C. Chen, L. Dou, R. Zhu, C.-H. Chung, T.-B. Song, Y. B. Zheng, S. Hawks, G. Li, P. S. Weiss and Y. Yang, ACS Nano, 2012, 6, 7185–7190 CrossRef CAS PubMed.
- L. Dou, J. You, J. Yang, C.-C. Chen, Y. He, S. Murase, T. Moriarty, K. Emery, G. Li and Y. Yang, Nat. Photonics, 2012, 6, 180–185 CrossRef CAS.
- C.-C. Chueh, S.-C. Chien, H.-L. Yip, J. F. Salinas, C.-Z. Li, K.-S. Chen, F.-C. Chen, W.-C. Chen and A. K.-Y. Jen, Adv. Energy Mater., 2013, 3, 417–423 CrossRef CAS.
- C.-Y. Chang, L. Zuo, H.-L. Yip, Y. Li, C.-Z. Li, C.-S. Hsu, Y.-J. Cheng, H. Chen and A. K.-Y. Jen, Adv. Funct. Mater., 2013, 23, 5084–5090 CrossRef CAS.
- L. Dou, C.-C. Chen, K. Yoshimura, K. Ohya, W.-H. Chang, J. Gao, Y. Liu, E. Richard and Y. Yang, Macromolecules, 2013, 46, 3384–3390 CrossRef CAS.
- Y. Xie, R. Xia, T. Li, L. Ye, X. Zhan, H.-L. Yip and Y. Sun, Small Methods, 2019, 3, 1900424 CrossRef CAS.
- S.-H. Liao, H.-J. Jhuo, Y.-S. Cheng and S.-A. Chen, Adv. Mater., 2013, 25, 4766–4771 CrossRef CAS PubMed.
- H. Yao, L. Ye, H. Zhang, S. Li, S. Zhang and J. Hou, Chem. Rev., 2016, 116, 7397–7457 CrossRef CAS PubMed.
- X. Ma, Z. Xiao, Q. An, M. Zhang, Z. Hu, J. Wang, L. Ding and F. Zhang, J. Mater. Chem. A, 2018, 6, 21485–21492 RSC.
- W. Liu, S. Sun, S. Xu, H. Zhang, Y. Zheng, Z. Wei and X. Zhu, Adv. Mater., 2022, 34, 2200337 CrossRef CAS PubMed.
- Y. Li, X. Guo, Z. Peng, B. Qu, H. Yan, H. Ade, M. Zhang and S. R. Forrest, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 21147–21154 CrossRef CAS PubMed.
- X. Huang, Y. Cheng, Y. Fang, L. Zhang, X. Hu, S. Y. Jeong, H. Zhang, H. Y. Woo, F. Wu and L. Chen, Energy Environ. Sci., 2022, 15, 4776–4788 RSC.
- M. Zhang, X. Guo, W. Ma, H. Ade and J. Hou, Adv. Mater., 2015, 27, 4655–4660 CrossRef CAS PubMed.
- J. Jing, S. Dong, K. Zhang, Z. Zhou, Q. Xue, Y. Song, Z. Du, M. Ren and F. Huang, Adv. Energy Mater., 2022, 12, 2200453 CrossRef CAS.
- S. Guan, Y. Li, K. Yan, W. Fu, L. Zuo and H. Chen, Adv. Mater., 2022, 34, 2205844 CrossRef CAS PubMed.
- X. Liu, Z. Zhong, R. Zhu, J. Yu and G. Li, Joule, 2022, 6, 1918–1930 CrossRef CAS.
- J. Liu, L. Liu, C. Zuo, Z. Xiao, Y. Zou, Z. Jin and L. Ding, Sci. Bull., 2019, 64, 1655–1657 CrossRef CAS PubMed.
- J. Xiong, K. Jin, Y. Jiang, J. Qin, T. Wang, J. Liu, Q. Liu, H. Peng, X. Li, A. Sun, X. Meng, L. Zhang, L. Liu, W. Li, Z. Fang, X. Jia, Z. Xiao, Y. Feng, X. Zhang, K. Sun, S. Yang, S. Shi and L. Ding, Sci. Bull., 2019, 64, 1573–1576 CrossRef CAS PubMed.
- J. Xiong, J. Xu, Y. Jiang, Z. Xiao, Q. Bao, F. Hao, Y. Feng, B. Zhang, Z. Jin and L. Ding, Sci. Bull., 2020, 65, 1792–1795 CrossRef PubMed.
- Y. Jiang, K. Jin, X. Chen, Z. Xiao, X. Zhang and L. Ding, J. Semicond., 2021, 42, 070501 CrossRef CAS.
- X. Chen, Y. Luo, M. Lv, C. Yi, M. Yin, S. Liu, Z. Xiao and L. Ding, Mater. Chem. Front., 2022, 6, 802–806 RSC.
- K. Jin, Z. Ou, L. Zhang, Y. Yuan, Z. Xiao, Q. Song, C. Yi and L. Ding, J. Semicond., 2022, 43, 050501 CrossRef.
- Z. Ou, J. Qin, K. Jin, J. Zhang, L. Zhang, C. Yi, Z. Jin, Q. Song, K. Sun, J. Yang, Z. Xiao and L. Ding, J. Mater. Chem. A, 2022, 10, 3314–3320 RSC.
- G. Zong, M. Li, K. Jin, Z. Xu, L. Zhang, N. Ma, J. Wang, G.-W. Wang, Z. Xiao and L. Ding, Mater. Chem. Front., 2022, 6, 1858–1864 RSC.
- K. Jiang, Q. Wei, J. Y. L. Lai, Z. Peng, H. K. Kim, J. Yuan, L. Ye, H. Ade, Y. Zou and H. Yan, Joule, 2019, 3, 3020–3033 CrossRef CAS.
- W. Yang, W. Wang, Y. Wang, R. Sun, J. Guo, H. Li, M. Shi, J. Guo, Y. Wu, T. Wang, G. Lu, C. J. Brabec, Y. Li and J. Min, Joule, 2021, 5, 1209 CrossRef.
- R. Po, G. Bianchi, C. Carbonera and A. Pellegrino, Macromolecules, 2015, 48, 453 CrossRef CAS.
- Z. Chiguvare and V. Dyakonov, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 235207 CrossRef.
- Y. Rui, Z. Jin, X. Fan, W. Li, B. Li, T. Li, Y. Wang, L. Wang and J. Liang, Mater. Futures, 2022, 1, 045101 CrossRef.
- J. Wang, D. Li, J. Wang and J. Peng, Mater. Futures, 2022, 1, 045301 CrossRef.
- X. Meng, M. Li, K. Jin, L. Zhang, J. Sun, W. Zhang, C. Yi, J. Yang, F. Hao, G.-W. Wang, Z. Xiao and L. Ding, Angew. Chem., Int. Ed., 2022, 61, e202207762 CAS.
- Z. Hu, Z. Wang and F. Zhang, J. Mater. Chem. A, 2019, 7, 7025–7032 RSC.
- N. Schopp, G. Akhtanova, P. Panoy, A. Arbuz, S. Chae, A. Yi, H. J. Kim, V. Promarak, T.-Q. Nguyen and V. V. Brus, Adv. Mater., 2022, 34, 2203796 CrossRef CAS PubMed.
- R. Xu, J. Guo, S. Mi, H. Wen, F. Pang, W. Ji and Z. Cheng, Mater. Futures, 2022, 1, 032302 CrossRef.
- C. Xu, K. Jin, Z. Xiao, Z. Zhao, X. Ma, X. Wang, J. Li, W. Xu, S. Zhang, L. Ding and F. Zhang, Adv. Funct. Mater., 2021, 31, 2107934 CrossRef CAS.
- Z. Wen, T. Wang, Z. Chen, T. Jiang, L. Feng, X. Feng, C. Qin and X. Hao, Chin. Chem. Lett., 2021, 32, 529–534 CrossRef CAS.
- G. Chai, Y. Chang, J. Zhang, X. Xu, L. Yu, X. Zou, X. Li, Y. Chen, S. Luo, B. Liu, F. Bai, Z. Luo, H. Yu, J. Liang, T. Liu, K. S. Wong, H. Zhou, Q. Peng and H. Yan, Energy Environ. Sci., 2021, 14, 3469–3479 RSC.
- A. Shang, S. Luo, J. Zhang, H. Zhao, X. Xia, M. Pan, C. Li, Y. Chen, J. Yi, X. Lu, W. Ma, H. Yan and H. Hu, Sci. China: Chem., 2022, 65, 1758–1766 CrossRef CAS.
- Z. Hu, J. Wang, X. Ma, J. Gao, C. Xu, K. Yang, Z. Wang, J. Zhang and F. Zhang, Nano Energy, 2020, 78, 105376 CrossRef CAS.
- L. Vitos, A. V. Ruban, H. L. Skriver and J. Kollár, Surf. Sci., 1998, 411, 186–202 CrossRef CAS.
- S. Schubert, J. Meiss, L. Müller-Meskamp and K. Leo, Adv. Energy Mater., 2013, 3, 438–443 CrossRef CAS.
- X. Huang, L. Zhang, Y. Cheng, J. Oh, C. Li, B. Huang, L. Zhao, J. Deng, Y. Zhang, Z. Liu, F. Wu, X. Hu, C. Yang, L. Chen and Y. Chen, Adv. Funct. Mater., 2021, 32, 2108634 CrossRef.
- A. Colsmann, A. Puetz, A. Bauer, J. Hanisch, E. Ahlswede and U. Lemmer, Adv. Energy Mater., 2011, 1, 599 CrossRef CAS.
- G. Xu, L. Shen, C. Cui, S. Wen, R. Xue, W. Chen, H. Chen, J. Zhang, H. Li, Y. Li and Y. Li, Adv. Funct. Mater., 2017, 27, 1605908 CrossRef.
- J. Zhang, G. Xu, F. Tao, G. Zeng, M. Zhang, Y. Yang, Y. Li and Y. Li, Adv. Mater., 2019, 31, 1807159 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2167880. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ma00179b |
| ‡ M. Li and T. An contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |