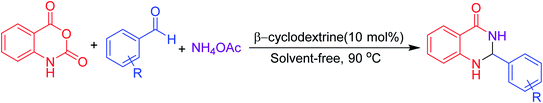
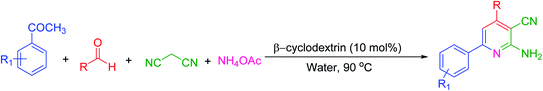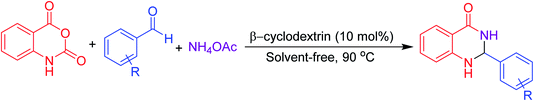Open Access Article
Open Access Articleβ-Cyclodextrin: a supramolecular catalyst for metal-free approach towards the synthesis of 2-amino-4,6-diphenylnicotinonitriles and 2,3-dihydroquinazolin-4(1H)-one†
Bijeta Mitraa,
Gyan Chandra Pariyarb and
Pranab Ghosh *a
*a
aDepartment of Chemistry, University of North Bengal, Dist. Darjeeling, West Bengal, India. E-mail: pizy12@yahoo.com; Fax: +91 353 2699001; Tel: +91 353 2776381
bDepartment of Food Technology, University of North Bengal, Dist. Darjeeling, West Bengal, India
First published on 5th January 2021
Abstract
β-Cyclodextrin, a green and widespread supramolecular catalyst, has been explored as a highly proficient promoter for the metal-free one-pot multi-component synthesis of a vast range of highly functionalized bioactive heterocyclic moiety, 2-amino-4,6-diphenylnicotinonitriles and 2,3-dihydroquinazolin-4(1H)-one, from easily available precursor aldehydes. The main endeavor of these protocols is to explore this organic supramolecule in one-pot multi-component synthesis. Absence of metal catalyst or toxic acid and harsh reaction conditions, excellent functional group tolerance, inexpensive, greener and environmentally safe protocol are the key advantages of this work.
1. Introduction
In organic transformations, a major apprehension focuses on catalysts and solvents. Pyrophoric property and the hazardous nature of solvent with high volatility and poor recovery are the major limitations of solvents. The main limitation of the metal catalyst is that it leads to metal contagion at the end of the reaction, which is not good for our Mother Earth. These disadvantages could be overcome using any green solvent and organocatalyst, which is good for the environment. We have developed a metal-free protocol using a supramolecular catalyst, β-cyclodextrin.1 In terms of environmentally friendly and atom economy, water2 as a reaction medium is highly advantageous.Given the advancements in supramolecular chemistry and homogeneous catalysis, the supramolecular catalyst has been emerging as an important tool in synthesizing organic heterocyclic compounds.3 Among the array of supramolecular hosts, cyclodextrins (CDs) are one of the important enzyme models.4–7 Being cyclic oligosaccharides, cyclodextrins possess hydrophobic cavities, which enable them to bind with substrates selectively, because of which they are able to catalyze chemical reactions and ensure high selectivity.8–12 During the course of the reaction, CDs can reversibly bind to a host–guest complex with the substrates via non-covalent bonding, thus forming a complex, which is responsible for altering product distribution during organic reactions. The characteristics are attributed to the development of geometrical constraints developed in the guest molecules because it gets included within the cavity of CDs. Similar to β-cyclodextrin, it uses the hydrophobic cavity to encapsulate biologically active molecules from aqueous solutions, which eventually enhances the bioavailability and stability of drug molecules. These properties have made CDs an important asset for pharmaceutical industries. Moreover, CDs are easily recyclable, easily available, cheaper, and non-toxic.13,14
In the past few decades, for producing a diverse array of heterocyclic molecules and making the synthetic route simpler, one-pot multi-component organic synthesis has played an imperative role in chemistry. Three or sometimes more starting materials coalesce together in the same pot to exclusively develop the target molecule in multi-component synthesis without separating the intermediate, which aids in reducing the reaction time, solvent waste, and energy consumption and therefore have considerable compensation over the multistep procedure.15
The pyridine motif is an essential scaffold because of its occurrence in natural products, biological and medicinal products. Among pyridine ring systems, 2-aminonicotinonitrile derivatives have achieved elevated synthetic attention as an imperative heterocyclic molecule because of its existence as bio-active species such as IKK-β inhibitors,16 potent inhibitor of HIV-1 integrase,17 A2A adenosine receptor antagonist18 as well as in antitubercular,19 anticancer, anticardiovascular20 and antimicrobial drugs.21 Moreover, pyridines are valuable in materials and surfaces,22 organocatalysis,23 supramolecular structures,24 and coordination chemistry.25 Furthermore, these compounds serve as useful intermediates for preparing diverse heterocyclic compounds.26
For the past few years, 2,3-dihydroquinazolin-4(1H)-one has gained considerable interest and has been utilized in preparing various drug molecules27,28 due to its potential biological and pharmaceutical activities, which may be either antidepressant, analgesic, sedative, diuretic, or antihistamine.29–31 Anticancer activities, such as cell proliferation, inhibition of tubulin formation,32 and inhibition against VEGFR2 tyrosine kinase, are extraordinarily important features of these compounds. Plant growth regulatory33 is another important activity of this molecule with a huge application. Some examples are given below in Fig. 1.
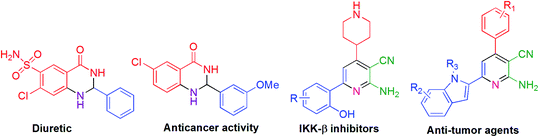 | ||
| Fig. 1 Some bioactive molecules having 2-amino-3-cyanopyridine and 2,3-dihydroquinazolin-4(1H)-one skeleton. | ||
Many diverse protocols have been reported for synthesizing 2-amino-3-cyanopyridine derivatives such as Fe3O4,34 acetic acid,35 ultrasound irradiation,36,37 DMF,38 Lewis acid catalysts,39 and graphene oxide.40 However, a straightforward and efficient one-pot reaction without a transition metal catalyst and hazardous solvent in mild conditions is still unexplored. Although several methods for preparing 2-amino-4,6-diphenylnicotinonitrile are well documented in the literature, most of them have a few limitations such as the use of hazardous solvents such as benzene or toluene, very high temperature, multiple-step pathway, long reaction times with low yields, use of transition metal that leads to metal contamination at the end of the reaction, and harsh reaction conditions that are not environmentally friendly.
Many strategies have been designed for synthesizing 2,3-dihydroquinazolin-4(1H)-ones in the literature; however, the most widely used method is the straightforward condensation reaction between 2-aminobenzamide and aldehyde. Most of these methodologies use metal salts,41 metal nano-particles42 of Co, Fe, La, Ag, In, and Cu, p-TSA,43 amberlyst-15,44 acidic silica,45 trifluoroethanol,46 cyanuric chloride,47 heteropoly acid,48 organic acid,49 I2,50 NH4Cl,51 and β-cyclodextrin.52 Synthesis of this molecule via a one-pot three-component protocol in a greener pathway is very tricky. Few methodologies for synthesizing 2,3-dihydroquinazolin-4(1H)-one via a one-pot three-component strategy using aldehydes, isatoic anhydride, and amines are reported in the literature.53 We have utilized the environmentally benign supramolecular catalyst β-cyclodextrin as a promoter for these two different one-pot multi-component organic reactions using aldehyde and ammonium acetate as the two main precursors (Schemes 1 and 2).
2. Experimental
2.1. Materials and apparatus
All compounds were purchased from commercial suppliers and used without further purification. 1H NMR, 13C NMR, and 19F NMR were recorded using 300 MHz, 400 MHz and 75 MHz, 100 MHz and 376 MHz, respectively, on Bruker AV 300 NMR spectrometer and Bruker AV 400 NMR spectrometer using TMS as the internal standard. IR spectra were recorded on KBr disc in the range of 4000–400 cm−1 on a Shimadzu FT-IR 8300 Spectrometer. The splitting patterns of protons were described as s (singlet), d (doublet), t (triplet), br (broad), and m (multiplet).2.2. General procedure for the synthesis of 2-amino-4,6-disubstituted nicotinonitriles
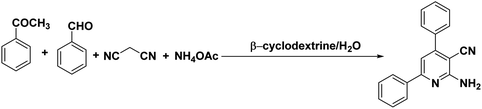
β-Cyclodextrin (10 mol%) and water were added to a mixture of aromatic aldehydes (1 mmol), acetophenone (1 mmol), malononitrile (1 mmol), and ammonium acetate (1 mmol) in a round-bottom flask. The resulting mixture was stirred at 90 °C for 2 h in the open air. The reaction progress was monitored using TLC with a mixture of ethyl acetate and n-hexane as the eluent system. After reaction completion, the mixture was quenched to room temperature and extracted with ethyl acetate twice (2 × 20 ml). The combined extracts were washed with distilled water, dried over anhydrous Na2SO4, and concentrated. The crude product was then purified by passing through a column packed with silica gel. The products obtained were known compounds and identified by melting points, FT-IR and 1H, 13C NMR spectroscopy.2.3. General procedure for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones
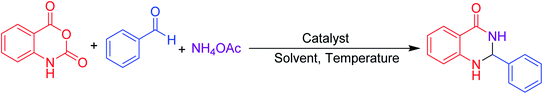
Aldehydes (1 mmol), isatoic anhydride (1 mmol), and ammonium acetate (1 mmol) were added in a round-bottom flask and then β-cyclodextrin (10 mol%) was added to the mixture under a solvent-free condition. The resulting mixture was stirred at 90 °C for 1 h in the open air. The reaction progress was monitored using TLC with a mixture of ethyl acetate and n-hexane as the eluent system. After reaction completion, the mixture was quenched to room temperature and extracted with ethyl acetate twice (2 × 20 ml). The combined extracts were washed with distilled water, dried over anhydrous Na2SO4 and concentrated. The residue obtained was recrystallized using a mixture of petroleum ether and ethyl acetate and then filtered to afford the desired pure solid products. All products were separated without column chromatography. The products obtained were known compounds and identified by melting point, FT-IR and 1H, 13C NMR spectroscopy.2.4. Spectroscopic data of the synthesized compounds
3. Results and discussion
3.1. Optimisation of reaction conditions
To begin our study, we have taken benzaldehyde, acetophenone, malononitrile and ammonium acetate for a model reaction in the water medium. For the optimization of reaction conditions, parameters such as temperature, time and amount of catalyst were reported to have an influence on the reaction and it was monitored by TLC. Because β-cyclodextrin is soluble in water than any other organic solvent, we performed all reactions in water. The experimental results are summarized in Table 1. Initially, the reaction was performed at 90 °C with 30 mol% of catalyst for 8 h, and 88% product was then formed (Table 1, entry 1). Then, we reduced the amount of the catalyst; when only 10 mol% of the catalyst was used, the yield was almost comparable with the yield of 30 mol% of catalyst (Table 1, entry 3). The same observation was seen in the case of optimization of time. By decreasing the time to 2 h, the yield was still 84% (Table 1, entry 4). Further decrease in the temperature (Table 1, entries 10, 11) and time (Table 1, entry 5) of the reaction did not increase the yield of the desired product. In the absence of a catalyst, no product was formed and only intermediates were formed, which was concluded by monitoring TLC (Table 1, entry 9). When we performed the same reaction in the solvent-free condition, a gummy solid was obtained (Table 1, entry 12). From this, it can be concluded that the reaction catalyzed by β-cyclodextrin and water is mandatory to perform the conversion.| Entry | Catalyst (mol%) | Temperature (°C) | Time (h) | Yieldc (%) |
|---|---|---|---|---|
| a The bold numbers represent the most optimized protocol/conditions.b Reaction of benzaldehyde (1 mmol), acetophenone (1 mmol), malononitrile (1 mmol), and ammonium acetate (1 mmol) in water with β-cyclodextrin.c Isolated yield of the product by column chromatography.d Solvent-free reaction. | ||||
| 1 | 30 | 90 | 8 | 88 |
| 2 | 20 | 90 | 8 | 86 |
| 3 | 10 | 90 | 8 | 86 |
| 4 | 10 | 90 | 2 | 84 |
| 5 | 10 | 90 | 1 | 74 |
| 6 | 5 | 90 | 8 | 62 |
| 7 | 4 | 90 | 8 | 40 |
| 8 | 2 | 90 | 8 | Trace |
| 9 | — | 90 | 12 | — |
| 10 | 10 | 50 | 2 | 73 |
| 11 | 10 | Room temperature | 12 | 68 |
| 12d | 10 | 90 | 2 | Trace |
Subsequently, the scope and efficiency of the reagent were explored under the optimized reaction conditions for the condensation of acetophenone with a broad range of structurally diverse aldehydes to furnish the related products. The structural diversity of the reactants is displayed in Table 2. The reactants bearing both electron-withdrawing and electron-donating substituents on the phenyl ring and the heterocyclic, aliphatic aldehydes are well tolerated under the reaction conditions to afford the final products in moderate to good yields (Table 2, entries 2–20). Electronic effects could be noticed in the reaction process. Aldehydes having electron-withdrawing groups give a better performance than those having electron-donating groups. Aldehydes having a hydroxyl group at the 2-position gave a better result, which may be attributed to H-bonding, whereas those with a hydroxyl group at the 4-position, the yield was moderate. Because of steric hindrance, 2-naphthylaldehyde moiety gave a better result than 1-naphthylaldehyde. 4-Substituted acetophenone gave a better result except for the amino group, which yielded only a trace product because of a self-condensation reaction.
| a Reaction condition: aldehyde (1 mmol), acetophenone (1 mmol), malononitrile (1 mmol), and ammonium acetate (1 mmol) in the presence of β-CD (10 mol%) in water at 90 °C for 2 h. |
|---|
 |
For screening reaction conditions, isatoic anhydride, benzaldehyde and ammonium acetate are considered for the model reaction (Table 3). Reaction parameters such as temperature, time, solvent, and amount of catalyst showed a profound effect in performing the reaction, which was evident while monitoring the reaction using TLC. We started investigating the reaction under solvent-free condition using a different loading of catalyst. At first, we attempted the reaction with 20 mol% of catalyst per mmol of substrates at 90 °C where a good yield of the product was observed (Table 3, entry 1). We then started to reduce the amount of catalyst. Surprisingly, 10 mol% of catalyst at the same temperature yielded almost the same yield (Table 3, entry 2). Keeping the temperature constant and further reducing the amount of catalyst, the yield decreased (Table 3, entries 3, 4). Furthermore, in the absence of a catalyst, only the intermediate was formed, which was confirmed from TLC (Table 3, entry 5). After fixing the amount of catalyst, we started to screen the time and temperature. With reduction in temperature to room temperature, the yield again decreased (Table 3, entries 7, 8). From this, temperature has considerable influence on this protocol. The further reduction of time from 5 h to 1 h with 10 mol% of catalyst loading at 90 °C yielded the same yield; therefore, 1 h may be enough to perform this protocol (Table 3, entry 6).
| Entry | Catalyst (mol%) | Temperature (°C) | Time (h) | Yieldc (%) |
|---|---|---|---|---|
| a The bold numbers represent the most optimized protocol/conditions.b Reaction of isatoic anhydride (1 mmol), benzaldehyde (1 mmol), ammonium acetate (1 mmol), and β-cyclodextrin.c Isolated yield of the product by column chromatography. | ||||
| 1 | 20 | 90 | 5 | 92 |
| 2 | 10 | 90 | 5 | 90 |
| 3 | 5 | 90 | 5 | 78 |
| 4 | 2 | 90 | 8 | Trace |
| 5 | — | 90 | 8 | — |
| 6 | 10 | 90 | 1 | 90 |
| 7 | 10 | 50 | 2 | 74 |
| 8 | 10 | rt | 5 | 58 |
With these optimized reaction conditions, we tested the reactions with various aldehydes in Table 4, and all the aldehydes gave good yields. The aldehydes used were aromatic aldehydes having electron-withdrawing and electron-donating groups, aliphatic aldehyde (Table 4, entry 18), and five- and six-membered heterocyclic aldehydes (Table 4, entries 13, 14, 15, 16). The electron-withdrawing group in the benzene ring gave a slightly better yield than the electron-donating group. This may be due to the increase in the electrophilicity of carbonyl carbon of the aldehyde moiety. Substitution at the ortho position gave a poor yield than others, which may be due to the steric repulsion (Table 4, entries 6, 10). Amazingly, the hydroxyl group at the 2-position increased the yield than others, which may be due to its H-bonding effect (Table 4, entry 8).
| Entry | Reactant | Product | Yieldb (%) |
|---|---|---|---|
| a Reaction of isatoic anhydride (1 mmol), aldehyde (1 mmol), and ammonium acetate (1 mmol) in the presence of β-CD (10 mol%) for 1 h at 90 °C under solvent-free condition.b Isolated yield of the product by column chromatography. | |||
| 1 |  |
 |
90 |
| 2 |  |
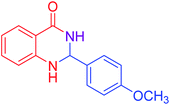 |
84 |
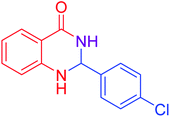
| 3 | 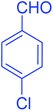 |
 |
88 |
| 4 | 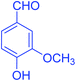 |
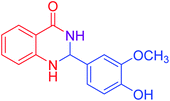 |
78 |
| 5 | 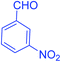 |
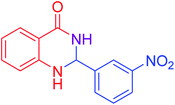 |
92 |
| 6 | 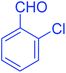 |
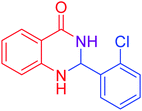 |
75 |
| 7 | 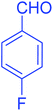 |
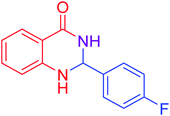 |
89 |
| 8 | 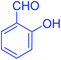 |
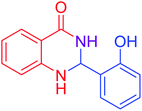 |
90 |
| 9 | 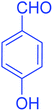 |
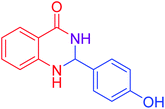 |
86 |
| 10 | 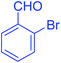 |
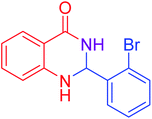 |
72 |
| 11 | 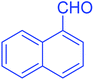 |
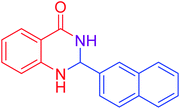 |
79 |
| 12 | 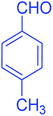 |
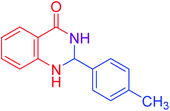 |
83 |
| 13 | 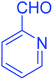 |
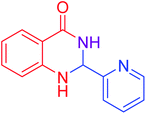 |
80 |
| 14 | 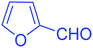 |
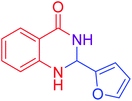 |
92 |
| 15 | 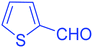 |
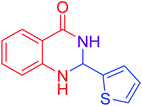 |
95 |
| 16 | 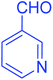 |
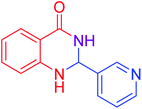 |
84 |
| 17 | 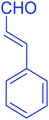 |
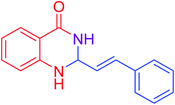 |
89 |
| 18 |  |
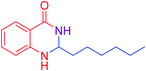 |
93 |
3.2. Mechanism
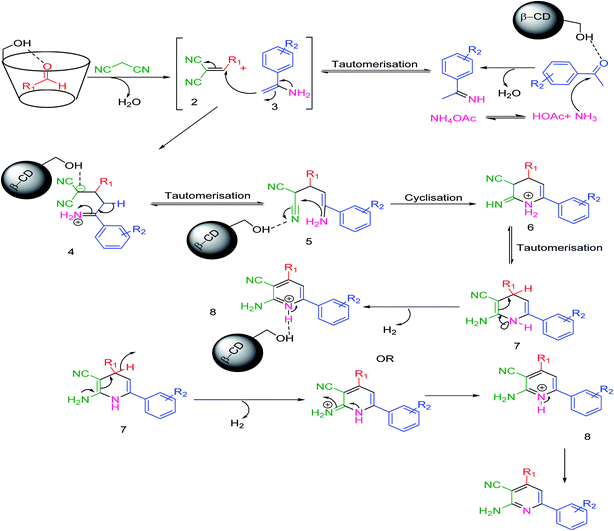
A plausible mechanism54 has been discussed in Scheme 3. β-CD with its seven free primary –OH groups synergistically behaves as an efficient host and a supramolecular catalyst. β-CD, our potential catalyst, simultaneously activates both the aryl aldehyde and acetophenone derivatives as the active electrophile species. The reaction of malononitrile and ammonium acetate with these two activated electrophiles generates the corresponding intermediates 2 and 3, respectively; intermediate 2 was then isolated. Afterwards, the reaction between these two intermediates will produce the corresponding intermediate 4. A sequence of tautomerization, cyclization, and again tautomerization generates the intermediate 7, which possesses a structure having a lone pair on the nitrogen atom sharing electrons from both –NH2 and –NH functional groups through C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds in the presence of the described catalyst and yields the desired products.
C double bonds in the presence of the described catalyst and yields the desired products.
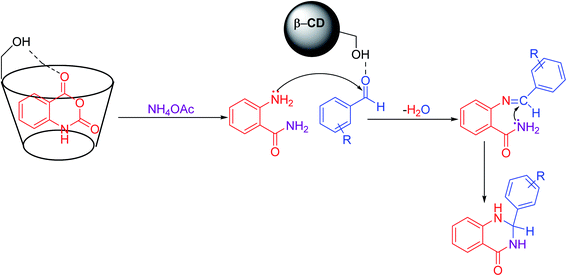
A plausible mechanism was described for synthesizing 2,3-dihydroquinazolin-4(1H)-one (Scheme 4). In the first step, isatoic anhydride coordinates to the β-CD cavity; by the reaction of ammonium acetate, it forms 2-aminobenzamide as an intermediate, which is then isolated. In the next step, it facilitates the nucleophilic attack by the electron-rich nitrogen of the amine group to the electrophilic carbonyl carbon centre of aldehydes, which is activated by β-CD. Then, the elimination of water followed by another nucleophilic attack by the amine group of the amide to the carbon centre on the substituted imine leads to the expected product 2,3-dihydroquinazolin-4(1H)-one.
4. Conclusion
In conclusion, we have developed a straightforward, robust and facile eco-friendly strategy for preparation of 2-amino-4,6-diphenylnicotinonitrile and 2,3-dihydroquinazolin-4(1H)-one derivatives using a bio-based supramolecule, β-cyclodextrin, in water and solvent-free condition, respectively, without using any additional catalyst or metal salt, which further enhances the advantage of the protocol. The replacement of toxic and expensive metal catalysts by an environment-friendly and inexpensive organocatalyst is the novelty of this protocol. This environmentally benign protocol is expected to achieve wide applications in the pharmaceutical industry and natural product synthesis.Author contributions
BM and GCP contribute equally to these methodologies. Scheme 1 is done by BM, and Scheme 2 is done by GCP under the guidance of Prof. P. Ghosh.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
B. M. is thankful to CSIR, New Delhi for financial support.References
- Y. A. Tayade, A. D. Jangale and D. S. Dalal, ChemistrySelect, 2018, 3, 8895–8900 CrossRef CAS.
- (a) P. Basak, S. Dey and P. Ghosh, ChemistrySelect, 2020, 5, 626–636 CrossRef CAS; (b) R. Singha, P. Basak, M. Bhattacharya and P. Ghosh, ChemistrySelect, 2020, 5, 6514–6525 CrossRef CAS; (c) P. Basak and P. Ghosh, Synth. Commun., 2018, 48, 2584–2599 CrossRef CAS; (d) G. C. Pariyar, B. Mitra, S. Mukherjee and P. Ghosh, ChemistrySelect, 2020, 5, 104–108 CrossRef.
- (a) S. S. Reddy, M. V. K. Reddy and P. V. G. Reddy, ChemistrySelect, 2018, 3, 4283–4288 CrossRef CAS; (b) S. N. Murthy and Y. V. D. Nageswar, Tetrahedron Lett., 2011, 52, 4481–4484 CrossRef; (c) M. Abbasi, J. Chin. Chem. Soc., 2017, 64, 896–917 CrossRef CAS; (d) S. V. Akolkar, N. D. Kharat and A. A. Nagargoje, Catal. Lett., 2020, 150, 450–460 CrossRef CAS; (e) A. Ghorad, S. Mahalle and L. D. Khillare, Catal. Lett., 2017, 147, 640–648 CrossRef CAS; (f) G. Dhananjaya, A. V. D. Rao, K. A. Hossain, V. R. Anna and M. Pal, Tetrahedron Lett., 2020, 61, 151972, DOI:10.1016/j.tetlet.2020.151972; (g) Y. A. Tayade, S. A. Padvi, Y. B. Wagh and D. S. Dalal, Tetrahedron Lett., 2020, 56, 2441–2447 CrossRef.
- L. R. Reddy, N. Bhanumathi and K. R. Rao, Chem. Commun., 2000, 2321–2322 RSC.
- M. A. Reddy, N. Bhanumathi and K. R. Rao, Chem. Commun., 2001, 1974–1975 RSC.
- K. Surendra, N. S. Krishnaveni, R. Sridhar and K. R. Rao, Tetrahedron Lett., 2006, 47, 2125–2127 CrossRef CAS.
- O. Z. Tee, C. Mazza, R. Lozano Hemmer and J. B. Giorgi, J. Org. Chem., 1994, 59, 7602–7608 CrossRef CAS.
- L. Marchetti and M. Levine, ACS Catal., 2011, 1, 1090–1118 CrossRef CAS.
- R. Breslow and U. Maitra, Tetrahedron Lett., 1983, 24, 1901–1904 CrossRef CAS.
- A. Gonzalez and S. Holt, J. Org. Chem., 1982, 47, 3186–3188 CrossRef CAS.
- H. J. Schneider and N. K. Sangwan, J. Chem. Soc., Chem. Commun., 1986, 24, 1787–1789 RSC.
- D. D. Sternbach and D. M. Rossana, J. Am. Chem. Soc., 1982, 104, 5853–5854 CrossRef CAS.
- A. L. Laza Knoerr, R. Gref and P. J. Couvreur, J. Drug Targeting, 2010, 18, 645–656 CrossRef CAS.
- J. Heng-Bing, S. DongPo, S. Ming, L. Zhong, W. Le-Fu, H. B. Ji, D. P. Shi, M. Shao, Z. Li and L. F. Wang, Tetrahedron Lett., 2005, 46, 2517–2520 CrossRef.
- (a) P. Choudhury, P. Ghosh and B. Basu, Mol. Diversity, 2020, 24, 283–294 CrossRef CAS; (b) B. Mitra, S. Mukherjee, G. C. Pariyar and P. Ghosh, Tetrahedron Lett., 2018, 59, 1385–1389 CrossRef CAS.
- T. Murata, M. Shimada, S. Sakakibara, T. Yoshino, H. Kadono, T. Masuda, M. Shimazaki, T. Shintani, K. Fuchikami, K. Sakai, H. Inbe, K. Takeshita, T. Niki, M. Umeda, K. B. Bacon, K. B. Ziegelbauer and T. B. Lowinger, Bioorg. Med. Chem. Lett., 2003, 13, 913–918 CrossRef CAS.
- M. Mantri, O. De Graaf, J. Van Veldhoven, A. Göblyös, J. K. Von Frijtag Drabbe Künzel, T. Mulder-Krieger, R. Link, H. De Vries, M. W. Beukers, J. Brussee and A. P. Ijzerman, J. Med. Chem., 2008, 51, 4449–4455 CrossRef CAS.
- J. Deng, T. Sanchez, L. Q. Al-Mawsawi, R. Dayam, R. A. Yunes, A. Garofalo, M. B. Bolger and N. Neamati, Bioorg. Med. Chem., 2007, 15, 4985–5002 CrossRef CAS.
- H. M. Kanjariya, T. V. Radhakrishnan, K. R. Ramchandran and P. Hansa, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2004, 43, 1569–1573 Search PubMed.
- J. Stoltefuss, S. Goldmann, A. Straub, H. Boeshagen, M. Bechem, R. Gross, S. Hebisch, J. Huetter and H. P. Rounding, US Pat., 5432282, 1995 Search PubMed.
- T. Murata, M. Shimada, H. Kadono, S. Sakakibara, T. Yoshino, T. Masuda, M. Shimazaki, T. Shintani, K. Fuchikami, K. B. Bacon and T. B. Z. Lowinger, Bioorg. Med. Chem. Lett., 2004, 14, 4013–4017 CrossRef CAS.
- R. Makiura, S. Motoyama, Y. Umemura, H. Yamanaka, O. Sakata and H. Kitagawa, Nat. Mater., 2010, 9, 565–571 CrossRef CAS.
- N. De Rycke, F. Couty and O. R. P. David, Chem.–Eur. J., 2011, 17, 12852–12871 CrossRef CAS.
- T. Šmejkal and B. Breit, Angew. Chem., Int. Ed., 2008, 47, 311–315 CrossRef.
- D. Bora, B. Deb, A. L. Fuller, A. M. Z. Slawin, J. D. Woollins and D. K. Dutta, Inorg. Chim. Acta, 2010, 363, 1539–1546 CrossRef CAS.
- C. J. Shishoo, M. B. Devani, V. S. Bhadti, S. Ananthan and G. V. Ullas, Tetrahedron Lett., 1983, 24, 4611–4612 CrossRef CAS.
- J. F. Wolfe, T. L. Rathman, M. C. Sleevi, J. A. Campbell and T. D. Greenwood, J. Med. Chem., 1990, 33, 161–166 CrossRef CAS.
- J. K. Padia, M. Field, J. Hinton, K. Meecham, J. Pablo, R. Pinnock, B. D. Roth, L. Singh, N. Suman-Chauhan, B. K. Trivedi and L. Webdale, J. Med. Chem., 1998, 41, 1042–1049 CrossRef CAS.
- E. Hamel, C. M. Lin, J. Plowman, H. Wang and K. D. Pad, Biochem. Pharmacol., 1996, 51, 53–59 CrossRef CAS.
- J. A. Lowe, R. L. Archer, D. S. Chapin, J. B. Cheng, D. Helweg, J. L. Johnson, B. K. Koe, L. A. Lebel and P. F. Moore, J. Med. Chem., 1991, 34, 624–628 CrossRef CAS.
- H. J. Havera, J. Med. Chem., 1979, 22, 1548–1550 CrossRef CAS.
- G. Wagner and I. Wunderlich, Pharmazie, 1978, 33, 15 CAS.
- C. H. Boehringer Sohn, Chem. Abstr., 1964, 61, 16075h Search PubMed.
- M. M. Heravi, S. Y. S. Beheshtiha, M. Dehghani and N. Hosseintash, J. Iran. Chem. Soc., 2015, 12, 2075–2081 CrossRef CAS.
- A. N. Vasiliev, Y. S. Kayukov, O. E. Nasakin, A. N. Lyshchikov, V. N. Nesterov, O. V. Kayukova and O. V. Poulkherovskaya, Chem. Heterocycl. Compd., 2011, 37, 309–314 CrossRef.
- F. Shi, V. Tu, F. Fang and T. Li, ARKIVOC, 2005, 137–142 Search PubMed.
- W. J. Zhou, S. J. Ji and Z. L. Shen, J. Organomet. Chem., 2006, 691, 1356–1360 CrossRef CAS.
- A. M. Shestopalov and O. A. Naumov, Russ. Chem. Bull., 2003, 52, 1380–1385 CrossRef CAS.
- J. Tang, L. Wang, Y. Yao, L. Zhang and W. Wang, Tetrahedron Lett., 2011, 52, 509–511 CrossRef CAS.
- D. Khalili, Tetrahedron Lett., 2016, 57, 1721–1723 CrossRef CAS.
- (a) J. X. Chen, D. Z. Wu, F. He, M. C. Liu, H. Y. Wu, J. C. Ding and W. K. Su, Tetrahedron Lett., 2019, 49, 3814–3818 CrossRef; (b) D. Zhan, T. Li, H. Wei, W. Weng, K. Ghandi and Q. Zeng, RSC Adv., 2013, 3, 9325–9329 RSC; (c) J. X. Chen, H. Y. Wu and W. K. Su, Chin. Chem. Lett., 2007, 18, 536–538 CrossRef CAS; (d) M. Prakesh and V. Kesavan, Org. Lett., 2012, 7, 1896–1899 CrossRef; (e) R. J. Abdel, W. Voelter and M. A. Saeed, Tetrahedron Lett., 2004, 45, 3475–3476 CrossRef; (f) G.-P. Cai, X.-L. Xu, Z.-F. Li, P. Willam, P. Weber and J. Lu, J. Heterocycl. Chem., 2002, 39, 1271–1272 CrossRef CAS; (g) C. L. Yoo, J. C. Fettinger and M. J. Kurth, J. Org. Chem., 2005, 70, 6941–6943 CrossRef CAS; (h) A. J. A. Watson, A. C. Maxwell and J. M. J. Williams, Org. Biomol. Chem., 2012, 10, 240–243 RSC; (i) J. Zhou and J. Fang, J. Org. Chem., 2011, 76, 7730–7736 CrossRef CAS; (j) H. Li, L. He, H. Neumann, M. Beller and X.-F. Wu, Green Chem., 2014, 16, 1336–1343 RSC; (k) H. Hikawa, Y. Ino, H. Suzuki and Y. Yokoyama, J. Org. Chem., 2012, 77, 7046–7051 CrossRef CAS; (l) X. Jiang, T. Tang, J.-M. Wang, Z. Chen, Y.-M. Zhu and S.-J. Ji, J. Org. Chem., 2014, 79, 5082–5087 CrossRef CAS; (m) M. Dabiri, P. Salehi, S. Otokesh, M. Baghbanzadeh, G. Kozehgary and A. A. Mohammadi, Tetrahedron Lett., 2005, 46, 6123–6126 CrossRef CAS; (n) D. Shi, L. Rong, J. Wang, Q. Zhuang, X. Wang and H. Hu, Tetrahedron Lett., 2003, 44, 3199–3201 CrossRef CAS.
- (a) J. Safari and S. G. Ravandi, C. R. Chim., 2013, 16, 1158–1164 CrossRef CAS; (b) A. G. Choghamarani and M. Norouzi, J. Mol. Catal. A: Chem., 2014, 395, 172–179 CrossRef; (c) J. Safari and S. Gandomi-Ravandi, J. Mol. Catal. A: Chem., 2013, 371, 135–140 CrossRef CAS; (d) J. Safari and S. G. Ravandi, RSC Adv., 2014, 4, 11654–11660 RSC; (e) S. Santra, M. Rahman, A. Roy, A. Majee and A. Hajra, Catal. Commun., 2014, 49, 52–57 CrossRef CAS; (f) S. Tarannum, N. Ahmed and Z. N. Siddiqui, Catal. Commun., 2015, 66, 60–66 CrossRef CAS; (g) A. G. Choghamarani and G. Azadi, RSC Adv., 2015, 5, 9752–9756 RSC.
- R. Cheng, T. Guo, D. Zhang-Negrerie, Y. Du and K. Zhao, Synthesis, 2013, 45, 2998–3006 CrossRef CAS.
- P. V. Murthy, D. Rambabu, G. RamaKrishna, C. M. Reddy, K. R. Prasad, M. V. B. Rao and M. Pal, Tetrahedron Lett., 2012, 53, 863–867 CrossRef CAS.
- M. Dabiri, P. Salehi, M. Baghbanzadeh, M. A. Zolfigol, M. Agheb and S. Heydari, Catal. Commun., 2008, 9, 785–788 CrossRef CAS.
- (a) R. Z. Qiao, B. L. Xu and Y. H. Wang, Chin. Chem. Lett., 2007, 18, 656–658 CrossRef CAS; (b) P. Salehi, M. Dabiri, M. A. Zolfigol and M. Baghbanzadeh, Synlett, 2019, 1155–1157 Search PubMed.
- M. Sharma, S. Pandey, K. Chauhan, D. Sharma, B. Kumar and P. M. Chauhan, J. Org. Chem., 2012, 77, 929–937 CrossRef CAS.
- Y.-X. Zong, Y. Zhao, W.-C. Luo, X. H. Yu, J.-K. Wang and Y. Pan, Chin. Chem. Lett., 2010, 21, 778–781 CrossRef CAS.
- G. C. Pariyar, B. Mitra, S. Mukherjee and P. Ghosh, ChemistrySelect, 2020, 5, 104–108 CrossRef.
- (a) S. Rostamizadeh, A. M. Amani, R. Aryan, H. R. Ghaieni and N. Shadjou, Synth. Commun., 2008, 38, 3567–3576 CrossRef CAS; (b) X. S. Wang, K. Yang, J. Zhou and S. J. Tu, J. Comb. Chem., 2010, 12, 417–421 CrossRef CAS.
- A. Shaabani, A. Maleki and H. Mofakham, Synth. Commun., 2008, 38, 3751–3755 CrossRef CAS.
- K. Ramesh, K. Karnakar, G. Satish, B. S. P. Anil Kumar and Y. V. D. Nageswar, Tetrahedron Lett., 2012, 53, 6936–6939 CrossRef CAS.
- (a) R. Sharma, A. K. Pandey and P. M. S. Chauhan, Synlett, 2012, 23, 2209–2214 CrossRef CAS; (b) F. Tamaddon and M. T. K. Varnamkhasti, Synlett, 2016, 27, 2510–2514 CrossRef CAS; (c) Z.-H. Zhang, H.-Y. Lü, S.-H. Yang and J.-W. Gao, J. Comb. Chem., 2010, 12, 643–646 CrossRef CAS; (d) C. K. Khatri, M. S. Patil and G. U. Chaturbhuj, J. Iran. Chem. Soc., 2017, 14, 1683–1689 CrossRef CAS; (e) N. Razavi and B. Akhlaghinia, New J. Chem., 2016, 40, 447–457 RSC; (f) S. Zhaleh, N. Hazeri and M. T. Maghsoodlou, Res. Chem. Intermed., 2016, 42, 6381–6390 CrossRef CAS; (g) S. Karhale, D. Survase and R. Bhat, Res. Chem. Intermed., 2017, 43, 3915–3924 CrossRef CAS; (h) A. Sahu, S. Mishra, P. Sahu, A. Gajbhiye and R. K. Agrawal, Curr. Organocatal., 2018, 5, 137–144 CrossRef CAS; (i) S. J. Wu, Z. Q. Zhao and J. S. Gao, Res. Chem. Intermed., 2019, 45, 2327–2339 CrossRef CAS; (j) F. Tamaddon and M. T. K. Varnamkhasti, Synlett, 2016, 27, 2510–2514 CrossRef CAS; (k) Y. Yang, R. Fu, Y. Liu, J. Cai and X. Zeng, Tetrahedron, 2020, 76, 131312 CrossRef CAS; (l) J. Wu, X. Du, J. Ma, Y. Zhang, Q. Shi, L. Luo, B. Song, S. Yanga and D. Hu, Green Chem., 2014, 16, 3210–3217 RSC; (m) D. R. Patil, P. G. Ingole, K. Singh and D. S. Dalal, J. Inclusion Phenom. Macrocyclic Chem., 2013, 76, 327–332 CrossRef CAS.
- M. A. Zolfigol, M. Kiafar, M. Yarie, A. Taherpour, T. Fellowes, A. N. Hancok and A. Yari, J. Mol. Struct., 2017, 1137, 674–680 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra09562a |
| This journal is © The Royal Society of Chemistry 2021 |