Open Access Article
Open Access ArticlePicolylamine–Ni(II) complex attached on 1,3,5-triazine-immobilized silica-coated Fe3O4 core/shell magnetic nanoparticles as an environmentally friendly and recyclable catalyst for the one-pot synthesis of substituted pyridine derivatives†
Sobhan Rezayatia,
Fatemeh Kalantaria and
Ali Ramazani *ab
*ab
aDepartment of Chemistry, Faculty of Science, University of Zanjan, Zanjan 45371-38791, Iran. E-mail: aliramazani@gmail.com; aliramazani@znu.ac.ir
bDepartment of Biotechnology, Research Institute of Modern Biological Techniques (RIMBT), University of Zanjan, Zanjan 45371-38791, Iran
First published on 25th April 2023
Abstract
In the current study, an environmentally friendly and facile method was proposed for designing and constructing a catalyst with Ni(II) attached to a picolylamine complex on 1,3,5-triazine-immobilized Fe3O4 core–shell magnetic nanoparticles (NiII-picolylamine/TCT/APTES@SiO2@Fe3O4) via a stepwise procedure. The as-synthesized nanocatalyst was identified and characterized via Fourier-transform infrared (FT-IR), X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TGA), vibrating-sample magnetometry (VSM), transmission electron microscopy (TEM), X-ray diffraction (XRD), Brunauer–Emmett–Teller (BET), field-emission scanning electron microscopy (FE-SEM), inductively coupled plasma (ICP), and energy-dispersive X-ray spectrometry (EDX). The obtained results from the BET analysis indicated that the synthesized nanocatalyst had high specific area (53.61 m2 g−1) and mesoporous structure. TEM observations confirmed the particle size distribution was in the range 23–33 nm. Moreover, the binding energy peaks observed at 855.8 and 864.9 eV in the XPS analysis confirmed the successful and stable attachment of Ni(II) on the surface of the picolylamine/TCT/APTES@SiO2@Fe3O4. The as-fabricated catalyst was used to produce pyridine derivatives by the one-pot pseudo-four component reaction of malononitrile, thiophenol, and a variety of aldehyde derivatives under solvent-free conditions or EG at 80 °C. The highest yield achieved was 97% for compound 4d in EG at 80 °C with a TOF of 823 h−1 and TON of 107. It was found that the used catalyst was recyclable for eight consecutive cycles. On the basis of ICP analysis, the results indicated that the Ni leaching was approximately 1%.
Introduction
The focus on sustainable chemistry and green chemistry in modern organic synthesis has intensified in recent years, and includes not using dangerous or toxic solvents and reagents, the avoidance of non-recyclable and expensive catalysts, and the avoidance of harsh reaction conditions.1–4 Over the past few decades, there has been increasing interest in the use of eco-friendly solvents or reagents, good atom economy, and measures that can save energy consumption in the synthesis of organic compounds.5–7 The use of clean and green chemical technologies both in organic transformations and in wider industry still faces some significant challenges to produce valuable chemicals. One of the important factors in sustainable chemistry and green chemistry is the use of catalysts. Recently, research on catalysts, especially heterogeneous catalysts, in research transformations and in most chemical industrial processes has gained high significance. Likewise, for supporting the global community, catalysts can help the production of sustainable fuels and many indispensable chemicals.8–13 Therefore, efficient, green, and suitable catalysts have a great role to play in promoting sustainability. In this regard, nanocatalysts have many suitable unique features, including efficient recovery, low cost of production, excellent selectivity, high activity and stability, and good recyclability.One of the most important modern science areas is nanoscience. Nanotechnology allows physicians, engineers, scientists, and chemists to do work at the cellular and molecular levels to make important advances in the healthcare and science fields, among others. In recent years, due to their electrical characteristics, distinctive magnetic properties, unique size, and high surface area, the utilization of magnetic nanoparticles (MNPs) has attracted considerable attention. They also have particular properties in industrial applications and biomedical applications, such as drug delivery systems, separation of metal ions for environmental remediation, bio-isolation, saving information, biomolecular sensing, magneto-heat therapy, targeted gene therapy, and sensors.14–16 A high biocompatibility and sensitivity to postsynthetic surface functionalization have made magmite (γ-Fe2O3) and magnetite (Fe3O4) among the range of iron oxides, great candidates for many significant applications.17–22 One of the important advantages of MNPs as catalysts is their easy recovery using a magnetic field. The two intrinsic properties of paramagnetism and the insolubility of nanomagnets facilitate the separation of such magnetic catalysts from reaction mixtures. In particular, silica-modified MNPs have received widespread attention due to their various important advantages, such as ease and simplicity of synthesis, functionalization, and easy separation from the reaction medium employing a simple magnet, thermal stability, and low toxicity.
One of the best ways to synthesize new heterocyclic compounds from available and simple raw materials is through multicomponent reactions (MCRs), which have been widely employed in the preparation of biologically active molecules, natural products, and organic matter.23,24 The development of one-pot MCRs is one of the best methods for the synthesis of desired products due to certain salient features of this method, such as high selectivity, facile automation, simplicity, and high atomic economy.25–27 Other benefits of one-pot MCRs over multistep syntheses include their short reaction time and high chemical yields, which also leads to reduced waste generation, manpower, and energy consumption. Hence, MCRs have become a very powerful tool in combinational chemistry and drug discovery for the synthesis of biologically important heterocyclic compounds.
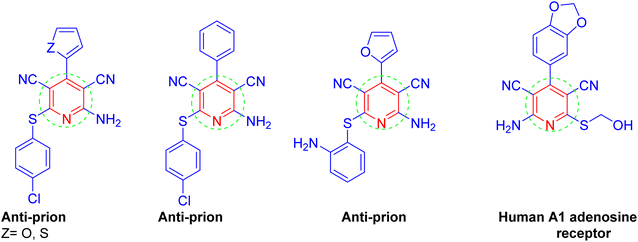
N-Heterocyclic scaffolds are very common in naturally occurring compounds. Among the heterocyclic compounds, the 2-amino-3,5-dicarbonitrile-6-thio-pyridines ring system has attracted much attention due to its widespread occurrence in numerous bioactive natural products. Such compounds have enjoyed wide applications in diverse pharmacological activities, such as anti-bacterial,28 anti-prion,29 anticancer agents,30 and anti-hepatitis B virus.31 (Scheme 1).
Furthermore, they can act as potassium channel openers for the treatment of urinary incontinence. In addition, some of these compounds were discovered to be candidates for the development of new drugs for the treatment of cancer, Parkinson's disease, epilepsy, hypoxia/ischemia, kidney disease, and asthma.32 For example, there are some naturally occurring compounds I, II, and III that bear a pyridine group as a structural subunit;33–35 while compounds IV and V are commercially available drug molecules that have the pyridine skeleton (Scheme 2).36
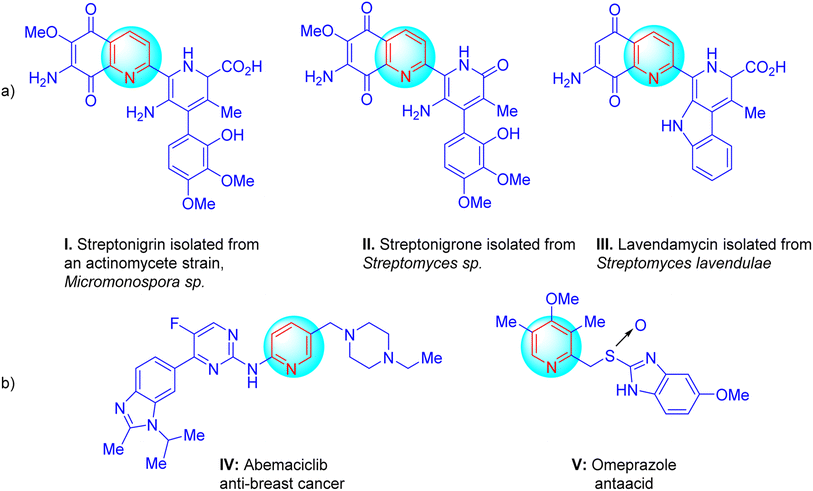 | ||
| Scheme 2 (a) Some naturally occurring compounds (I, II, and III) bearing pyridine groups and (b) some drug molecules with a pyridine skeleton (IV and V). | ||
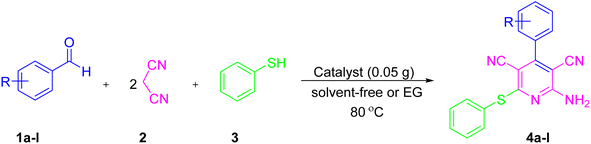
Diverse approaches have been developed for preparing pyridine derivatives with multistep methods.37–39 Recently, the reaction between aldehyde, malononitrile, and thiol via a one-pot pseudo-four component reaction was evaluated as an efficient and simple method for the preparation of 2-amino-3,5-dicarbonitrile-6-thio-pyridines in the presence of a catalyst. In recent years, more effective catalysts for the preparation of pyridine derivatives have been reported, such as ZnCl2,40 NAP-MgO,41 iodoxybenzoic acid (IBX),42 DBU,43 KF/alumina,44 scandium(III) triflate,45 [bmIm]OH,46 DABCO,47 WEB,48 Fe2O3@Fe3O4@Co3O4,49 NaNH2,50 Cu(I) nanoparticles,51 Zn(II) and Cd(II) MOFs,52 microporous molecular sieves 4A,53 and phosphotungstic acid/CTAB.54 Nevertheless, some previously reported procedures remain valuable, albeit many of these procedures have significant drawbacks, such as low yields, tedious workup procedures, high temperatures, long reaction times, and the use of phosphine ligands and copper salts. For example, Evodkimov et al. developed a new method for the synthesis of pyridines derivatives using DABCO or Et3N as a base catalyst. One of the limitations of that method was that the corresponding products were obtained in yields of only 20–48%.47 Sridhar et al. reported a new ZnCl2 as a Lewis acid catalyst for the synthesis of pyridines derivatives under exotic reaction conditions (conventional heating or microwave heating). The ZnCl2 catalyst gave the products in 45–73% and 46–67% yields, respectively.40 It was further observed that some methods used expensive or toxic catalysts and reagents.43 Therefore, the development of an environmentally friendly and green route for the synthesis of pyridine derivatives is still a desirable goal.
Continuing our endeavors for preparing and designing new magnetic nanoparticles as nanocatalysts,55–58 herein, for the first time, we report the synthesis of NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 as a heterogeneous nanocatalyst. This catalyst could be successfully used in the one-pot pseudo-four component synthesis of substituted pyridine derivatives (Scheme 3).
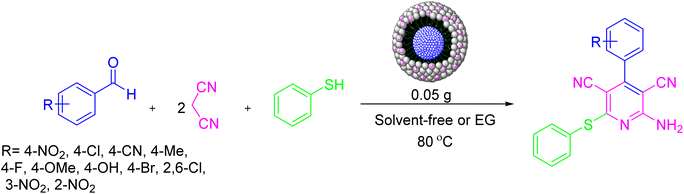 | ||
| Scheme 3 Activity of the NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 catalyst for the synthesis of substituted pyridine derivatives. | ||
Results and discussion
Preparation of NiII-picolylamine/TCT/APTES@SiO2@Fe3O4
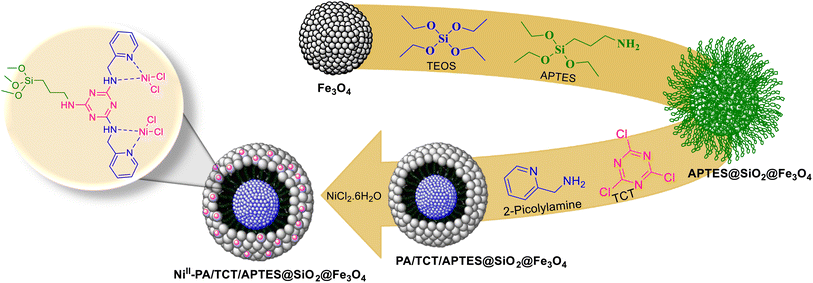
NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 was made according to a convenient and simple four-step operation process, as illustrated in Scheme 4. Silica-coated Fe3O4 was initially obtained by attaching Fe3O4 to tetraethyl orthosilicate. In the next step, the resulting Fe3O4@SiO2 was coated with (3-aminopropyl)triethoxysilane (APTES), followed by treatment with 2,4,6-trichloro-1,3,5-triazine (TCT) as a cross-linking agent in THF at room temperature. The chloride atoms in the cyanuric chloride agent were alternatively substituted to amine groups in APTES and 2-picolylamine at diverse temperatures, respectively. Picolylamine/TCT/APTES@SiO2@Fe3O4 was formed through the reaction between Fe3O4@SiO2@NH2@TCT and 2-picolylamine under reflux conditions in CH3CN. Finally, the reaction with a solution of nickel(II) chloride hexahydrate and picolylamine/TCT/APTES@SiO2@Fe3O4 in EtOH at room temperature gave NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 (Scheme 4).Characterization of NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 catalyst
The as-prepared nanocatalyst was characterized using various techniques, including FT-IR, XPS, TGA, VSM, TEM, XRD, BET, FE-SEM, ICP, and EDX. FT-IR spectra were obtained for assessing the chemical changes during the synthesis of the NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 catalyst in the range of 400 to 3900 cm−1, and the results are displayed in Fig. 1. As can be seen in the FT-IR spectrum of pure magnetic nanoparticles, the strong absorption at 610 cm−1 was associated with the Fe–O bond, and confirmed the synthesis of iron oxide (Fig. 1a).57,58 Two absorption peaks at 1109 and 3414 cm−1 were observed, attributed to the presence of Si–O–Si and OH groups, respectively (Fig. 1b).57,58 After immobilizing APTES on the silica-coated Fe3O4, new bands appeared at 3445 and 1635 cm−1, which were assigned to N–H stretching vibration and N–H bending vibration of NH2, respectively. Furthermore, the new band detected at 2922 cm−1 was due to the C–H(sp3) of a –CH2 group, indicating the APTES coating on the SiO2@Fe3O4 (Fig. 1c).57,58 The absorption peaks about 1300–1600 cm−1 in the TCT/APTES@SiO2@Fe3O4 spectra were associated with the C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrations, proving the TCT presence on the TCT/APTES@SiO2@Fe3O4 structure. Besides, the absorption band at 866 cm−1 was related to the unreacted C–Cl bond (Fig. 1d).57,58 After the reaction between TCT/APTES@SiO2@Fe3O4 with 2-picolylamine to give picolylamine/TCT/APTES@SiO2@Fe3O4, as expected, the absorption peak for C–Cl disappeared (Fig. 1e). After the coordination of the Ni(II) metal ion, the imine peak shifted from 1626 to 1639 cm−1. These obtained result indicated the coordination of Ni(II) metal ion on the surface of picolylamine/TCT/APTES@SiO2@Fe3O4 (Fig. 1f).57,58
N stretching vibrations, proving the TCT presence on the TCT/APTES@SiO2@Fe3O4 structure. Besides, the absorption band at 866 cm−1 was related to the unreacted C–Cl bond (Fig. 1d).57,58 After the reaction between TCT/APTES@SiO2@Fe3O4 with 2-picolylamine to give picolylamine/TCT/APTES@SiO2@Fe3O4, as expected, the absorption peak for C–Cl disappeared (Fig. 1e). After the coordination of the Ni(II) metal ion, the imine peak shifted from 1626 to 1639 cm−1. These obtained result indicated the coordination of Ni(II) metal ion on the surface of picolylamine/TCT/APTES@SiO2@Fe3O4 (Fig. 1f).57,58
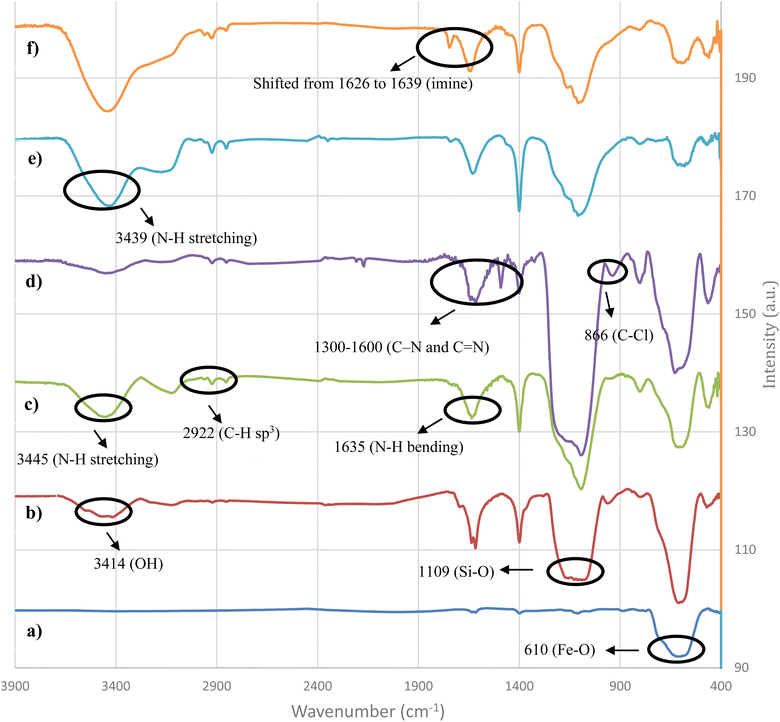 | ||
| Fig. 1 FT-IR spectra of (a) Fe3O4, (b) SiO2@Fe3O4, (c) APTES@SiO2@Fe3O4, (d) TCT/APTES@SiO2@Fe3O4, (e) picolylamine/TCT/APTES@SiO2@Fe3O4, and (f) NiII-picolylamine/TCT/APTES@SiO2@Fe3O4. | ||
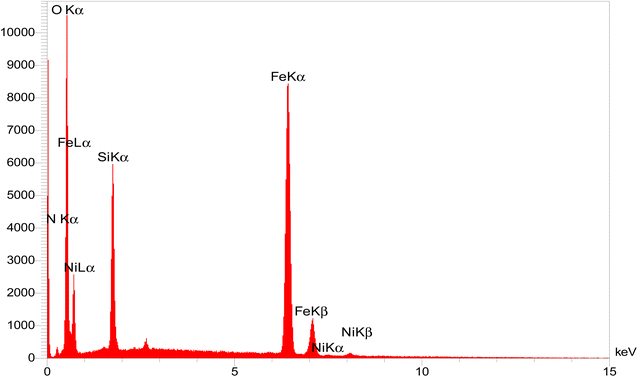
EDX spectroscopy is one of the most significant elemental analyses to chemically characterize samples with high accuracy. The EDX spectrum clearly showed the presence of O, Fe, Si, C, N, and Ni elements in the catalyst with mass percentages of 36.0, 39.0, 19.71, 2.83, 1.45, and 1.01, respectively, indicating the catalyst had been successfully prepared. The Ni loading onto the NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 catalyst was confirmed by the peak presence of the Ni element in the EDX spectrum (Fig. 2). Moreover, ICP was used to confirm the exact amount of Ni loading of the catalyst. The ICP results clearly showed the presence of Ni (1.77%) in the catalyst.
Further, the presence of O, Fe, Si, C, N, and Ni elements in the NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 catalyst was confirmed by another elemental analysis, namely elemental mapping analysis (Fig. 3). Fig. 3 clearly indicates the presence of O, Fe, Si, C, N, and Ni elements in the catalyst with a suitable dispersity. Moreover, the results from the elemental mapping analysis comfirmed the EDX analysis.
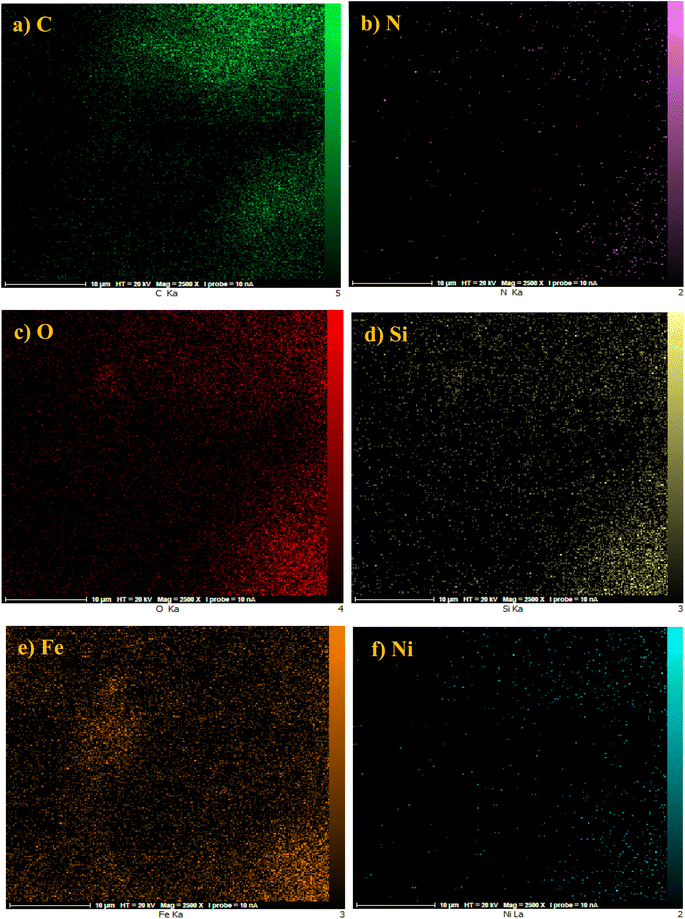 | ||
| Fig. 3 Elemental mapping analysis of NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 catalyst: (a) C, (b) N, (c) O, (d) Si, (e) Fe, and (f) Ni. | ||
XPS was performed from 0 to 1200 eV for the NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 catalyst to obtain the oxidation of the Ni state together with elemental analysis (Fig. 4). Fig. 4a–f illustrate the spectra for (a) Fe 2p, (b) C 1s, (c) N 1s, (d) Ni 2p, (e) Si 2p, and (f) O 1s elements in NiII-picolylamine/TCT/APTES@SiO2@Fe3O4. Fig. 4a demonstrates the high-resolution spectra of Fe 2p with two peaks at 712.6 and 726.4 eV, attributed to 2p3/2 and 2p1/2, respectively.58 The peaks at binding energies of 283.8 and 286.4 eV were attributed to C–C and C–O, respectively (Fig. 4b).58 Fig. 4c depicts the high-resolution spectrum of N 1s with two peaks at 401.2 and 404.6 eV related to C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, respectively.58 The XPS spectrum for Ni showed doublet peaks at 855.8 and 864.9 eV related to Ni 2p3/2 and Ni 2p1/2, respectively (Fig. 4d).59,60 These peaks well confirmed the oxidation state of Ni(II) in the catalyst. The Si 2p appear at ≈102.7 eV and 104.8 eV, respectively related to the Si–C and Si–O, respectively (Fig. 4e).58 Finally, Fig. 4f reveals the O atoms contained in as-prepared nanocatalyst. O 1s at 530.2 eV, 534.8 eV and 535.2 eV corresponding to Fe–O, Si–O and O–H, respectively.58
N, respectively.58 The XPS spectrum for Ni showed doublet peaks at 855.8 and 864.9 eV related to Ni 2p3/2 and Ni 2p1/2, respectively (Fig. 4d).59,60 These peaks well confirmed the oxidation state of Ni(II) in the catalyst. The Si 2p appear at ≈102.7 eV and 104.8 eV, respectively related to the Si–C and Si–O, respectively (Fig. 4e).58 Finally, Fig. 4f reveals the O atoms contained in as-prepared nanocatalyst. O 1s at 530.2 eV, 534.8 eV and 535.2 eV corresponding to Fe–O, Si–O and O–H, respectively.58
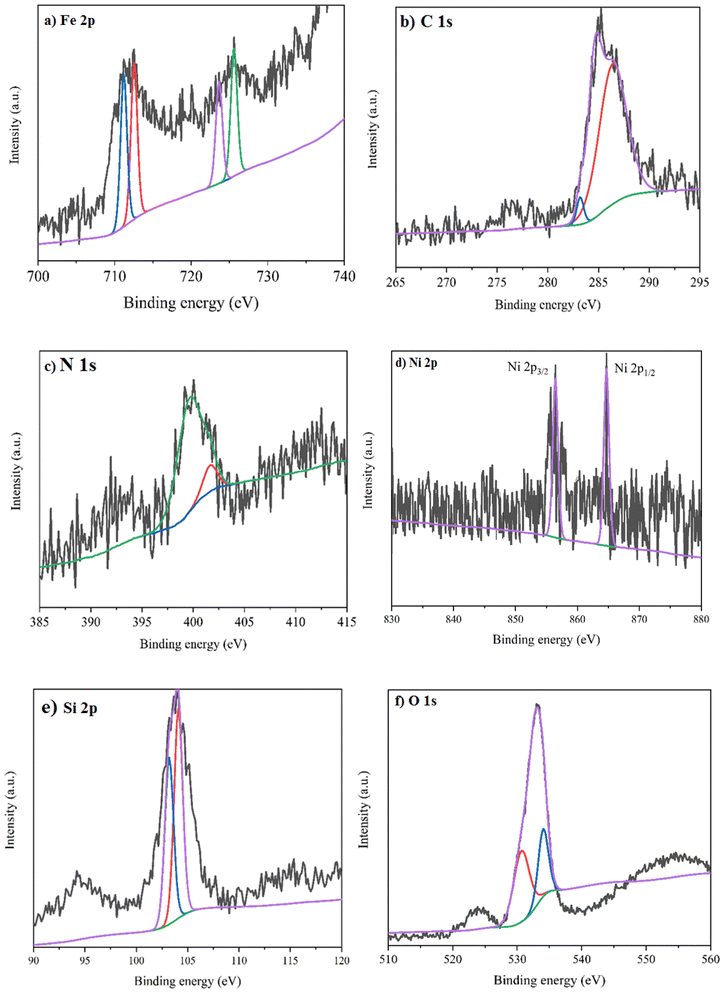 | ||
| Fig. 4 XPS spectra of NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 catalyst: (a) Fe 2p, (b) C 1s, (c) N 1s, (d) Ni 2p, (e) Si 2p, and (f) O 1s. | ||
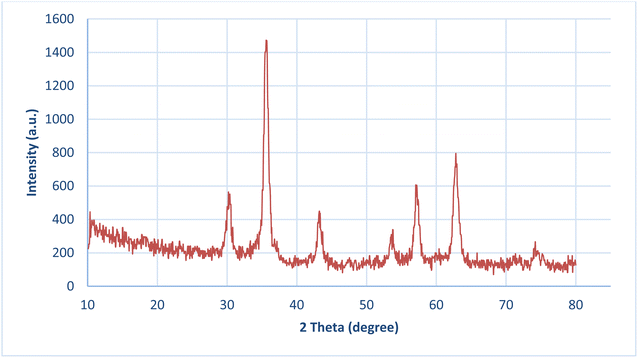
The crystalline structure of NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 was investigated by powder X-ray diffraction (PXRD) (Fig. 5). Fig. 5 displays strong and sharp peaks at 2θ = 30.45°, 35.44°, 35.68°, 43.24°, 53.69°, 57.14°, and 62.86° belonging to NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 which was well consistent with the Fe3O4 nanoparticle crystal structure. Besides, the obtained result demonstrated that the designed catalyst had been successfully synthesized and there was no damage to the crystalline structure of Fe3O4, and the crystallographic faces were in good agreement with the XRD pattern of cubic Fe3O4 NP (JCPDS 88-0866).57,58 The crystallite average size and interplaner distance of the catalyst were determined to be 28.7 nm and 0.208 nm from the XRD spectra according to FWHM calculations using the Scherrer formula and Bragg equation, respectively (Table S1, ESI†).
The magnetic properties of Fe3O4 and NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 were studied at ambient temperature by VSM analysis (Fig. 6). Fig. 6a clearly indicates that these nanoparticles of (a) Fe3O4 and (b) NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 had a superparamagnetic nature. The magnetic saturation value for pure Fe3O4 was observed at 58.74 emu g−1. After coating various organic compounds on the surface of pure Fe3O4 to synthesize the NiII-picolylamine/TCT/APTES@SiO2@Fe3O4, the magnetic saturation value was observed at 37.02 emu g−1. Nevertheless, this magnetic saturation value was sufficient for the catalyst's easy separation from the reaction mixture by employing a magnetic bar (Fig. 6b).
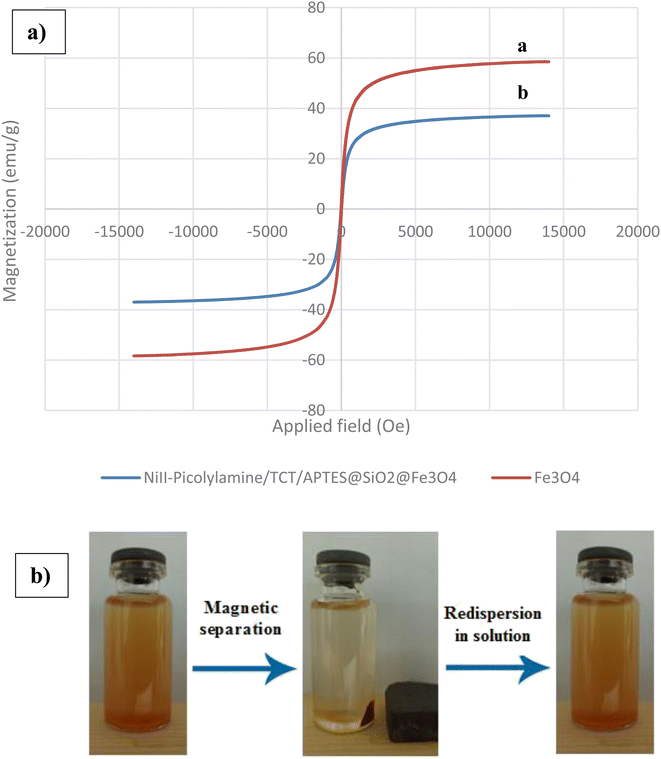 | ||
| Fig. 6 (a) VSM analysis, (b) image of the magnetic separation and redispersion of NiII-picolylamine/TCT/APTES@SiO2@Fe3O4. | ||
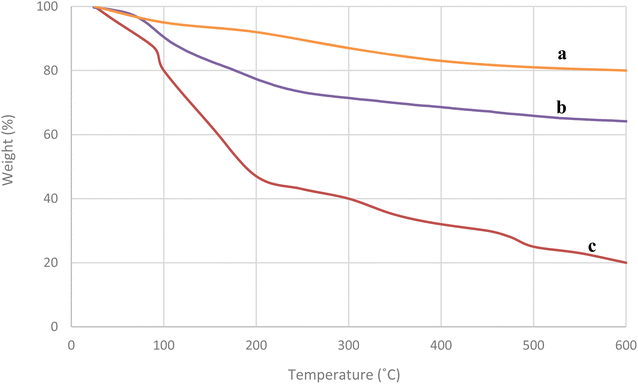
The thermal stability of Fe3O4, SiO2@Fe3O4, and NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 were investigated through TGA analysis in the temperature range of 25–600 °C (Fig. 7). Both the TGA diagram of Fe3O4 and silica-coated SiO2 indicated a small weight loss below 100 °C, probably due to the removal of surface hydroxyl groups and physically adsorbed and trapped water molecules. For NiII-picolylamine/TCT/APTES@SiO2@Fe3O4, there were two mass reductions: (i) below 100 °C, probably due to the evaporation of surface hydroxyl groups and physically adsorbed and trapped water molecules in the structure of the catalyst and (ii) between 180–600 °C, which was the main weight loss and probably due to the thermal decomposition of the functional groups and organic compounds supported on the catalyst surface. This thermal decomposition indicated the coating of various organic compounds on the iron oxide support. Moreover, the complete decomposition of the as-prepared nanocatalyst happened at about 500 °C.
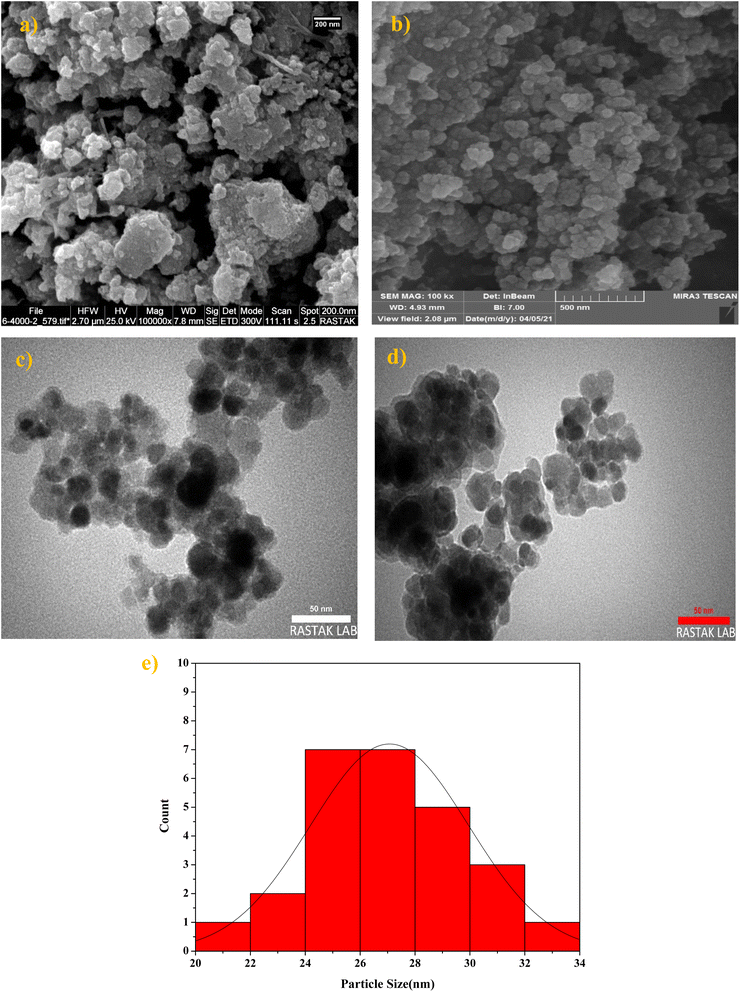
The structural features, morphologies, and particle sizes of Fe3O4 and NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 were investigated by FE-SEM, TEM, and size-distributions images (Fig. 8). The FE-SEM images indicated that Fe3O4 and NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 had been synthesized with spherical shapes with the particle sizes in the 20–33 nm and of 31–42 nm ranges, respectively (Fig. 8a and b). In another study, the uniformity and morphology of Fe3O4 and NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 were further investigated by TEM (Fig. 8c and d). Fig. 8d clearly indicates that the particles had been synthesized with a uniform size and core/shell. Further, the size distribution of the NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 was measured by TEM to be over 28 nm, which suitably matched with the results obtained from the XRD analysis (Fig. 8e).
The isotherm type, total pore volume, mean pore diameter, and surface area of NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 were determined by BET analysis (Fig. 9). As can be seen from Fig. 9a, the structure of the as-prepared nanocatalyst could be ascribed to the type IV isotherm with a distinct hysteresis loop indicating mesoporous structure.57,58 Fig. 9b shows that the as-prepared nanocatalyst sat in the P/P0 range of 0.006–0.007 with a strong inflection, which may have been because the capillary condensation of N2 was reflected in the mesopores. Further, the corresponding structural parameters of the as-prepared nanocatalyst included a total pore volume, mean pore diameter, and surface area of 0.189 cm3 g−1, 22.31 nm, and 53.61 m2 g−1, respectively.
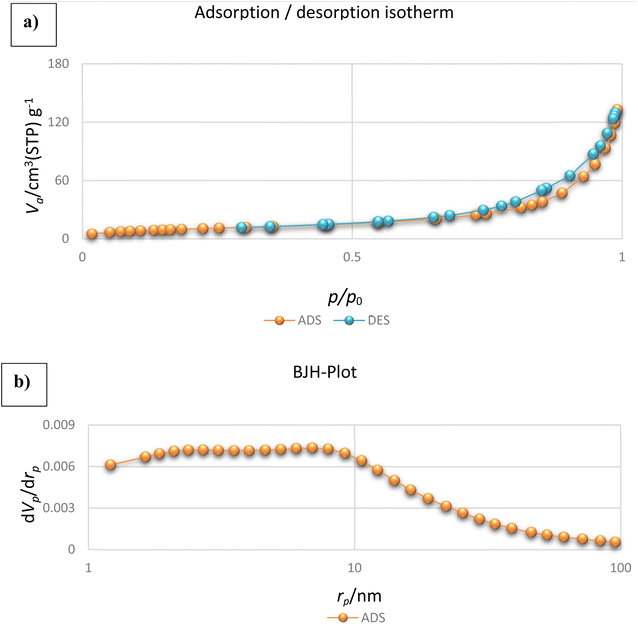 | ||
| Fig. 9 (a) BET plot of the nitrogen adsorption–desorption and (b) pore-size distribution curve of the NiII-picolylamine/TCT/APTES@SiO2@Fe3O4. | ||
Catalytic activity test
Activity tests for the prepared NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 catalyst were performed for the one-pot synthesis of substituted pyridine derivatives. For this, a one-pot pseudo-four component reaction of malononitrile (2 mmol), thiophenol (1 mmol), and benzaldehyde (1 mmol) was tested initially as a model reaction for the synthesis of 4a. In this study, we investigated the effect of various solvents, loadings of catalyst, and temperatures, and the obtained results are summarized in Table 1. When the reaction was stirred in the absence of the as-prepared nanocatalyst under solvent-free conditions at room temperature, a trace amount of 4a was obtained even after 24 h (Table 1, entry 1). Similarly, when the reaction was stirred under the same condition at 80 °C, only a trace amount of 4a was obtained even after 24 h (Table 1, entry 2). It was observed that in the presence of the catalyst, the product was indeed obtained. Initially, we tried the reaction with 0.005 g of the as-prepared nanocatalyst under solvent-free conditions at 80 °C and a yield of 43% of 4a was obtained (Table 1, entry 3). When the catalyst loading was increased to 0.01 g and 0.03 g under the same conditions, 62% and 75% yields of 4a were obtained (Table 1, entries 4 and 5). The best result was obtained when the reaction was carried out with 0.05 g of the as-prepared nanocatalyst under solvent-free conditions, whereby the reaction yield increases to 95% after 30 min (Table 1, entry 6). On increasing the amount of catalyst to 0.07–0.1 g of the as-prepared nanocatalyst, 95%, 93%, and 91% product yields were obtained, respectively (Table 1, entries 7–9). In the next study, we examined the effect of various solvents, such as MeOH, H2O, CH2Cl2, CHCl3, H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1
EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), EtOH, and EG on the reaction. The obtained results indicated the products were obtained in moderate yield (Table 1, entries 10–14). In EtOH produced a 92% product yield after 60 min (Table 1, entry 15). The screening of various solvents indicated that the EG as the solvent was more effective than the other solvents. In addition, in the solvent-free condition, when the reaction was performed by EG in the presence of 0.05 g of as-prepared nanocatalyst under reflux conditions, 96% product was obtained after 35 min (Table 1, entry 16).
1), EtOH, and EG on the reaction. The obtained results indicated the products were obtained in moderate yield (Table 1, entries 10–14). In EtOH produced a 92% product yield after 60 min (Table 1, entry 15). The screening of various solvents indicated that the EG as the solvent was more effective than the other solvents. In addition, in the solvent-free condition, when the reaction was performed by EG in the presence of 0.05 g of as-prepared nanocatalyst under reflux conditions, 96% product was obtained after 35 min (Table 1, entry 16).
| Entry | Catalyst (g) | Solvent (3 mL) | Temperature (°C) | Time (min) | Yieldb (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: malononitrile (2 mmol), thiophenol (1 mmol), benzaldehyde (1 mmol), catalyst, and solvent (3 mL).b Isolated yield. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | None | Solvent-free | Room temperature | 24 (h) | Trace | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | None | Solvent-free | 80 | 24 (h) | Trace | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 0.005 | Solvent-free | 80 | 130 | 43 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 0.01 | Solvent-free | 80 | 90 | 62 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | 0.03 | Solvent-free | 80 | 60 | 75 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | 0.05 | Solvent-free | 80 | 30 | 95 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 0.07 | Solvent-free | 80 | 35 | 95 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | 0.09 | Solvent-free | 80 | 40 | 93 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | 0.1 | Solvent-free | 80 | 40 | 91 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | 0.05 | MeOH | Reflux | 120 | 45 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 | 0.05 | H2O | Reflux | 90 | 78 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 | 0.05 | CH2Cl2 | Reflux | 180 | 51 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 | 0.05 | CHCl3 | Reflux | 180 | 44 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 | 0.05 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1 EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 60 | 83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 | 0.05 | EtOH | Reflux | 60 | 92 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16 | 0.05 | EG | 80 | 20 | 96 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In the next study, we investigated the one-pot pseudo-four component reaction of malononitrile (2 mmol), thiophenol (1 mmol), and benzaldehyde (1 mmol), using various catalysts, such as Fe3O4, SiO2@Fe3O4, APTES@SiO2@Fe3O4, TCT/APTES@SiO2@Fe3O4, picolylamine/TCT/APTES@SiO2@Fe3O4, and NiCl2·6H2O for the synthesis of 4a under solvent-free conditions at 80 °C, and the results are listed in Table 2. As can be seen, it was found that NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 catalyze the reaction with high yield and short reaction time than the other catalysts.
| Entry | Catalyst | Time (h) | Yieldb (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: malononitrile (2 mmol), thiophenol (1 mmol), benzaldehyde (1 mmol), and catalyst (0.05 g) under solvent-free conditions at 80 °C.b Isolated yield. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | Fe3O4 | 4 | 41 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | SiO2@Fe3O4 | 3 | 55 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | APTES@SiO2@Fe3O4 | 1 | 71 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | TCT/APTES@SiO2@Fe3O4 | 24 | 10 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | Picolylamine/TCT/APTES@SiO2@Fe3O4 | 6 | 25 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | NiCl2·6H2O | 6 | 45 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 | 30 min | 95 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Knowing the optimized conditions (using 0.05 g of the as-prepared nanocatalyst under solvent-free conditions or EG at 80 °C), a range of aromatic aldehydes containing electron-withdrawing and electron-donating groups were reacted with malononitrile and thiophenol, and afforded the corresponding products (4a–l), and good results were obtained (Table 3). In method A, NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 as a high-performance heterogeneous nanocatalyst was used for the reaction of malononitrile, thiophenol, and various aldehydes under solvent-free conditions at 80 °C and gave satisfactory yields (91–97%) after 10–55 min. Moreover, NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 as a high-performance heterogeneous nanocatalyst showed excellent activity for the reaction of malononitrile, thiophenol, and various aldehydes in EG at 80 °C. The corresponding products were furnished in short reaction times (8–45 min) and in excellent yields (89–97%). Generally, NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 displayed remarkable efficiency for the one-pot synthesis of substituted pyridine derivatives in both methods A and B.
| Entry | Product | Method Aa | Method Bb | Melting point (°C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Yieldc (reaction time) | % Yieldc (reaction time) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| a Reaction conditions: Malononitrile (2 mmol), thiophenol (1 mmol), various aldehydes (1 mmol), and NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 (0.05 g) under solvent-free conditions at 80 °C.b Reaction conditions: Malononitrile (2 mmol), thiophenol (1 mmol), various aldehydes (1 mmol), and NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 (0.05 g) in EG (3 mL) at 80 °C.c Isolated yield. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | 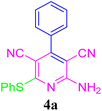 |
95 (30 min) | 96 (20 min) | 217–219 (ref. 51) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 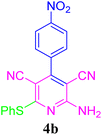 |
97 (15 min) | 96 (10 min) | 285–287 (ref. 51) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 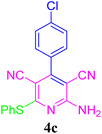 |
94 (35 min) | 93 (30 min) | 220–222 (ref. 51) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 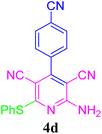 |
96 (10 min) | 97 (8 min) | 271–273 (ref. 51) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | 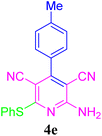 |
93 (50 min) | 95 (40 min) | 209–211 (ref. 51) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | 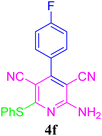 |
91 (30 min) | 92 (25 min) | 224–226 (ref. 53) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | 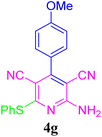 |
92 (50 min) | 91 (40 min) | 238–240 (ref. 51) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | 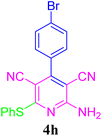 |
95 (35 min) | 94 (30 min) | 256–258 (ref. 51) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | 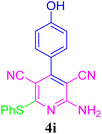 |
94 (55 min) | 89 (45 min) | 314–316 (ref. 51) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 | 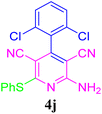 |
96 (30 min) | 92 (25 min) | 183–185 (ref. 51) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 | 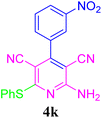 |
96 (20 min) | 96 (15 min) | 217–219 (ref. 51) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 |  |
93 (20 min) | 93 (10 min) | — | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Due to environmental concerns, the catalyst reusability was tested (Fig. 10). For this, we carried out the reaction between 4-nitrobenzaldehyde (1 mmol), malononitrile (2 mmol), and thiophenol (1 mmol) in the presence of NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 in EG at 80 °C. After the reaction completion, the catalyst could be separated easily from the reaction mixture by using a simple magnet. Then, the recovered catalyst was washed with EtOH (3 × 10 mL) and dried at room temperature for reused in the next run. This catalyst was reused up to eight times with only a slight decrease in activity, whereby the yields from the reaction smoothly decreased from 97% to 87% during 25–33 min (Fig. 10a). This slight decrease in the catalytic activity of the recovered nanocatalyst was due to the small leaching of Ni (1%) in the reaction after eight runs. The nanocatalyst was investigated by various analyses, including TEM and size distribution after each run for eight times. The TEM image was obtained for the recovered nanocatalyst after the eight runs (Fig. 10b), and indicated that the recovered nanocatalyst had a nearly uniform size and spherical shape, similar to the fresh catalyst. Likewise, the size distribution of the recovered nanocatalyst was measured by TEM, and was nearly same as the fresh catalyst (Fig. 10c).
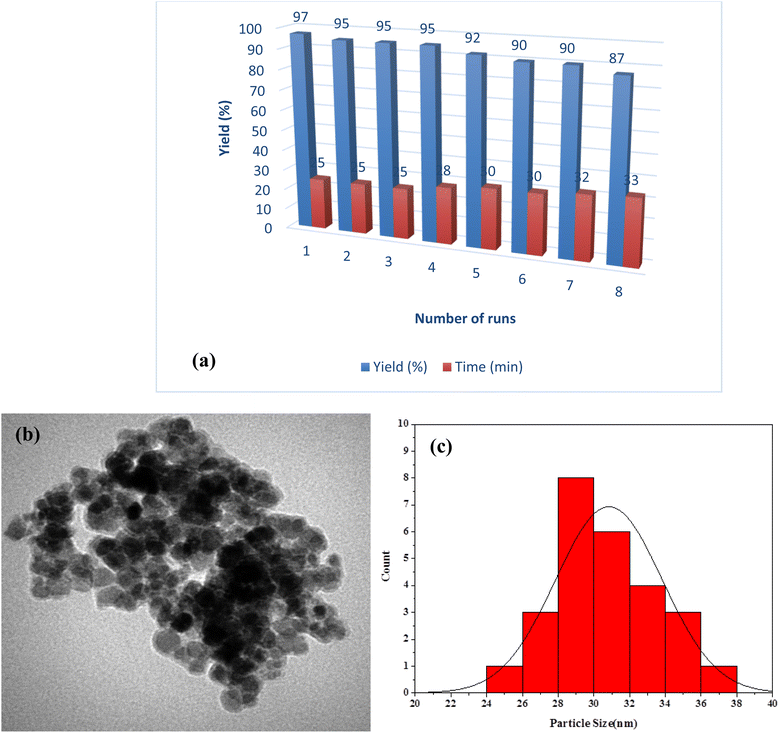 | ||
| Fig. 10 (a) Reusability results of NiII-picolylamine/TCT/APTES@SiO2@Fe3O4, (b) TEM image, and (c) size distribution of the reused catalyst. | ||
In another study, the effect of leaching of the active species in the reaction mixture on the stability of the as-prepared nanocatalyst was investigated. To address this possibility, we used ICP-AES analysis of the fresh state of the catalyst and also the recovered catalyst after eight runs. The reaction on the basis of ICP-AES analysis showed a leaching of Ni of 1 wt% after the eight runs. This result obtained could explain the negligible decrease in the yield of the product 4b with the increased recycling.
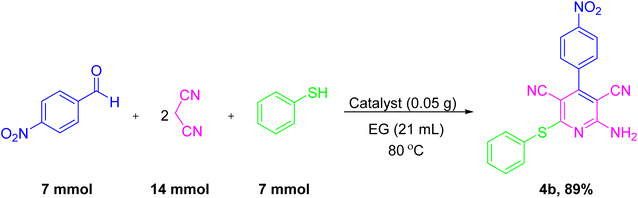
Large-scale synthesis of the reaction between 4-nitrobenzaldehyde (7 mmol, 1.05 g), malononitrile (14 mmol, 0.92 g), and thiophenol (7 mmol, 0.77 g) was performed in the presence of NiII-picolylamine/TCT/APTES@ SiO2@Fe3O4 in EG (21 mL) at 80 °C to give the corresponding 4b to prove the scalability and the prospective use of the current method (Scheme 5). The obtained results indicated that when performing the large-scale synthesis, the desired product was obtained with an 89% yield.
A plausible mechanism for obtaining the substituted pyridine derivatives in the reaction catalyzed by NiII-picolylamine/TCT/APTES@ SiO2@Fe3O4 is indicated in Scheme 6. The reaction was accelerated via the surface properties, including by Ni2+ acting as a Lewis acid. Initially, the Lewis acid site of the as-prepared nanocatalyst activated the aldehyde A and malonontrile B, and produced the 2-arylidene malononitrile C via a Knoevenagel condensation. In the next step, Ni2+ catalyzed the thiophenol D and malononitrile B and via a Michael addition to the intermediate C led to producing the intermediate F. Then, dihydropyridine G was formed via the tautomerization of the Michael product F, followed by yielding the desired product H.
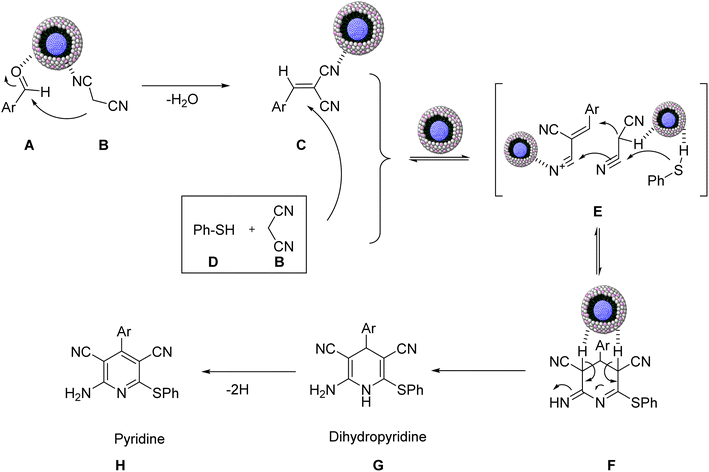 | ||
| Scheme 6 Plausible mechanism for the construction of substituted pyridine derivatives catalyzed by NiII-picolylamine/TCT/APTES@ SiO2@Fe3O4. | ||
Experimental
Materials and methods
All of the used materials were purchased from Fluka (Switzerland), Sigma-Aldrich, and Merck (Germany): iron(III) chloride, 25% ammonia, FeSO4·7H2O, (3-aminopropyl)triethoxysilane, ethanol, tetraethyl orthosilicate, cyanuric chloride, tetrahydrofuran, 2-picolylamine, NiCl2·6H2O, malononitrile, various aromatic aldehydes, and thiophenol. 1H and 13C NMR spectra were obtained applying CDCl3 or DMSO-d6 solvent on a Bruker DRX-250 AVANCE spectrometer (at 250 and 62.5 MHz). FT-IR spectra were acquired by employing a PerkinElmer 597 spectrophotometer. The EDAX spectra were applied for the elemental analysis of NiII-picolylamine/TCT/APTES@SiO2@Fe3O4. PXRD analysis was performed on an X'Pert-PRO advanced diffractometer operated at 40 kV and 40 mA at r.t to investigate the crystalline structure of the synthesized catalyst. The surface atomic concentration and chemical composition of NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 were investigated using XPS at an energy of 1253.6 eV (Esfahan University, Esfahan, Iran). SEM analysis was recorded on a TE-SCAN system (Brno, Czech Republic). TEM images were recorded using a Zeiss EM10C microscope operating at 80 kV. VSM analysis was applied to verify the magnetic properties of NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 (VSM, Taban, Tehran, Iran).Fe3O4 synthesis
The coprecipitation procedure was applied for the preparation of magnetic Fe3O4. First, 0.9 g of FeSO4·7H2O with 0.97 g of FeCl3 in 120 mL of deionized H2O were dissolved at 80 °C under a N2 gas atmosphere, and then a 25% aqueous ammonia solution (120 mL, 1.5 M) was added dropwise into the solution under stirring. In the following, the obtained black mixture was stirred under N2 gas for 30 min. The reaction mixture was cooled to 25 °C, and the resulting black precipitate was isolated by applying a simple magnet and washed several times with deionized H2O and EtOH, and dried at 40 °C for 24 h (yield: 92%).Synthesis of SiO2@Fe3O4
First, 1 g of Fe3O4 was dispersed in a mixture of EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled H2O (80
distilled H2O (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20; 100 mL) with applying sonification for 30 min. Then, 2 mL of NH3 solution 25% and 2 mL of TEOS (tetraethyl orthosilicate) were added and the obtaining solution was stirred under N2 for 12 h. Finally, the obtained products were collected by employing a simple magnet and washed five times with distilled H2O and once with EtOH. Eventually, SiO2@Fe3O4 was afforded and dried in an oven at 40 °C for 24 h.
20; 100 mL) with applying sonification for 30 min. Then, 2 mL of NH3 solution 25% and 2 mL of TEOS (tetraethyl orthosilicate) were added and the obtaining solution was stirred under N2 for 12 h. Finally, the obtained products were collected by employing a simple magnet and washed five times with distilled H2O and once with EtOH. Eventually, SiO2@Fe3O4 was afforded and dried in an oven at 40 °C for 24 h.
Synthesis of APTES@SiO2@Fe3O4
First, 1 g of SiO2@Fe3O4 in 100 mL of EtOH was suspended under ultrasonic radiation for 30 min, and then 4 mL of APTES under a nitrogen gas atmosphere was added to the dispersed solid NPs at 35 °C. Next, 4 mL of distilled H2O was added to increase the hydrolysis rate of APTES, and the combination was stirred for 12 h under reflux conditions. Finally, the resulting APTES@SiO2@Fe3O4 was obtained by applying a simple magnet, washed with several runs of deionized H2O and EtOH, and dried in a vacuum at ambient temperature for 24 h (yield: 91%).Synthesis of TCT/APTES@SiO2@Fe3O4
First, 0.79 g of APTES@SiO2@Fe3O4 in 100 mL of dry THF was suspended, and then 0.35 g of TCT was added and the resulting mixture was stirred for 16 h at room temperature. The afforded catalyst was washed three times with EtOH and dried under vacuum at ambient temperature (yield: 90%).Synthesis of PA/TCT/APTES@SiO2@Fe3O4
First, 0.5 mL of 2-picolylamine was slowly added to 100 mL of the ethanolic solution of TCT/APTES@SiO2@Fe3O4 (0.1 g), and then the obtained mixture was stirred at ambient temperature for 24 h. The obtained PA/TCT/APTES@SiO2@Fe3O4 was isolated magnetically and washed several times with EtOH and dried under vacuum.Synthesis of NiII-picolylamine/TCT/APTES@SiO2@Fe3O4
First, 1 g of PA/TCT/APTES@SiO2@Fe3O4 in 100 mL of EtOH was suspended by employing sonication for 30 min. On the other hand, 2.5 mmol of NiCl2·6H2O in 10 mL of EtOH was dissolved under vigorous stirring at ambient temperature to obtain a blue solution. Then, the solution of NiCl2·6H2O in EtOH was quickly added to the synthesized picolylamine/TCT/APTES@SiO2@Fe3O4, which was then stirred under reflux conditions for 48 h. The solvent was evaporated under vacuum, and the NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 was washed three times with EtOH and then dried in air at ambient temperature.Synthesis of pyridine derivatives
A mixture of the as-prepared nanocatalyst (0.05 g), malononitrile (2 mmol), thiophenol (1 mmol), and a variety of aldehyde derivatives (1 mmol) under solvent-free conditions or EG (3 mL) was heated to 80 °C in an oil bath until reaction completion. To evaluate the progress of the reaction, the mixture was monitored by thin-layer chromatography (TLC). After completion of the reaction, ethanol (15 mL) was added to the contents of the reaction container and stirred for 5 min. To reuse the as-prepared nanocatalyst in the next run, it was conveniently recovered from the reaction mixture by employing a magnetic bar and washed three times with ethanol. The synthesized products were recrystallized from hot ethanol to afford the pure product.Conclusion
The synthesis of NiII-picolylamine/TCT/APTES@SiO2@Fe3O4 was reported according to a convenient and simple four-step operation process. The as-prepared nanocatalyst was characterized by FT-IR, XPS, TGA, VSM, TEM, XRD, BET, FE-SEM, ICP-AES, and EDX analyses. The activity of the as-prepared nanocatalyst was investigated in the one-pot pseudo-four component reaction of malononitrile, thiophenol, and a variety of aldehyde derivatives under solvent-free conditions or EG at 80 °C, and the corresponding products were obtained in excellent yields. This method is safe and environmentally benign and offers various advantages, such as no use of column chromatography, easy workup procedure, multiple carbon–heteroatom and carbon–carbon bond formation, usage of green solvent, environmental friendliness, economical processing, high product yield, and easy separation of the catalyst from the reaction environment. The as-synthesized nanocatalyst indicated good reusability by employing a magnetic bar, with only small decreases in the yields of the reaction from 97% to 87%. Likewise, a negligible loss of Ni (1% leaching) was observed on the basis of ICP analysis after eight runs.Author contributions
S. Rezayati and A. Ramazani conceived the idea and designed the research. S. Rezayati and F. Kalantari carried out the research. S. Rezayati analyzed the data. S. Rezayati wrote the original draft. A. Ramazani reviewed the manuscript and editing. A. Ramazani was responsible for the funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The present study, derived from PhD thesis by Sobhan Rezayati, was supported by Iran National Science Foundation INSF and the University of Zanjan.References
- R. S. Varma, ACS Sustainable Chem. Eng., 2019, 7, 6458–6470 CrossRef CAS.
- S. A. Nafiu, S. Shaheen Shah, A. Aziz and M. Nasiruzzaman Shaikh, Chem.–Asian J., 2021, 16, 1956–1966 CrossRef PubMed.
- W. Wang, M. Luo, W. Yao, M. Ma, S. A. Pullarkat, L. Xu and P. H. Leung, New J. Chem., 2019, 43, 10744–10749 RSC.
- B. Baruah and M. L. Deb, Org. Biomol. Chem., 2021, 19, 1191–1229 RSC.
- Z. Ma, B. Zhao, Y. Gong, J. Deng and Z. Tan, J. Mater. Chem. A, 2019, 7, 22826–22847 RSC.
- H. Tian, S. Liu, Z. Zhang, T. Dang, Y. Lu and S. Liu, ACS Sustainable Chem. Eng., 2021, 9, 4660–4667 CrossRef CAS.
- Q. Yang, G. Pan, J. Wei, W. Wang, Y. Tang and Y. Cai, ACS Sustainable Chem. Eng., 2021, 9, 2367–2377 CrossRef CAS.
- M. Nasiruzzaman Shaikh, A. N. Kalanthoden, M. Ali, Md. A. Haque, Md. Abdul Aziz, M. M. Abdelnaby, S. K. Rani and A. Idris Bakare, ChemistrySelect, 2020, 5, 14827–14838 CrossRef.
- M. Nasiruzzaman Shaikh, Md. Abdul Aziz and Z. H. Yamani, Catal. Sci. Technol., 2020, 10, 6544–6551 RSC.
- M. Nasiruzzaman Shaikh, A. Helal, A. N. Kalanthoden, B. Najjar, Md. Abdul Aziz and H. D. Mohamed, Catal. Commun., 2019, 119, 134–138 CrossRef.
- M. Nasiruzzaman Shaikh, Md. Abdul Aziz, A. N. Kalanthoden, A. Helal, A. S. Hakeema and M. Bououdina, Catal. Sci. Technol., 2018, 8, 4709–4717 RSC.
- J. P. K. Reynhardt, Y. Yang, A. Sayari and H. Alper, Adv. Synth. Catal., 2005, 347, 1379–1388 CrossRef CAS.
- A. N. Kalanthoden, Md. H. Zahir, Md. Abdul Aziz, B. Al-Najar, S. K. Rani and M. Nasiruzzaman Shaikh, Chem.–Asian J., 2022, 17, e202101195 CrossRef CAS PubMed.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J. M. Basset, Chem. Rev., 2011, 111, 3036–3075 CrossRef CAS PubMed.
- M. Gawande, A. Rathi, P. Branco and R. Varma, Appl. Sci., 2013, 3, 656–674 CrossRef CAS.
- M. Esmaeilpour, J. Javidi, F. N. Dodeji and M. M. M. Abarghoui, Transition Met. Chem., 2014, 39, 797–809 CrossRef CAS.
- S. Rezayati, Y. Ahmadi and A. Ramazani, Inorg. Chim. Acta, 2023, 544, 121203 CrossRef CAS.
- R. Abu-Reziq, H. Alper, D. Wang and M. L. Post, J. Am. Chem. Soc., 2006, 128, 5279–5282 CrossRef CAS PubMed.
- M. Nasiruzzaman Shaikh, M. Bououdina, A. Azeez Jimoh, Md. Abdul Aziz, A. Helal, A. S. Hakeem, Z. H. Yamania and T. J. Kim, New J. Chem., 2015, 39, 7293–7299 RSC.
- M. Nasiruzzaman Shaikh, Md. Abdul Aziz, A. Helal, M. Bououdina, Z. H. Yamani and T. J. Kim, RSC Adv., 2016, 6, 41687–41695 RSC.
- A. Nasar Kalanthoden, Md. Hasan Zahir, Md. Abdul Aziz, B. Al-Najar, S. K. Rani and M. Nasiruzzaman Shaikh, Chem.–Asian J., 2022, 17, e202101195 Search PubMed.
- N. Griffete, F. Herbst, J. Pinson, S. Ammar and C. Mangeney, J. Am. Chem. Soc., 2011, 133, 1646–1649 CrossRef CAS PubMed.
- B. B. Toure and D. G. Hall, Chem. Rev., 2009, 109, 4439–4486 CrossRef CAS PubMed.
- E. Ruijter, R. Scheffelaar and R. V. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234–6246 CrossRef CAS PubMed.
- A. Maleki, Z. Hajizadeh and R. Firouzi-Haji, Microporous Mesoporous Mater., 2018, 259, 46–53 CrossRef CAS.
- A. Maleki, M. Niksefat, J. Rahimi and R. Taheri-Ledari, Mater. Today Chem., 2019, 13, 110–120 CrossRef CAS.
- A. Maleki, R. Firouzi-Haji and Z. Hajizadeh, Int. J. Biol. Macromol., 2018, 116, 320–326 CrossRef CAS PubMed.
- S. B. Levy, M. N. Alekshun, B. L. Podlogar, K. Ohemeng, A. K. Verma, T. Warchol, B. Bhatia, T. Bowser and M. U. S. Grier, Patent Appl., 2005124678 A1 20050609, 2005.
- T. R. K. Reddy, R. Mutter, W. Heal, K. Guo, V. J. Gillet, S. Pratt and B. Chen, J. Med. Chem., 2006, 46, 607–615 CrossRef PubMed.
- M. T. Cocco, C. Congiu, V. Lilliu and V. Onnis, Eur. J. Med. Chem., 2005, 40, 1365–1372 CrossRef CAS PubMed.
- H. Chen, W. Zhang, R. Tam and A. K. Raney, PCT Int. Appl., WO 2005058315 A1, 20050630, 2005.
- B. B. Fredholm, A. P. Ijzerman, K. A. Jacobson and K. N. Klotz, J. Pharmacol. Rev., 2001, 53, 527–552 CAS.
- A. J. Herlt, R. W. Rickards and J. P. J. Wu, Antibiot, 1985, 38, 516–518 CrossRef CAS PubMed.
- H. Wang, S. L. Yeo, J. Xu, X. Xu, H. He, F. Ronca, A. E. Ting, Y. Wang, V. C. Yu and M. M. Sim, J. Nat. Prod., 2002, 65, 721–724 CrossRef CAS PubMed.
- D. L. Boger, S. D. Burke, T. A. Engler, B. Fraser-Reid, M. N. Greco, P. Helquist, P. A. Jacobi, M. E. Jung, A. P. Kozikowski, S. E. Martin, W. R. Roush, A. B. Smith III, R. Tsang, D. R. Williams and T. Lindberg, ed. Academic Press, San Diego, 1988, 2, 1.
- E. S. Kim, Drugs, 2017, 77, 2063–2070 CrossRef PubMed.
- M. W. Beukers, L. C. W. Chang, J. K. von Frijtag Drabbe Künzel, T. Mulder-Krieger, R. F. Spanjersberg, J. Brussee and A. P. Ijzerman, J. Med. Chem., 2004, 47, 3707–3709 CrossRef CAS PubMed.
- L. C. W. Chang, J. K. von Frijtag Drabbe Künzel, T. Mulder-Krieger, R. F. Spanjersberg, S. F. Roerink, G. van den Hout, M. W. Beukers, J. Brussee and A. P. Ijzerman, J. Med. Chem., 2005, 48, 2045–2053 CrossRef CAS PubMed.
- S. Tu, B. Jiang, H. Jiang, Y. Zhang, R. Jia, J. Zhang, Q. Shao, C. Li, D. Zhou and L. Cao, Tetrahedron, 2007, 63, 5406–5414 CrossRef CAS.
- M. Sridhar, B. C. Ramanaiah, C. Narsaiah, B. Mahesh, M. Kumaraswamy, K. R. R. Mallu, V. M. Ankathi and P. S. Rao, Tetrahedron Lett., 2009, 50, 3897 CrossRef CAS.
- M. L. Kantam, K. Mahendar and S. Bhargava, J. Chem. Sci., 2010, 122, 63–69 CrossRef CAS.
- S. Takale, J. Patil, V. Padalkar, R. Pisal and A. Chaskar, J. Braz. Chem. Soc., 2012, 23, 966–969 CrossRef CAS.
- R. Mamgain, R. Singh and D. S. Rawat, J. Heterocycl. Chem., 2009, 46, 69–73 CrossRef CAS.
- K. N. Singh and S. K. Singh, ARKIVOC, 2009, xiii, 153–160 Search PubMed.
- S. Shrinivas Kottawar, A. Shapi Siddiqui and R. Sudhakar Bhusare, Heterocycl. Commun., 2012, 18, 249–252 CrossRef.
- B. C. Ranu, R. Jana and S. Sowmiah, J. Org. Chem., 2007, 72, 3152–3154 CrossRef CAS PubMed.
- N. M. Evdokimov, I. V. Magedov, A. S. Kireev and A. Konienko, Org. Lett., 2006, 8, 899–902 CrossRef CAS PubMed.
- A. Allahi and B. Akhlaghinia, Phosphorus, Sulfur, and Silicon and the Related Elements, 2021, 196, 328–336 CrossRef CAS.
- B. Maleki, H. Natheghi, R. Tayebee, H. Alinezhad, A. Amiri, S. A. Hossieni and S. M. Mahdi Nouri, Polycyclic Aromat. Compd., 2020, 40, 633–643 CrossRef CAS.
- M. R. Poor Heravi and F. Fakhr, Tetrahedron Lett., 2011, 52, 6779–6782 CrossRef CAS.
- J. Safaei-Ghomi and M. A. Ghasemzadeh, J. Sulfur Chem., 2013, 34, 233–241 CrossRef CAS.
- P. V. Shinde, S. S. Sonar, B. B. Shingate and M. S. Shingare, Tetrahedron Lett., 2012, 53, 4870–4872 CrossRef.
- P. V. Shinde, V. B. Labade, B. B. Shingate and M. S. Shingare, J. Mol. Catal. A: Chem., 2011, 336, 100–105 CrossRef CAS.
- M. S. Su, X. J. Ji, B. B. Zhao, M. Tian and J. J. Ma, J. Chem. Soc. Pak., 2015, 37, 1130–1134 CAS.
- F. Kalantari, S. Rezayati, A. Ramazani, H. Aghahosseini, K. Ślepokura and T. Lis, ACS Appl. Nano Mater., 2022, 5, 1783–1797 CrossRef CAS.
- F. Kalantari, A. Ramazani, M. R. Poor Heravi, H. Aghahosseini and K. Ślepokura, Inorg. Chem., 2021, 60, 15010–15023 CrossRef CAS PubMed.
- S. Rezayati, A. Ramazani, S. Sajjadifar, H. Aghahosseini and A. Rezaei, ACS Omega, 2021, 6, 25608–25622 CrossRef CAS PubMed.
- S. Rezayati, F. Kalantari, A. Ramazani, S. Sajjadifar, H. Aghahosseini and A. Rezaei, Inorg. Chem., 2022, 61, 992–1010 CrossRef CAS PubMed.
- Y. Zhang, D. Li, M. Yu, W. Ma, J. Guo and C. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 8836–8844 CrossRef CAS PubMed.
- F. Wu, J. Tian, Y. Su, J. Wang, C. Zhang, L. Bao, T. He, J. Li and S. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 7702–7708 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of FT-IR, 1H NMR (250 MHz, CDCl3 or DMSO-d6) and 13C NMR (62.5 MHz, CDCl3 or DMSO-d6) spectra of the synthesized compounds, and XRD data for the NiII-picolylamine/TCT/APTES@SiO2@Fe3O4. See DOI: https://doi.org/10.1039/d3ra01826a |
| This journal is © The Royal Society of Chemistry 2023 |