Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Post-synthetic modification of a macrocyclic receptor via regioselective imidazolium ring-opening†
Jia
Shang
a,
Brett M.
Rambo
b,
Xiang
Hao
c,
Jun-Feng
Xiang
c,
Han-Yuan
Gong
*a and
Jonathan L.
Sessler
*bd
aCollege of Chemistry, Beijing Normal University, Xinjiekouwaidajie 19, Beijing 100875, P. R. China. E-mail: hanyuangong@bnu.edu.cn
bDepartment of Chemistry, The University of Texas at Austin, 105 East 24th Street, Stop A5300, Austin, Texas 78712-1224, USA. E-mail: sessler@cm.utexas.edu
cInstitute of Chemistry, Chinese Academy of Sciences, Zhongguancunbeiyijie 2, Beijing 100190, P. R. China
dDepartment of Chemistry, Shanghai University, Shanghai 200444, China
First published on 9th March 2016
Abstract
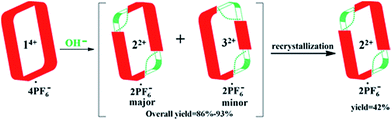
A facile post-synthetic modification of a tetracationic tetraimidazolium macrocycle, 14+ (i.e., the “Texas-sized” molecular box (cyclo[2](2,6-di(1H-imidazol-1-yl)pyridine)[2](1,4-dimethylenebenzene)), is described. Under mild basic conditions, ring-opening of the imidazolium moieties occurs. This results in two new isomeric dicationic macrocycles. This simple yet efficient modification serves to alter the size of the molecular cavity, the charge of the macromolecular receptor, and the manner whereby it interacts with dianionic guest molecules. The isomeric mixture of imidazolium ring opened macrocycles can be synthesized in relatively high overall yield (86–93%). The reaction shows regioselectivity and the ratio of major to minor (i.e., trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ring-opened products) was determined to be ca. 3
cis ring-opened products) was determined to be ca. 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 via1H NMR spectroscopy. The major isomer, trans-cyclo[2]((Z)-N-(2-((6-(1H-imidazol-1-yl)pyridin-2-yl)amino)vinyl)formamide)[2](1,4-bismethylbenzene) hexafluorophosphate (22+·2PF6−), was isolated in its pure form in 42% yield via recrystallization. The molecular recognition properties of 22+ were investigated using a series of dianionic guests (i.e., 2,6-naphthalenedicarboxylate (4), 2,6-naphthalenedisulfonate (5), and 1,5-naphthalenedisulfonate (6)) whose binding interactions with 14+ have been previously reported. This allowed us to evaluate how imidazolium ring-opening affects the inherent host/guest interactions of 14+. On the basis of solution spectroscopic studies (e.g., 1H NMR, 1H–1H COSY NMR, DOSY NMR, and NOESY NMR), in tandem with mass spectrometric analyses (ESI-MS) and single-crystal X-ray diffraction studies, we conclude that opening up the macrocyclic structure (i.e., converting 14+ to 22+) leads to considerable changes in the recognition behavior, with so-called outside binding or weak ion pair interactions, rather than pseudorotaxane formation, being favored both in solution and the solid-state. We postulate that methodologies such as those described herein could provide a means to control the molecular interactions of both free-standing macrocycles and those used to construct mechanically-interlocked molecules. Indeed, the application of hydroxide anion under the present conditions not only serves to effect the ring-opening of 14+, but also pseudorotaxane structures, such as, e.g., [14+·4] or [14+·5] derived there from.
1 via1H NMR spectroscopy. The major isomer, trans-cyclo[2]((Z)-N-(2-((6-(1H-imidazol-1-yl)pyridin-2-yl)amino)vinyl)formamide)[2](1,4-bismethylbenzene) hexafluorophosphate (22+·2PF6−), was isolated in its pure form in 42% yield via recrystallization. The molecular recognition properties of 22+ were investigated using a series of dianionic guests (i.e., 2,6-naphthalenedicarboxylate (4), 2,6-naphthalenedisulfonate (5), and 1,5-naphthalenedisulfonate (6)) whose binding interactions with 14+ have been previously reported. This allowed us to evaluate how imidazolium ring-opening affects the inherent host/guest interactions of 14+. On the basis of solution spectroscopic studies (e.g., 1H NMR, 1H–1H COSY NMR, DOSY NMR, and NOESY NMR), in tandem with mass spectrometric analyses (ESI-MS) and single-crystal X-ray diffraction studies, we conclude that opening up the macrocyclic structure (i.e., converting 14+ to 22+) leads to considerable changes in the recognition behavior, with so-called outside binding or weak ion pair interactions, rather than pseudorotaxane formation, being favored both in solution and the solid-state. We postulate that methodologies such as those described herein could provide a means to control the molecular interactions of both free-standing macrocycles and those used to construct mechanically-interlocked molecules. Indeed, the application of hydroxide anion under the present conditions not only serves to effect the ring-opening of 14+, but also pseudorotaxane structures, such as, e.g., [14+·4] or [14+·5] derived there from.
Introduction
Macrocyclic compounds play central roles in organic, inorganic, and supramolecular chemistry. To date, macrocycles have found application in areas as diverse as host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest chemistry, self-assembly, and the preparation of mechanically-interlocked molecules (MIMs).1 Tailor-made macrocycles can act as selective hosts for target analytes, as well as building blocks for complex supramolecular architectures and advanced materials.1c–f,2 There is thus an ongoing need to prepare new macrocycles, as well as to create analogues of existing structures with recognized utility. We believe that post-synthetic modification (PSM) strategies may have a role to play in these efforts. The appeal of post-synthetic strategies is that once a synthetic route is established, modification of the core structure may be used to access new analogues without a need to rework the synthesis. PSM may also provide a means of modulating the intrinsic binding properties of the core system in question. In the case of macrocycle-based MIMs, for instance, the ability to control recognition via PSM could find application in the areas of molecular switching1g,h,2f,3 and shuttling.1h,3a,4 While functionalized cyclodextrins,5 (hetero)calix[n]aromatics,6,7 and pillar[n]arene,8 have been made in this manner and the resulting systems support MIM formation, examples of PSM being used to change the macrocyclic core itself are, to our knowledge, lacking.
guest chemistry, self-assembly, and the preparation of mechanically-interlocked molecules (MIMs).1 Tailor-made macrocycles can act as selective hosts for target analytes, as well as building blocks for complex supramolecular architectures and advanced materials.1c–f,2 There is thus an ongoing need to prepare new macrocycles, as well as to create analogues of existing structures with recognized utility. We believe that post-synthetic modification (PSM) strategies may have a role to play in these efforts. The appeal of post-synthetic strategies is that once a synthetic route is established, modification of the core structure may be used to access new analogues without a need to rework the synthesis. PSM may also provide a means of modulating the intrinsic binding properties of the core system in question. In the case of macrocycle-based MIMs, for instance, the ability to control recognition via PSM could find application in the areas of molecular switching1g,h,2f,3 and shuttling.1h,3a,4 While functionalized cyclodextrins,5 (hetero)calix[n]aromatics,6,7 and pillar[n]arene,8 have been made in this manner and the resulting systems support MIM formation, examples of PSM being used to change the macrocyclic core itself are, to our knowledge, lacking.
There are a variety of ways to prepare macrocyclic receptors. One common approach involves the use of a template, typically a loosely bound species that favors formation of a single product.2e,9,10 Many systems have been prepared using template-based approaches. In contrast, relatively few new macrocyclic systems have been prepared via post-synthetic modification (PSM) of known macrocyclic precursors. However, the PSM approach is attractive because it can reduce the overall synthetic burden while providing a means to alter the recognition features of the initial macrocyclic system. In an elegant example of PSM, Kohnke et al. used calix[6]furan as a precursor to generate efficiently calix[6]pyrrole, as well as a series of calix[m]furan[n]pyrroles (m = 2 or 3, n = 2 or 4).11a Prior to that report, our group prepared several calix[4]pyridine and calix[m]pyridine[n]pyrroles (m + n = 4) derivatives by treating calix[4]pyrrole with a carbene to induce an insertion-based pyrrole-to-pyridine transformation.11b Recently, Wang and colleagues reported converting a tetrazine-containing macrocycle into a novel macrocyclic structure with diaziene fragments.11c–f
PSM reactions involving macrocycle-directed transformations are even less common and, in the case of charged macrocyclic species are, in fact, all but unknown. One important exception involves Lehn's polyamonium macrocycles, which acted as catalysts for phosphoryl transfer in ATP hydrolysis. In this case, transient modification of the macrocycle is thought to play a role in directing the overall transformation.12 Other charged macromolecular receptors, including Stoddart's “blue box” (i.e., cyclobis(paraquat-p-phenylene) or CBPQT4+)13 and the imidazolium receptors developed by Yoon, K. S. Kim and others,14–18 and triazole or triazolium receptors gained by our group,19a Flood,19b–d and Beer,20 have not yet, apparently, been subject to post-synthetic modification. On the other hand, PSM has been widely applied in the field of metal–organic frameworks as a method of functionalizing the basic system after it has been synthesized.9v,21
MIMs constitute another set of well-defined constructs where post-synthetic modification could be useful in terms of modulating the inherent properties. As a test of this latter hypothesis, we sought to apply a PSM approach to the modification of macrocycles that are predisposed to MIM formation. As detailed below we have found that the tetracationic tetraimidazolium macrocycle, known as the “Texas-sized box” (i.e., 14+·4PF6−),22a,b undergoes PSM under mild basic conditions to produce isomeric dicationic products wherein two of the four imidazolium subunits have undergone ring opening to produce a pair of “cis-” (22+) and “trans-” (32+) constitutional isomers (Scheme 1). One of these products, 22+, could be obtained in decent yield in purified form as its bis-PF6− salt. On the basis of solution phase spectroscopic studies and X-ray diffraction analyses, it was inferred that 22+ is much more flexible that its precursor 14+. This structural change is reflected in the binding properties. Specifically, for dianionic guests whose binding interactions with 14+ have been previously reported (i.e., 2,6-naphthalenedicarboxylate (4), 2,6-naphthalenedisulfonate (5), and 1,5-naphthalenedisulfonate (6); cf.Scheme 1),22b,c so-called outside binding, involving weak electrostatic ion pairing, rather than pseudorotaxane formation, is favored after imidazolium ring opening. Furthermore, it was found that hydroxide anion-induced imidazolium ring-opening causes irreversible disassembly of the original pseudorotaxane structures (e.g., [14+·4] or [14+·5]). Previously, we showed proton-induced dethreading of [14+·4].22b The susceptibility to effective pH seen for [14+·4] under acidic and now basic conditions mimics in a rudimentary fashion many biological interpenetrated structures, which only maintain physiological activity over a tightly regulated pH range.
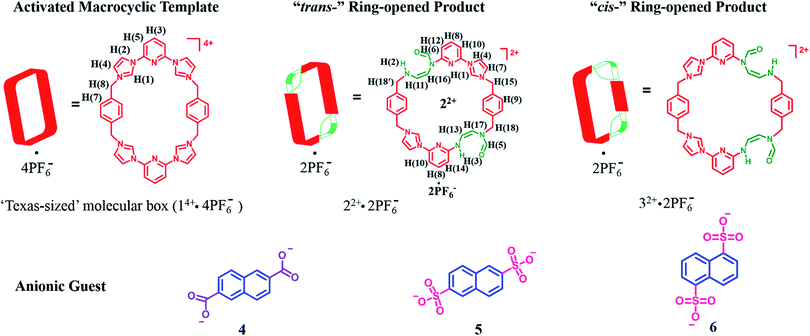 | ||
| Scheme 1 Structures of the “Texas-sized” molecular box (14+·4PF6−) and its “trans-” and “cis-” imidazolium ring-opened products. Also shown are the dianionic guest molecules used in this study. | ||
Results and discussion
The starting material for the present PSM study was 14+·4PF6− (i.e., the hexafluorophosphate salt of cyclo[2](2,6-di(1H-imidazol-1-yl)pyridine)[2](1,4-dimethylenebenzene); Scheme 1). This tetracationic macrocycle is readily obtained via a two step procedure. It acts as an effective receptor for dicarboxylate and disulfonate guests, as reported recently.22b,cIn the case of more highly basic guests, evidence of receptor decomposition was seen. We considered that this could reflect ring-opening reaction at the imidazolium sites present in 14+. While not hitherto exploited for the purposes of post-synthetic receptor modification, ring-opening reactions involving imidazolium species (cf.Scheme 2) have been known for at least 25 years.23–26 However, typically hydrolysis of common imidazole-2-ylidenes requires strongly basic aqueous media.26 We were thus keen to explore whether the unique macrocyclic nature of 14+·4PF6− would allow viable PSM imidazolium ring-opening conversions to be carried out under milder and hence more accessible conditions.
 | ||
| Scheme 2 Graphical comparison of this study to prior work involving non-macrocyclic imidazolium substrates. | ||
Initial experiments in pursuit of this goal were carried out in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of acetonitrile and an aqueous medium, which consisted of water containing varying concentrations of NH3·H2O (25–28% by mass). The effects of base concentration, temperature, and reaction time on the ring-opening reaction of 14+·4PF6− were then explored. It was found that macrocycle 14+ underwent essentially complete conversion in 45 h to a mixture of isomeric imidazolium ring-opened products. This occurred even when the pH of the initial solvent system was as low as 11 at 298 K (cf. ESI†). This facility stands in contrast to what is seen in the case of the non-macrocyclic control, imidazole-2-ylidene, where even at a pH as high as 13, no appreciable hydrolysis was reported to take place at room temperature.26 For preparative PSM conversions involving 14+, a pH of 11.6 at 313 K and a reaction time of 24 h were employed.27
1 mixture of acetonitrile and an aqueous medium, which consisted of water containing varying concentrations of NH3·H2O (25–28% by mass). The effects of base concentration, temperature, and reaction time on the ring-opening reaction of 14+·4PF6− were then explored. It was found that macrocycle 14+ underwent essentially complete conversion in 45 h to a mixture of isomeric imidazolium ring-opened products. This occurred even when the pH of the initial solvent system was as low as 11 at 298 K (cf. ESI†). This facility stands in contrast to what is seen in the case of the non-macrocyclic control, imidazole-2-ylidene, where even at a pH as high as 13, no appreciable hydrolysis was reported to take place at room temperature.26 For preparative PSM conversions involving 14+, a pH of 11.6 at 313 K and a reaction time of 24 h were employed.27
The preparative reactions were worked up easily by using compressed air to blow off the residual acetonitrile and other volatiles. The light yellow precipitate obtained from the remaining aqueous layer was then collected and analyzed. 1H NMR spectroscopic analyses led us to conclude that the precipitate obtained in this way consisted of a mixture of related products (cf. ESI† for spectra). An effort was made to purify and characterize these products as described below.
To determine the structures of the ring-opened products, 1H NMR and diffusion ordered spectroscopic (DOSY) analyses were carried out on the crude products obtained from the preparative-scale reaction outlined above. It was found that the proton signals of the products had essentially the same diffusion coefficients as the starting tetracationic macrocycle (D = 1.1 × 10−10 m2 s−1 in both cases). On this basis, we conclude that the crude product or products obtained after treatment of 14+·4PF6− with aqueous base and the starting macrocycle (i.e., 14+) have very similar molecular weights, shapes, and polarities.
In an effort to purify the crude material, it was subjected to recrystallization using a mixture of acetonitrile and dioxane (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). This allowed isolation of pure trans-cyclo[2]((Z)-N-(2-((6-(1H-imidazol-1-yl)pyridin-2-yl)amino)vinyl)formamide)[2](1,4-bi-methylbenzene) hexafluorophosphate (i.e., 22+·2PF6−), as inferred from 1H NMR spectroscopic analysis (cf.Scheme 3 and ESI†). This particular imidazolium ring-opened product was isolated in an overall yield of 42%. Further support for the formation of 22+ as its PF6− salt came from mass spectrometric studies using ESI-MS. These analyses revealed a peak corresponding to [22+·PF6−]+ (m/z = 809.2657) in the gas phase.
1, v/v). This allowed isolation of pure trans-cyclo[2]((Z)-N-(2-((6-(1H-imidazol-1-yl)pyridin-2-yl)amino)vinyl)formamide)[2](1,4-bi-methylbenzene) hexafluorophosphate (i.e., 22+·2PF6−), as inferred from 1H NMR spectroscopic analysis (cf.Scheme 3 and ESI†). This particular imidazolium ring-opened product was isolated in an overall yield of 42%. Further support for the formation of 22+ as its PF6− salt came from mass spectrometric studies using ESI-MS. These analyses revealed a peak corresponding to [22+·PF6−]+ (m/z = 809.2657) in the gas phase.
Analysis of the residual material via1H NMR and DOSY spectroscopy led to the conclusion that there was a second minor product present in the original crude product. This minor product was identified as 32+·2PF6− on the basis of a single crystal X-ray diffraction analysis and ESI-MS studies wherein a peak corresponding to [32+·PF6−]+ (m/z = 809.2670) was seen. The overall yield of crude product (mixture of 22+·2PF6− and 32+·2PF6−) was 86–93%, with the ratio of major to minor isomer being roughly 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 based on 1H NMR spectral integrations. Unfortunately, it proved difficult to obtain significant quantities of pure 32+·2PF6−via recrystallization or high performance liquid chromatography (HPLC).28 This difficulty is ascribed to the fact that 32+·2PF6− has physical properties that are similar to those of the other regioisomer (i.e., 22+·2PF6−).
1 based on 1H NMR spectral integrations. Unfortunately, it proved difficult to obtain significant quantities of pure 32+·2PF6−via recrystallization or high performance liquid chromatography (HPLC).28 This difficulty is ascribed to the fact that 32+·2PF6− has physical properties that are similar to those of the other regioisomer (i.e., 22+·2PF6−).
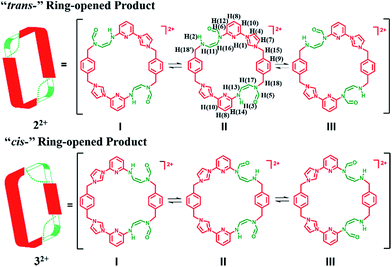
Initial characterization of 22+·2PF6− was carried out in DMSO-d6 using a variety of techniques, including 1H NMR, 13C NMR, heteronuclear singular quantum correlation (HSQC), 2D correlation (COSY), one-dimensional and two-dimensional nuclear Överhauser effect (1D NOE and NOESY) spectroscopies. The 1H NMR spectrum proved complex, leading us to suggest that 22+ is highly flexible in solution (as inferred from the observation of correlations between H(1), H(4) and H(10)) and exist in several different inter converting configurational forms. Further evidence of this proposed intramolecular conversion came from NOESY and 1D NOE spectral studies. Here, evidence of rapid exchange between the signals of H(5) and H(6), namely the proton signals on the formoxyl group, was observed. On this basis we propose that upon ring opening, macrocycle 22+ undergoes rapidly structural interconversion wherein the formoxyl group and the neighbouring amino moiety exchange, as shown in Scheme 4. Additional insights into this proposed intramolecular conversion came from temperature dependent 1H NMR and 13C NMR spectroscopic analyses carried out at 278 K and 373 K, HSQC analyses, as well as 1H,15N-heteronuclear multiple bond correlation (1H,15N-HMBC), COSY, and NOESY studies, all of which were carried out in DMF-d7 (cf. ESI†). In this solvent system the signal ascribed to H(1) on 22+ is split into four peaks at 278 K, a finding that is consistent with the intramolecular conversion shown in Scheme 4. Additional evidence in support of this conclusion came from a 13C NMR spectroscopic study carried out at 278 K, wherein more than 50 signals were observed. The 1H,15N-HMBC spectrum was also recorded. Here, 7 distinct 15N signals were seen, a finding that provides additional support for the proposed intramolecular conversion of 22+. Furthermore, the 1H and 13C signals ascribed to the differing isomers of 22+ become degenerate at 373 K (cf. ESI†). This is consistent with higher temperatures serving to accelerate the intramolecular conversions involving the various isomeric forms of 22+.
Support for the above conversion process involving an exchange between isomers, rather than an equilibrium between two or more chemically distinct products came from electrospray ionization mass spectrometry (ESI-MS), which revealed a signal with a m/z = 663.2934 ([(22+ − H)]+˙) without evidence of interfering species (cf. ESI†). A single crystal X-ray diffraction analysis of 22+·2PF6− was also carried out, as discussed below.
In the case of the minor product, 32+·2PF6−, it was not possible to isolate enough material to successfully conduct 1H NMR spectroscopic analysis. However using the crude material, and accounting for the signals for 22+·2PF6−, it was inferred that 32+ displays at least 13 signals in the 1H NMR spectrum recorded in DMSO-d6 at 300 K (cf. ESI†). This leads us to suggest that interconversion between the formoxyl group isomers also takes place within macrocycle 32+, as illustrated in Scheme 4.
As a control study, 1,3-dimethyl-1H-imidazolium (studied as its respective PF6− salt) was tested under the same reaction conditions used for the preparative-scale PSM of 14+·4PF6−. In this case, no evidence of imidazolium ring opened products was observed (cf. ESI†). This result, which is in accord with what was expected based on the literature,26 leads us to suggest that the macrocyclic structure of 14+ with its relatively enhanced anion binding properties serves to activate the constituent imidazolium moieties and increase their propensity to undergo hydrolysis (cf.Scheme 5).
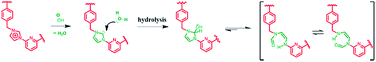 | ||
| Scheme 5 Schematic representation of the hydrolysis of the imidazole-2-ylidene moieties in 14+ that occurs in mild basic aqueous solution (pH = 11.6). | ||
Single crystals of 22+·2PF6− suitable for X-ray diffraction analysis were grown from two different solvent systems, namely (1) acetonitrile/dixoane (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and (2) a mixed solution of water/acetonitrile (1
1, v/v) and (2) a mixed solution of water/acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
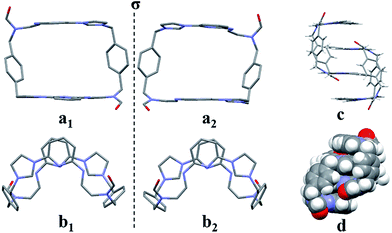
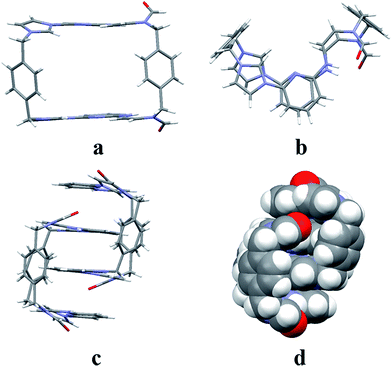
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) via slow evaporation. This yielded two independent sets of single crystals. Analysis of the single crystals obtained from the first crystallization method revealed a structure wherein macrocycle 22+ is arranged in a “box-like” conformation around molecules of dioxane (i.e., [22+·2PF6−·4dioxane]; cf.Fig. 1). The four bound dioxane cosolvent molecules appear to be stabilized by weak non-covalent O⋯π interactions, as inferred from the metric parameters (cf. ESI†). The width of the box was determined to be 5.814 Å with a length of 11.425 Å. The length of 22+ in this structure is larger than seen in the structure of free 14+ (7.699 or 10.136 Å in “complete chair” or “partial chair” conformation, respectively);22a,b however, the two macrocycles have approximately the same width (cf.Fig. 1 and the ESI†).
1, v/v) via slow evaporation. This yielded two independent sets of single crystals. Analysis of the single crystals obtained from the first crystallization method revealed a structure wherein macrocycle 22+ is arranged in a “box-like” conformation around molecules of dioxane (i.e., [22+·2PF6−·4dioxane]; cf.Fig. 1). The four bound dioxane cosolvent molecules appear to be stabilized by weak non-covalent O⋯π interactions, as inferred from the metric parameters (cf. ESI†). The width of the box was determined to be 5.814 Å with a length of 11.425 Å. The length of 22+ in this structure is larger than seen in the structure of free 14+ (7.699 or 10.136 Å in “complete chair” or “partial chair” conformation, respectively);22a,b however, the two macrocycles have approximately the same width (cf.Fig. 1 and the ESI†).
The second single crystal of macrocycle 22+ isolated and used for analysis contained a single acetonitrile cosolvent molecule per macrocycle, as well as two hexafluorophosphate counter anions (i.e., [22+·2PF6−·CH3CN·H2O]). In the resulting structure, macrocycle 22+ is seen to adopt a “clip-like” conformation around the acetonitrile cosolvent (cf.Fig. 2 and ESI†). It exists in the form of two enantiomeric conformations that are paired in the overall structure. As part of this pairing, two neighbouring molecules of 22+ are bound to each other via presumed π⋯π donor–acceptor interactions involving the stacked aromatic planes of ((Z)-N-(2-((6-(1H-imidazol-1-yl)pyridin-2-yl)amino)vinyl)formamide). The result is the formation of head-to-tail racemic dimer (i.e., 22), which contains a centre of symmetry (cf.Fig. 2). This second structure of 22+ was also found to differ significantly from that of 14+.22a Specifically, the width of the top of 22+ in its clip conformation is 7.485 Å, which is significantly smaller than what was observed in the case of 14+ in a structure wherein it also adopts a clip-like conformation (9.370 Å; cf. ESI†).
The two different conformations observed in the single crystal structures of 22+ were taken as evidence that this imidazolium ring-opened system is relatively flexible. The flexibility of 22+, which was originally inferred from the spectroscopic analyses discussed above, is reminiscent of Alcalde's dicationic imidazolium-based cyclophanes.29 In this structure, the carbonyl groups are located on the nitrogen atoms of the two ring-opened segments, which are linked via methylene units.
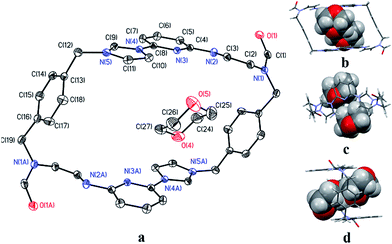
Even though it proved challenging to isolate preparative quantities of 32+·2PF6−, single crystals of [32+·2PF6−·CH3CN] suitable for X-ray diffraction analysis could be isolated after the bulk of the 22+·2PF6− product was removed by recrystallization. The single crystal used for structural analysis was obtained by running the ring opening reaction under the preparative conditions described above, subjecting the resulting crude product to recrystallization from dioxane/acetonitrile, collecting the solid material that consisted predominantly of 22+·2PF6−, removing the solvent, redissolving the remaining oil in a mixture of acetonitrile/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), and then subjecting the resulting solution to slow evaporation. X-ray diffraction structural analysis revealed that 32+ was the cis-isomer of 22+.
1, v/v), and then subjecting the resulting solution to slow evaporation. X-ray diffraction structural analysis revealed that 32+ was the cis-isomer of 22+.
In analogy to what was seen for [22+·2PF6−·CH3CN·H2O], it was found that in the solid state the dicationic macrocycle, 32+, adopts a “clip” conformation containing a plane of symmetry. As was true for the acetonitrile adduct of 22+, a head-to-tail dimer arrangement was seen (i.e.32). This dimer is presumably stabilized by multiple π⋯π donor–acceptor interactions as reflected in the short distances (≤3.5 Å) between the neighbouring planes (cf.Fig. 3 and ESI†).
The above studies provide support for the suggestion that the imidazolium ring-opening PSM reaction that serves to convert macrocycle 14+ into 22+ also serves to alter the structure of the host. It was expected that this would translate into a difference in molecular recognition properties. To test this hypothesis, we chose for study anionic guests that had been previously investigated with 14+, namely 2,6-naphthalenedicarboxylate (4), 2,6-naphthalenedisulfonate (5), and 1,5-naphthalenedisulfonate (6).22b,c As was true for this earlier work, the anions were studied in the form of their tetramethylammonium (TMA+) salts and all titrations were carried out in DMSO-d6.
Initially, NMR spectroscopic analyses were used to investigate the host–guest interactions between 22+ and 4. Upon titration of 22+ with 4, distinct shifts in the 1H NMR spectral features associated with both the host and the guest species were observed. The greatest changes were seen for the imidazole C–H and N–H resonances. Job plots were constructed and revealed a maximum value of 0.5 (for the ratio [H]/([H] + [G])), as would be expected for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (H
1 (H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G) binding stoichiometry. Fitting the 1H NMR spectral titration data to a 1
G) binding stoichiometry. Fitting the 1H NMR spectral titration data to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding profile, allowed association constants of Ka = (2.0 ± 0.1) × 102 M−1 to be calculated for the formation of a formally neutral host–guest complex (i.e., [22+·4]).
1 binding profile, allowed association constants of Ka = (2.0 ± 0.1) × 102 M−1 to be calculated for the formation of a formally neutral host–guest complex (i.e., [22+·4]).
To understand better the nature of this presumed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex, 1D-NOE and NOESY NMR spectroscopic studies were carried out. These analyses revealed no correlations between the protons on 22+ and those on 4. This finding is consistent with guest anion 4 being bound outside the central cavity of macrocycle 22+ (cf.Scheme 6). Further support for this 1
1 complex, 1D-NOE and NOESY NMR spectroscopic studies were carried out. These analyses revealed no correlations between the protons on 22+ and those on 4. This finding is consistent with guest anion 4 being bound outside the central cavity of macrocycle 22+ (cf.Scheme 6). Further support for this 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode came from ESI-MS studies, wherein a peak corresponding to [22+ + 4 + H]+˙ (m/z = 879.3366) was observed (cf. ESI†). The proposed outside binding mode in [22+·4] is distinctly different from the interpenetrated structure observed when 14+ is exposed to 4.22b This difference highlights the effect that the present PSM can have on the host–guest recognition properties of ostensibly related cationic receptor systems.
1 binding mode came from ESI-MS studies, wherein a peak corresponding to [22+ + 4 + H]+˙ (m/z = 879.3366) was observed (cf. ESI†). The proposed outside binding mode in [22+·4] is distinctly different from the interpenetrated structure observed when 14+ is exposed to 4.22b This difference highlights the effect that the present PSM can have on the host–guest recognition properties of ostensibly related cationic receptor systems.
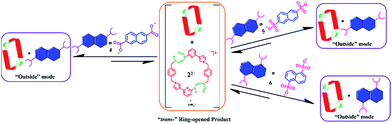 | ||
| Scheme 6 Schematic representation of the interactions between 22+ and guest species 4, 5, and 6, as inferred from 1H NMR spectroscopic analyses carried out in DMSO-d6. | ||
The molecular recognition features of 22+ were explored further using 5 as a guest (cf.Scheme 6). As in the case of 4, direct titration of 22+ with 5 resulted in chemical shifts in the 1H NMR spectral features associated with both the host and the guest. As was seen with 4, a Job-plot analysis of the 1H NMR spectral data revealed a maximum at ca. 0.5 ([H]/([H] + [G])). Such a finding is consistent with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (H
1 (H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G) binding stoichiometry. More detailed analysis of the 1H NMR spectral data, led to the conclusion that a 1
G) binding stoichiometry. More detailed analysis of the 1H NMR spectral data, led to the conclusion that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ([22+·5]) complex was being formed during the course of the titration. A binding constant of Ka = (1.7 ± 0.2) × 102 M−1 was calculated for the formation of [22+·5]. Separate NOESY and 1D NOE spectroscopic analyses provided support for the conclusion that in this 1
1 ([22+·5]) complex was being formed during the course of the titration. A binding constant of Ka = (1.7 ± 0.2) × 102 M−1 was calculated for the formation of [22+·5]. Separate NOESY and 1D NOE spectroscopic analyses provided support for the conclusion that in this 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex guest molecule 5 is located outside of the macrocyclic core (cf. ESI†). ESI-MS analyses revealed inter alia peaks corresponding to [22+ + 5 − 6H]+˙ (m/z = 944.2182) in the gas phase. Again, this binding behaviour was very different from that seen when 14+ was used as a receptor. In this case 1
1 complex guest molecule 5 is located outside of the macrocyclic core (cf. ESI†). ESI-MS analyses revealed inter alia peaks corresponding to [22+ + 5 − 6H]+˙ (m/z = 944.2182) in the gas phase. Again, this binding behaviour was very different from that seen when 14+ was used as a receptor. In this case 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pseudorotaxane complexes are seen with 5 in DMSO-d6.22c
1 pseudorotaxane complexes are seen with 5 in DMSO-d6.22c
The interactions between 22+ and 6 were also investigated. Studies analogous to those performed in the case of 4 and 5 revealed the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (H
1 (H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G) complex (cf. ESI†). Again, 1D-NOE and NOESY analyses revealed no correlation between protons on host 22+ and guest 6, leading us to conclude that guest binding takes place outside of the central cavity of 22+ (cf.Scheme 6). In this case, and in contrast to what is observed for 4 and 5 there is direct analogy to the binding behavior seen with 14+, which also interacts with 6via an outside binding mode. However, as might be expected given the charge disparity, complex [14+·6]2+ displayed greater thermodynamic stability than [22+·6] as inferred from the associated Ka values ((1.0 ± 0.1) × 103 M −1 for [14+·6]2+ as noted previously,22c and (4.0 ± 0.5) × 102 M−1 for [22+·6]; cf. ESI†). A diffusion ordered spectroscopic (DOSY) study (DMSO-d6) revealed that all of the proton signals located on 22+ and 6 have similar diffusion rates in the representative solution state mixtures (cf. ESI†). Evidence for the formation of the 1
G) complex (cf. ESI†). Again, 1D-NOE and NOESY analyses revealed no correlation between protons on host 22+ and guest 6, leading us to conclude that guest binding takes place outside of the central cavity of 22+ (cf.Scheme 6). In this case, and in contrast to what is observed for 4 and 5 there is direct analogy to the binding behavior seen with 14+, which also interacts with 6via an outside binding mode. However, as might be expected given the charge disparity, complex [14+·6]2+ displayed greater thermodynamic stability than [22+·6] as inferred from the associated Ka values ((1.0 ± 0.1) × 103 M −1 for [14+·6]2+ as noted previously,22c and (4.0 ± 0.5) × 102 M−1 for [22+·6]; cf. ESI†). A diffusion ordered spectroscopic (DOSY) study (DMSO-d6) revealed that all of the proton signals located on 22+ and 6 have similar diffusion rates in the representative solution state mixtures (cf. ESI†). Evidence for the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 species [22+·6] was again found in the gas phase via ESI-MS analysis. Specifically, a peak corresponding to [22+ + 6 + Na]+˙ (m/z = 973.2519) was observed (Table 1).
1 species [22+·6] was again found in the gas phase via ESI-MS analysis. Specifically, a peak corresponding to [22+ + 6 + Na]+˙ (m/z = 973.2519) was observed (Table 1).
| Guest | 1 4+ | 2 2+ | ||||
|---|---|---|---|---|---|---|
| Binding mode | Stoichiometric ratio/(H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G) G) |
Association constants (Ka)/M−1 | Binding mode | Stoichiometric ratio/(H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G) G) |
Association constants (Ka)/M−1 | |
| 2,6-Naphthalene dicarboxylate (4) | Pseudo rotaxane | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
(3.5 ± 0.2) × 104 | “Outside” | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
(2.0 ± 0.1) × 102 |
| 2,6-Naphthalene disulfonate (5) | Pseudo rotaxane | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
(1.6 ± 0.1) × 103 | “Outside” | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
(1.7 ± 0.2) × 102 |
| 1,5-Naphthalene disulfonate (6) | “Outside” | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
(1.0 ± 0.1) × 103 | “Outside” | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
(4.0 ± 0.5) × 102 |
Based on the above studies, we conclude that the recognition features of 14+ and its PSM derivative 22+ differ in (1) the basic binding mode and (2) the binding affinities, with those of macrocycle 22+ being weaker. We postulate that these differences result directly from the structural changes induced via imidazolium ring-opening, which results in a larger cavity, lower net positive charge, and weaker hydrogen bonding interactions in the case of 22+ as compared to 14+.
To provide further support for these hypotheses, we set out to isolate single crystals of the molecular complexes produced from 22+ and the test anions. Single crystals suitable for X-ray diffraction analysis were obtained in the case of [22+·5·5H2O] and [22+·6·2.5H2O]. The crystals were obtained via slow diffusion using a three-layer solution setup. Specifically, 1.0 molar equiv. of 5 or 6, and 2.0 molar equiv. of TMA+·OH− (based on 22+·2PF6−) were dissolved in water and placed in a small tube. A mixture of DMF and water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was added as the second layer, and 22+·2PF6− (1.0 molar equiv.) dissolved in CH3CN, DMF, and water (1
1, v/v) was added as the second layer, and 22+·2PF6− (1.0 molar equiv.) dissolved in CH3CN, DMF, and water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v/v) was then added as the third layer.
1, v/v/v) was then added as the third layer.
Structural analysis in the case of [22+·5·5H2O] (cf.Fig. 4), revealed that dianion 5 binds to the outside of macrocycle 22+ in the solid state, as was inferred from the solution NMR spectroscopic analyses discussed above. Host 22+ and guest 5 are bound primarily via intermolecular C–H⋯O hydrogen bonds characterized by relatively short C–O separations (≤3.63 Å). In the solid state, the complex exists as a dimeric species 5·(22+)2·5 stabilized via presumed π⋯π donor–acceptor interactions (cf.Fig. 4 and the ESI†). This solid state binding behaviour is distinctly different from what is seen in the case of 14+ and 5 which aggregate to form pseudorotaxane units (i.e., [14+·52·7.5H2O]), as noted in previous studies.22c
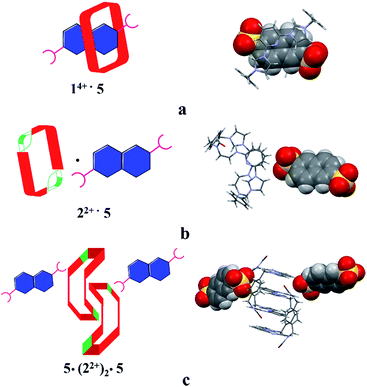 | ||
| Fig. 4 Schematic views and single crystal X-ray structures of (a) [14+·52·7.5H2O],22c (b) [22+·5·5H2O], and (c) 5·(22+)2·5. Most counter ions and solvent molecules have been omitted for clarity. | ||
The single-crystal X-ray structure of [22+·6·2.5H2O] (cf.Fig. 5) also served to confirm the inference drawn from the solution phase analyses (vide supra), namely that the dianionic guest species is bound to the periphery of macrocycle 22+. In this case the binding mode is similar to what was observed previously in the case of 14+, where outside binding is also seen (i.e., [14+·6]2+ in [14+·62·6H2O] or [14+·62·14H2O]) (cf.Fig. 5). However, different conformations are seen for the cationic receptors. In the case of [22+·6·2.5H2O], the macrocycle adopts a “clip” conformation where as a “chair” mode was seen in the case of [14+·6]2+ in [14+·62·6H2O] or [14+·62·14H2O] (cf.Fig. 5). The primary stabilizing interactions between 22+ and 6 are intermolecular C–H⋯O hydrogen bonds, as inferred from the short C–O distances (≤3.60 Å). Also present are π⋯π donor–acceptor interactions characterized by the short separation (≤3.8 Å) between the naphthalene ring on 6 and the neighbor benzene plane on 22+ (cf. ESI†). An overall head-to-tail racemic dimer (i.e.22) with a center of symmetry is seen in the solid state structure of [22+·6·2.5H2O] (cf.Fig. 5 and ESI† for further details). This mirrors what was seen for [22+·5·5H2O] as noted above.
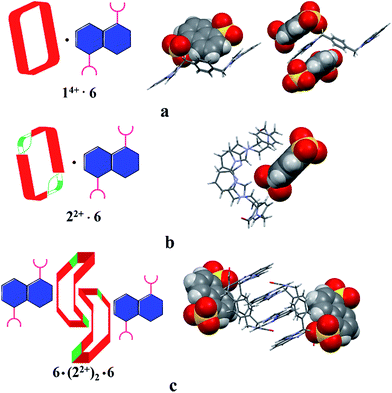 | ||
| Fig. 5 Schematic views and single crystal X-ray structure of (a) [14+·62·6H2O], [14+·62·14H2O],22c (b) [22+·6·2.5H2O] and (c) 6·(22+)2·6. Some of the counter ions and solvent molecules have been omitted for clarity. | ||
To test whether the present OH− anion-promoted ring-opening of 14+ would complement the previously observed acid-induced dethreading seen for pseudorotaxanes built up from this core, both [14+·4] and [14+·5] (obtained from a mixture of 14+·4PF6− and 1 molar equiv. of either 4 or 5in situ) were subject to treatment with OH−. The correlation between protons on the macrocycle as well as the guest species were monitored via 1D-NOE while irradiating the protons on anionic guest 4 or 5. It was found that over the course of the ring-opening process the correlated signals disappear. This was taken as evidence that the pseudorotaxne structures, i.e., [14+·4] or [14+·5], underwent disassembly under the aforementioned reaction conditions. Further proof of the proposed dethreading of anion 5 came from a single-crystal diffraction study involving [22+·5·5H2O] (cf.Fig. 4b). The single crystals used for this analysis were obtained from the contents of an NMR tube that was left standing for 12 h after the initial reaction between [14+·5] and OH−. Identical crystals were obtained when 22+ was mixed with 5 and subject to slow diffusion crystallization as detailed above.
As noted above, we previously reported that treatment of [14+·4] with a proton source leads to formation of 14+ and 2H+·4. Taken in conjunction with the previous findings, the present results serve to highlight that the fact that pseudorotaxane [14+·4] is only stable as a mechanically interlocked species over a limited effective pH range; thus mimicking many biological systems, which only maintain their unique structures and bioactivity at certain well-defined pH values.
Conclusions
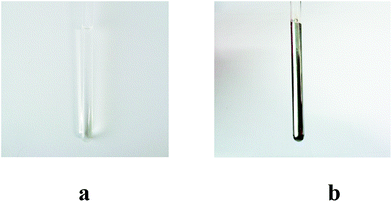
In summary, a new post-synthetic modification strategy is described that may provide a facile means for modulating the properties of cationic macrocycles. This strategy has been applied to the tetraimidazolium Texas-sized box, 14+ to give two regioisomeric imidazolim ring-opened products, 22+·2PF6− and 32+·2PF6−. The trans isomer (i.e., 22+·2PF6−) is the dominant product and could be obtained on a preparative scale. It displayed outside binding or only weak ion pairing when tested with the dianionic substrates 4 and 5. Its recognition behaviour thus proved to be distinct from its macrocyclic precursor (14+), a tetracationic systems that supports the formation of interpenetrated pseudorotaxane complexes with these same anionic guests. Moreover, the OH− ring-opening process can be used to irreversibly disassemble the interpenetrated structures [14+·4] and [14+·5].The unique nature of the PSM reaction reported here, which includes its relatively high yield, the mild reaction conditions it requires, and an ability to obtain differing regioisomers leads us to suggest that this simple yet versatile approach could prove to be of general utility and readily applied to other imidazolium-based receptor systems. More broadly, new methods that allow for the post-synthetic modification of known receptor systems are likely to allow for the exploration of new recognition and reactivity chemistry (e.g., the reaction of the –NH and/or –CHO), just as it has in the parallel area of MOFs. Indeed, in preliminary work, we have found that treatment of 22+·2PF6 with AgNO3 leads to formation of a sliver mirror (cf.Fig. 6).30
Acknowledgements
H.-Y. G. thanks the National Natural Science Foundation of China (21472014), National Basic Research Program of China (973 Program 2015CB856502), the Young One-Thousand-Talents Scheme, the Fundamental Research Funds for the Central Universities, the Beijing Municipal Commission of Education, and the Beijing Normal University for financial support. J. L. S. thanks the National Science Foundation (grant no. CHE-1402004) and the Robert A. Welch Foundation (grant F-1018) for support.Notes and references
- (a) J.-M. Lehn, Supramolecular Chemistry, Wiley-VCH, Weiheim, 1995 Search PubMed; (b) J. M. Abendroth, O. S. Bushuyev, P. S. Weiss and C. J. Barrett, ACS Nano, 2015, 9, 7746 CrossRef CAS PubMed; (c) X. Ma and Y. Zhao, Chem. Rev., 2015, 115, 7794 CrossRef CAS PubMed; (d) M. Xue, Y. Yang, X. Chi, X. Yan and F. Huang, Chem. Rev., 2015, 115, 7398 CrossRef CAS PubMed; (e) G. Yu, K. Jie and F. Huang, Chem. Rev., 2015, 115, 7240 CrossRef CAS PubMed; (f) L. Yang, X. Tan, Z. Wang and X. Zhang, Chem. Rev., 2015, 115, 7196 CrossRef CAS PubMed; (g) S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan and A. L. Nussbaumer, Chem. Rev., 2015, 115, 10081 CrossRef CAS PubMed; (h) C. Cheng, P. R. McGonigal, J. F. Stoddart and R. D. Astumian, ACS Nano, 2015, 9, 8672 CrossRef CAS PubMed; (i) W. Xue, Z. Li, G. Liu, X. Chen, T. Li, S. H. Liu and J. Yin, Org. Biomol. Chem., 2014, 12, 4862 RSC; (j) N. H. Evans and P. D. Beer, Chem. Soc. Rev., 2014, 43, 4658 RSC; (k) Z. T. Li, Beilstein J. Org. Chem., 2015, 11, 2057 CrossRef PubMed; (l) N. C. Frank, D. J. Mercer and S. J. Loeb, Chem.–Eur. J., 2013, 19, 14076 CrossRef CAS PubMed; (m) J. F. Stoddart, Angew. Chem., Int. Ed., 2014, 53, 11102 CrossRef CAS PubMed; (n) W. Wang, L. J. Chen, X. Q. Wang, B. Sun, X. Li, Y. Zhang, J. Shi, Y. Yu, L. Zhang, M. Liu and H. B. Yang, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 5597 CrossRef CAS PubMed; (o) B. Sun, M. Wang, Z. Lou, M. Huang, C. Xu, X. Li, L. J. Chen, Y. Yu, G. L. Davis, B. Xu, H. B. Yang and X. Li, J. Am. Chem. Soc., 2015, 137, 1556 CrossRef CAS PubMed; (p) M. Wang, C. Wang, X. Q. Hao, X. Li, T. J. Vaughn, Y. Y. Zhang, Y. Yu, Z. Y. Li, M. P. Song, H. B. Yang and X. Li, J. Am. Chem. Soc., 2014, 136, 10499 CrossRef CAS PubMed.
- (a) D. H. Qu, Q. C. Wang, Q. W. Zhang, X. Ma and H. Tian, Chem. Rev., 2015, 115, 7543 CrossRef CAS PubMed; (b) X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042 RSC; (c) S. L. Cai, W. G. Zhang, R. N. Zuckermann, Z. T. Li, X. Zhao and Yi. Liu, Adv. Mater., 2015, 27, 5762 CrossRef CAS PubMed; (d) W. Si, P. Xin, Z. T. Li and J. L. Hou, Acc. Chem. Res., 2015, 48, 1612 CrossRef CAS PubMed; (e) D. H. Qu and H. Tian, Chem. Sci., 2011, 2, 1011 RSC; (f) H. Tian and Q. C. Wang, Chem. Soc. Rev., 2006, 35, 361 RSC.
- (a) W. Zhou, J. Li, X. He, C. Li, J. Lv, Y. Li, S. Wang, H. Liu and D. Zhu, Chem.–Eur. J., 2008, 14, 754 CrossRef CAS PubMed; (b) J. Beswick, V. Blanco, G. De Bo, D. A. Leigh, U. Lewandowska, B. Lewandowski and K. Mishiro, Chem. Sci., 2015, 6, 140 RSC; (c) V. Blanco, A. Carlone, K. D. Hänni, D. A. Leigh and B. Lewandowski, Angew. Chem., Int. Ed., 2012, 51, 5166 CrossRef CAS PubMed; (d) P. R. McGonigal, P. Deria, I. Hod, P. Z. Moghadam, A. J. Avestro, N. E. Horwitz, I. C. Gibbs-Hall, A. K. Blackburn, D. Chen, Y. Y. Botros, M. R. Wasielewski, R. Q. Snurr, J. T. Hupp, O. K. Farha and J. F. Stoddart, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 11161 CrossRef CAS PubMed.
- (a) K. Zhu, V. N. Vukotic, N. Noujeim and S. J. Loeb, Chem. Sci., 2012, 3, 3265 RSC; (b) K. Zhu, C. A. O'Keefe, V. N. Vukotic, R. W. Schurko and S. J. Loeb, Nat. Chem., 2015, 7, 514 CrossRef CAS PubMed; (c) N. Dozova, R. Kumar, T. Pradhan, F. Lacombat, B. Valeur, J. S. Kim and P. Plaza, Chem. Commun., 2015, 51, 14859 RSC.
- (a) B. Andrade, Z.-Y. Song, J. Li, S.-C. Zimmerman, J.-J. Cheng, J. S. Moore, K. Harris and J. S. Katz, ACS Appl. Mater. Interfaces, 2015, 7, 6359 CrossRef CAS PubMed; (b) J. J. Gassensmith, H. Furukawa, R. A. Smaldone, R. S. Forgan, Y. Y. Botros, O. M. Yaghi and J. F. Stoddart, J. Am. Chem. Soc., 2011, 133, 15312 CrossRef CAS PubMed; (c) J. J. Gassensmith, J. Y. Kim, J. M. Holcroft, O. K. Farha, J. F. Stoddart, J. T. Hupp and N. C. Jeong, J. Am. Chem. Soc., 2014, 136, 8277 CrossRef CAS PubMed; (d) H.-L. Sun, Y. Chen, J. Zhao and Y. Liu, Angew. Chem., Int. Ed., 2015, 54, 9376 CrossRef CAS PubMed.
- (a) Y.-X. Wang, Y.-M. Zhang and Y. Liu, J. Am. Chem. Soc., 2015, 137, 4543 CrossRef CAS PubMed; (b) D.-S. Guo and Y. Liu, Acc. Chem. Res., 2014, 47, 1925 CrossRef CAS PubMed; (c) D.-S. Guo and Y. Liu, Chem. Soc. Rev., 2012, 41, 5907 RSC.
- (a) P. A. Gale, P. Anzenbacher Jr and J. L. Sessler, Coord. Chem. Rev., 2001, 222, 57 CrossRef CAS; (b) J. L. Sessler, S. Camiolo and P. A. Gale, Coord. Chem. Rev., 2003, 240, 17 CrossRef CAS; (c) P. Anzenbacher Jr, R. Nishiyabu and M. A. Palacios, Coord. Chem. Rev., 2006, 250, 2929 CrossRef; (d) G. Cafeo, F. H. Kohnke, G. Mezzatesta, A. Profumo, C. Rosano, A. Villari and A. J. P. White, Chem.–Eur. J., 2015, 21, 5323 CrossRef CAS PubMed; (e) M.-X. Wang, Chem. Commun., 2008, 4541 RSC; (f) L. Wang, D. Wang and M.-X. Wang, Chin. J. Chem., 2013, 32, 1033 CrossRef.
- (a) K. Jie, Y. J. Zhou, Y. Yao, B.-B. Shi and F. Huang, J. Am. Chem. Soc., 2015, 137, 10472 CAS; (b) G. Yu, M. Xue, Z. Zhang, J. Li, C. Han and F. Huang, J. Am. Chem. Soc., 2012, 134, 13248 CrossRef CAS PubMed; (c) C.-F. Zhang, S.-H. Li, C.-C. Zhang and Y. Liu, Org. Biomol. Chem., 2015, 13, 10808 RSC; (d) J. Han, X. Hou, C. Ke, H. Zhang, N. L. Strutt, C. L. Stern and J. F. Stoddart, Org. Lett., 2015, 17, 3260 CrossRef CAS PubMed.
- (a) F. Diederich and P. J. Stang, Templated Organic Synthesis, Wiley-VCH, Weiheim, 2000 Search PubMed; (b) P. E. Barran, H. L. Cole, S. M. Goldup, D. A. Leigh, P. R. McGonigal, M. D. Symes, J. Wu and M. Zengerle, Angew. Chem., Int. Ed., 2011, 50, 12280 CrossRef CAS PubMed; (c) T. Chen, G. B. Pan, H. Wettach, M. Fritzsche, S. Hoger, L. J. Wan, H. B. Yang, B. H. Northrop and P. J. Stang, J. Am. Chem. Soc., 2010, 132, 1328 CrossRef CAS PubMed; (d) S. Dasgupta and J. Wu, Org. Biomol. Chem., 2011, 9, 3504 RSC; (e) S. D. Han, W. C. Song, J. P. Zhao, Q. Yang, S. J. Liu, Y. Li and X. H. Bu, Chem. Commun., 2013, 49, 871 RSC; (f) E. A. Kataev, G. V. Kolesnikov, R. Arnold, H. V. Lavrov and V. N. Khrustalev, Chem.–Eur. J., 2013, 19, 3710 CrossRef CAS PubMed; (g) Y. Li, C. Liu, Y. Xie, X. Li, X. Fan, L. Yuan and Q. Zeng, Chem. Commun., 2013, 49, 9021 RSC; (h) W. Uhl, C. Stefaniak, M. Voß, J. Kösters and F. Rogel, Organometallics, 2012, 31, 6988 CrossRef CAS; (i) Y. W. Wu, P. N. Chen, C. F. Chang, C. C. Lai and S. H. Chiu, Org. Lett., 2015, 17, 2158 CrossRef CAS PubMed; (j) S. Chao, C. Romuald, K. Fournel-Marotte, C. Clavel and F. Coutrot, Angew. Chem., Int. Ed., 2014, 53, 6914 CrossRef CAS PubMed; (k) C. Clavel, C. Romuald, E. Brabet and F. Coutrot, Chem.–Eur. J., 2013, 19, 2982 CrossRef CAS PubMed; (l) N. H. Evans and P. D. Beer, Angew. Chem., Int. Ed., 2014, 53, 11716 CrossRef CAS PubMed; (m) N. H. Evans and P. D. Beer, Chem. Soc. Rev., 2014, 43, 4658 RSC; (n) Y. Geng, M. Liu, J. Xue, P. Xu, Y. Wang, L. Shu, Q. Zeng and C. Wang, Chem. Commun., 2015, 51, 6820 RSC; (o) T. H. Ho, C. C. Lai, Y. H. Liu, S. M. Peng and S. H. Chiu, Chem.–Eur. J., 2014, 20, 4563 CrossRef CAS PubMed; (p) Y. H. Lin, C. C. Lai and S. H. Chiu, Org. Biomol. Chem., 2014, 12, 2907 RSC; (q) A. Noor, W. K. Lo, S. C. Moratti and J. D. Crowley, Chem. Commun., 2014, 50, 7044 RSC; (r) B. Qiao, A. Sengupta, Y. Liu, K. P. McDonald, M. Pink, J. R. Anderson, K. Raghavachari and A. H. Flood, J. Am. Chem. Soc., 2015, 137, 9746 CrossRef CAS PubMed; (s) Y. Hua, Y. Liu, C. H. Chen and A. H. Flood, J. Am. Chem. Soc., 2013, 135, 14401 CrossRef CAS PubMed; (t) S. K. Kim and J. L. Sessler, Chem. Soc. Rev., 2010, 39, 3784 RSC; (u) S. K. Kim, V. M. Lynch, B. P. Hay, J. S. Kim and J. L. Sessler, Chem. Sci., 2015, 6, 1404 RSC; (v) E. F. V. Dry, J. K. Clegg, B. Breiner, D. E. Whitaker, R. Stefaky and J. R. Nitschke, Chem. Commun., 2011, 47, 6021 RSC.
- (a) B. C. Gibb, Chem.–Eur. J., 2003, 9, 5180 CrossRef PubMed; (b) X. Li, C. L. D. Gibb, T. Upton and B. C. Gibb, J. Am. Chem. Soc., 2003, 125, 650 CrossRef CAS PubMed; (c) P. Anzenbacher Jr, K. Jursíková, J. A. Shriver, H. Miyaji, V. M. Lynch, J. L. Sessler and P. A. Gale, J. Org. Chem., 2000, 65, 7641 CrossRef CAS; (d) J. Cho, T. Pradhan, Y. M. Lee, J. S. Kim and S. Kim, Dalton Trans., 2014, 43, 16178 RSC; (e) R. Kumar, Y. O. Lee, V. Bhalla, M. Kumar and J. S. Kim, Chem. Soc. Rev., 2014, 43, 4824 RSC.
- (a) G. Cafeo, F. H. Kohnke, G. L. La Torre, A. J. P. White and D. J. Williams, Angew. Chem., Int. Ed., 2000, 39, 1496 CrossRef CAS; (b) V. Král, P. A. Gale, P. Anzenbacher Jr, K. Jursíková, V. M. Lynch and J. L. Sessler, Chem. Comm, 1998, 9 RSC; (c) Q. H. Guo, L. Zhao and M. X. Wang, Angew. Chem., Int. Ed., 2015, 54, 8386 CrossRef CAS PubMed; (d) H. Zhang, B. Yao, L. Zhao, D. X. Wang, B. Q. Xu and M. X. Wang, J. Am. Chem. Soc., 2014, 136, 6326 CrossRef CAS PubMed; (e) D. X. Wang and M. X. Wang, J. Am. Chem. Soc., 2013, 135, 892 CrossRef CAS PubMed; (f) M. X. Wang, Acc. Chem. Res., 2012, 45, 182 CrossRef CAS PubMed.
- M. W. Hosseini, J.-M. Lehn, K. C. Jones, K. E. Plute, K. B. Mertes and M. P. Mertes, J. Am. Chem. Soc., 1989, 111, 6330 CrossRef CAS.
- (a) D. B. Amabilino and J. F. Stoddart, Chem. Rev., 1995, 95, 2725 CrossRef CAS, and references therein; (b) F. M. Raymo and J. F. Stoddart, Chem. Rev., 1999, 99, 1643 CrossRef CAS PubMed, and references therein; (c) J. F. Stoddart, Chem. Soc. Rev., 2009, 38, 1802 RSC.
- E. Alcalde, I. Dinares and N. Mesquida, Top. Heterocycl. Chem., 2010, 24, 267 CAS.
- C. E. Willans, K. M. Anderson, L. C. Potts and J. W. Steed, Org. Biomol. Chem., 2009, 7, 2756 CAS.
- L.-S. Jiang and D.-K. Yang, Acta Chim. Sin., 2012, 12, 1385 Search PubMed.
- (a) J. Yoon, Z. Xu, N. J. Singh, K. Sook, D. R. Spring and K. S. Kim, Chem.–Eur. J., 2011, 17, 1163 CrossRef PubMed; (b) J. Yoon, Z. Xu and S. K. Kim, Chem. Soc. Rev., 2010, 39, 1457 RSC; (c) J. Yoon, J. K. Kim, N. J. Singh and K. S. Kim, Chem. Soc. Rev., 2006, 35, 355 RSC.
- C. J. Serpell, J. Cookson, A. L. Thompsona and P. D. Beer, Chem. Sci., 2011, 2, 494 RSC.
- (a) J. Cai, B. P. Hay, N. J. Young, X. Yang and J. L. Sessler, Chem. Sci., 2013, 4, 1560 RSC; (b) Y. Li and A. H. Flood, Angew. Chem., Int. Ed., 2008, 47, 2649 CrossRef CAS PubMed; (c) K. P. McDonald, Y. Hua, S. Lee and A. H. Flood, Chem. Commun., 2012, 48, 5065 RSC; (d) R. O. Ramabhadran, Y. Liu, Y. Hua, M. Ciardi, A. H. Flood and K. Raghavachari, J. Am. Chem. Soc., 2014, 136, 5078 CrossRef CAS PubMed.
- N. G. White, S. Carvalho, V. Félixb and P. D. Beer, Org. Biomol. Chem., 2012, 10, 6951 CAS.
- (a) Z. Wang and S. M. Cohen, Chem. Soc. Rev., 2009, 38, 1315 RSC; (b) K. K. Tanabe and S. M. Cohen, Chem. Soc. Rev., 2011, 40, 498 RSC; (c) T. R. Cook and P. J. Stang, Chem. Rev., 2015, 115, 7001 CrossRef CAS PubMed; (d) A. J. McConnell, C. S. Wood, P. P. Neelakandan and J. R. Nitschke, Chem. Rev., 2015, 115, 7729 CrossRef CAS PubMed; (e) D. Schultz and J. R. Nitschke, J. Am. Chem. Soc., 2006, 128, 9887 CrossRef CAS PubMed; (f) A. D. Burrows and S. M. Cohen, CrystEngComm, 2012, 14, 4095 RSC.
- (a) H. Y. Gong, B. M. Rambo, E. Karnas, V. M. Lynch and J. L. Sessler, Nat. Chem., 2010, 2, 406 CrossRef CAS PubMed; (b) H. Y. Gong, B. M. Rambo, E. Karnas, V. M. Lynch, K. M. Keller and J. L. Sessler, J. Am. Chem. Soc., 2011, 133, 1526 CrossRef CAS PubMed; (c) H.-Y. Gong, F. Tang, B. M. Rambo, R. Cao, J.-F. Xiang and J. L. Sessler, Chem. Commun., 2015, 51, 1795 RSC.
- M. Tomasz, R. Lipman, G. L. Verdine and K. Nakanishi, J. Am. Chem. Soc., 1985, 107, 6120 CrossRef CAS.
- S. Gopalakrishnan, M. P. Stone and T. M. Harris, J. Am. Chem. Soc., 1989, 111, 7232 CrossRef CAS.
- K. L. Brown, J. Z. Deng, R. S. Iyer, L. G. Iyer, M. W. Voehler, M. P. Stone, C. M. Harris and T. M. Harris, J. Am. Chem. Soc., 2006, 128, 15188 CrossRef CAS PubMed.
- O. Hollóczki, P. Terleczky, D. Szieberth, G. Mourgas, D. Gudat and L. Nyulászi, J. Am. Chem. Soc., 2011, 133, 780 CrossRef PubMed.
- The reaction was also run for 48 at a pH value between 11.4 and 11.6 at 313 K. However, analysis of the data leads us to conclude that such long reaction times are not needed.
- HPLC studies were carried out in an effort to separate 22+·2PF6− and 32+·2PF6− without success. An octadecylsilyl (ODS) C18 analytical column (4.6 × 150 mm; 4.6 μm) was employed, along with eluents containing varying acetonitrile/water mixtures.
- C. Caltagirone and P. A. Gale, Chem. Soc. Rev., 2009, 38, 520 RSC , and references therein.
- Evidence of a reaction between 22+·2PF6− (8.7 mM L−1) and 2 molar equiv. of 9-(chloromethyl)anthracene in the presence of 2 molecules of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in DMSO-d6 has also been obtained as inferred from 1H NMR spectroscopic analyses. These results will be presented in greater detail in due course.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1433722–1433726. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc04860e |
| This journal is © The Royal Society of Chemistry 2016 |