Open Access Article
Open Access ArticleDifferential effects of zinc binding on structured and disordered regions in the multidomain STIL protein†
Hadar
Amartely
a,
Ahuvit
David
bc,
Mai
Shamir
a,
Mario
Lebendiker
d,
Shai
Izraeli
bc and
Assaf
Friedler
*a
aInstitute of Chemistry, Hebrew University of Jerusalem, Safra Campus, Givat Ram, Jerusalem 91904, Israel
bSheba Cancer Research Center and the Edmond and Lily Safra Children Hospital, Sheba Medical Center, Tel-Hashomer 52621, Israel
cDepartment of Molecular Genetics and Biochemistry, Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
dThe Wolfson Centre for Applied Structural Biology, Hebrew University of Jerusalem, Safra Campus, Givat Ram, Jerusalem 91904, Israel
First published on 4th March 2016
Abstract
Binding of metal ions is an important regulatory mechanism in proteins. Specifically, Zn2+ binding to disordered regions commonly induces a disorder to order transition and gain of structure or oligomerization. Here we show that simultaneous binding of Zn2+ ions has different effects on structured and disordered domains in the same multidomain protein. The centrosomal STIL protein bound Zn2+ ions via both its structured N-terminal domain (NTD) and disordered central region (IDR). Zn2+ binding induced structural rearrangement of the structured NTD but promoted oligomerization of the IDR. We suggest that by binding Zn2+ STIL acquires a different conformation, which allows its oligomerization and induces its activity. Sequence alignment of the oligomerization region revealed a new suggested motif, SxKxS/SxHxS/SxLxS, which may participate in STIL oligomerization. Binding of the same metal ion through a disordered and a structured domain in the same protein is a property that may have implications in regulating the protein activity. By doing so, the protein achieves two parallel outcomes: structural changes and oligomerization that can take place together. Our results describe a new important role of the delicate interplay between structure and intrinsic disorder in proteins.
Introduction
Binding of metal ions may induce structural changes in proteins as a means of regulating protein activity.1–3 Metal ions can also play a role in protein oligomerization.4–6 Binding of metal ions to disordered proteins may induce a disorder-to-order transition, stabilizing the active conformation and enabling the protein to function.7–9 Metal binding can also induce oligomerization of a disordered protein.10–12 Here we show that the binding of Zn2+ ions may have different effects on structured and disordered domains in the same multidomain protein. Our model protein in this study is STIL (SCL/TAL1 Interrupting Locus) which is a 150 kDa cytosolic protein that contains 1288 residues.13 STIL plays an essential role in cell proliferation and survival14–16 and is required for centrosomal biogenesis and centriolar duplication.17–19 STIL has two functional orthologue proteins, Drosophila ANA-2 and C. elegans SAS-5. Both are centrosomal proteins that play a role in centriolar assembly.20,21 STIL is involved in several types of cancer14,22 and in autosomal recessive primary microcephaly.23 We have previously shown that STIL contains an intrinsically disordered central region that mediates its protein interactions.24–26 In addition, it contains a structured N-terminal domain, a short coiled coil domain and a STAN motif27 (Fig. 1A). STIL was also shown to oligomerize.21,26,28 The STIL orthologues ANA-2 and SAS-5 also form oligomers and SAS-5 contains an intrinsically disordered region, like STIL.21,29 Here we show that STIL contains two Zn2+ binding sites: the structured N terminal domain (NTD) and the intrinsically disordered region (IDR). Binding of Zn2+ ions had two parallel and different effects on the two domains: it induced structural changes in the NTD and oligomerization of the IDR. The oligomerization was critical for the interaction of STIL with its partner proteins. We conclude that the presence of a disordered and a structured domain in the same protein is essential for achieving two outcomes, structural changes and oligomerization, by binding the same metal ion.Results
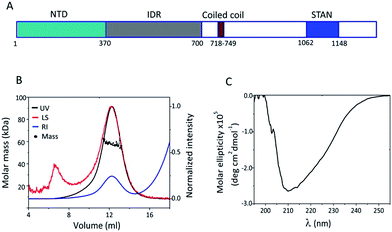
Structural characterization of STIL NTD and IDR
The N terminal domain of STIL (residues 2–370) and the central region of STIL (residues 450–700) were expressed and purified as HLT-fusion recombinant proteins.24 The structural properties of these two fragments were studied using SEC and CD spectroscopy. We have previously shown that HLT STIL 450–700 is intrinsically disordered24 and exists in an equilibrium between high and low oligomeric states (Fig. S1†). HLT STIL NTD, which is predicted to be fully structured,24 eluted in SEC as a monomer as confirmed by SEC-MALS experiments (Fig. 1B). CD experiments showed that HLT STIL NTD is composed of secondary structure elements and lacks disordered conformations (Fig. 1C). Taken together, our results provide experimental evidence that STIL NTD is a structured monomer while STIL 450–700 is disordered.Both STIL NTD and IDR bind Zn2+ ions
The amino acid composition of STIL contains a high percentage of negatively charged residues (Glu and Asp), which are common in disordered proteins and are known to participate in metal ion binding.30,31 STIL also has a relatively high proportion of Cys and His residues, known to participate in metal ion binding,31 similar to Zn2+ binding proteins (Fig. S2†). Since both Cys and His are common ligands of soft metal ions, we tested the possibility that STIL is a Zn2+ binding protein. STIL may be already bound to Zn2+ ions following its expression and thus we performed atomic absorption (AA) spectroscopy on HLT STIL NTD and HLT STIL IDR to detect the possible presence of the metal ions (Table 1). The results suggest a binding stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between HLT STIL NTD and Zn2+ as well as between the multimeric HLT STIL IDR and Zn2+. The zinc absorbance in the sample of monomeric STIL IDR was at the sensitivity limit of the instrument and therefore we concluded that the HLT STIL IDR monomer was not pre-bound to Zn2+ ions while the multimeric HLT STIL IDR and HLT STIL NTD were pre-bound to Zn2+ ions. The AA results combined with the SEC results (Fig. S1†) indicate that HLT STIL IDR exists in an equilibrium between a monomer that is unbound to Zn2+ ions and a Zn2+ pre-bound multimer. Thus, the IDR was studied in its monomeric and multimeric forms. AA studies showed no presence of other metal ions, including Fe2+, Ni2+, Cu2+ or Ca2+ in the protein samples. Zn2+ or Ni2+ ions that may be bound by the His-tag during the purification process were not detected in the sample of HLT alone. In the case of HLT STIL NTD, addition of Zn2+ ions was significantly important to prevent protein precipitation during the expression (Fig. S3†), suggesting that Zn2+ is important for the correct folding of the protein.
1 between HLT STIL NTD and Zn2+ as well as between the multimeric HLT STIL IDR and Zn2+. The zinc absorbance in the sample of monomeric STIL IDR was at the sensitivity limit of the instrument and therefore we concluded that the HLT STIL IDR monomer was not pre-bound to Zn2+ ions while the multimeric HLT STIL IDR and HLT STIL NTD were pre-bound to Zn2+ ions. The AA results combined with the SEC results (Fig. S1†) indicate that HLT STIL IDR exists in an equilibrium between a monomer that is unbound to Zn2+ ions and a Zn2+ pre-bound multimer. Thus, the IDR was studied in its monomeric and multimeric forms. AA studies showed no presence of other metal ions, including Fe2+, Ni2+, Cu2+ or Ca2+ in the protein samples. Zn2+ or Ni2+ ions that may be bound by the His-tag during the purification process were not detected in the sample of HLT alone. In the case of HLT STIL NTD, addition of Zn2+ ions was significantly important to prevent protein precipitation during the expression (Fig. S3†), suggesting that Zn2+ is important for the correct folding of the protein.
| STIL fragment | Protein concentration (μM) | AU | [Zn2+] (μM) |
|---|---|---|---|
| a Solutions of HLT STIL NTD and multimeric or monomeric HLT STIL IDR were tested in an atomic absorption spectrophotometer (Perkin Elmer). The table presents the absorption of the Zn atom at 213.9 nm (AU) and the calculated concentrations of the Zn2+ ion in the protein samples. | |||
| HLT STIL NTD | 20 | 0.145 ± 0.001 | 18.5 ± 0.1 |
| HLT STIL IDR multimer | 6 | 0.023 ± 0.002 | 6.2 ± 0.7 |
| HLT STIL IDR monomer | 3 | 0.005 ± 0.001 | — |
| HLT | 6 | Undetectable | — |
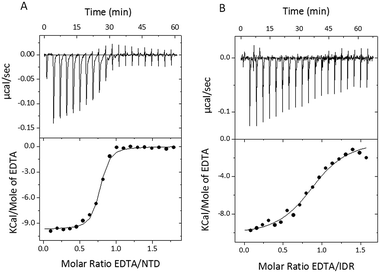
To characterize the binding of Zn2+ ions to the STIL fragments, we used isothermal titration calorimetry (ITC). Since HLT STIL NTD and the multimeric HLT STIL IDR are already pre-bound to Zn2+ ions, we titrated EDTA into the protein to complex the Zn2+ ions. The addition of EDTA to HLT STIL NTD and to HLT STIL IDR resulted in the dissociation of Zn2+ ions from the proteins (Fig. 2A and B). Fitting the curves to one set of sites revealed a stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between EDTA and the proteins, which is equal to the EDTA
1 between EDTA and the proteins, which is equal to the EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn2+ ratio (Table 2). Residual apo-STIL IDR or NTD that may exist in the samples would affect only the stoichiometry of the binding and not any of the thermodynamic parameters calculated from the ITC. We conclude that Zn2+ is bound to both the STIL NTD and IDR through one binding site in each domain. The thermodynamic parameters calculated from the fit are shown in Table 2 and represent the binding of EDTA to the Zn2+ ions that are released from the protein. While the changes in enthalpy and the changes in Gibbs free energy are very similar for the NTD and IDR, the changes in entropy (−2.6 cal mol−1 K−1 for the NTD and −8.67 cal mol−1 K−1 for the IDR) and the affinities were different (Kd of 0.12 ± 0.02 μM for the NTD and 0.8 ± 0.1 μM for the IDR). Titration of EDTA into free Zn2+ ions under the same experimental conditions revealed a Kd of 0.043 μM (data not shown). We calculated the Kd of Zn2+ binding to the STIL fragments using eqn (1)–(3) and found that the STIL NTD binds Zn2+ with a Kd of 0.36 μM and the STIL IDR binds Zn2+ with a Kd of 0.05 μM. This indicates that the STIL IDR binds Zn2+ 7 times more strongly than the STIL NTD. The higher change in entropy observed for the IDR may indicate that more significant conformational changes occur in the disordered STIL IDR compared to the structured STIL NTD. Other events coupled to the binding, such as proton exchange, may also contribute to this higher ΔS.
Zn2+ ratio (Table 2). Residual apo-STIL IDR or NTD that may exist in the samples would affect only the stoichiometry of the binding and not any of the thermodynamic parameters calculated from the ITC. We conclude that Zn2+ is bound to both the STIL NTD and IDR through one binding site in each domain. The thermodynamic parameters calculated from the fit are shown in Table 2 and represent the binding of EDTA to the Zn2+ ions that are released from the protein. While the changes in enthalpy and the changes in Gibbs free energy are very similar for the NTD and IDR, the changes in entropy (−2.6 cal mol−1 K−1 for the NTD and −8.67 cal mol−1 K−1 for the IDR) and the affinities were different (Kd of 0.12 ± 0.02 μM for the NTD and 0.8 ± 0.1 μM for the IDR). Titration of EDTA into free Zn2+ ions under the same experimental conditions revealed a Kd of 0.043 μM (data not shown). We calculated the Kd of Zn2+ binding to the STIL fragments using eqn (1)–(3) and found that the STIL NTD binds Zn2+ with a Kd of 0.36 μM and the STIL IDR binds Zn2+ with a Kd of 0.05 μM. This indicates that the STIL IDR binds Zn2+ 7 times more strongly than the STIL NTD. The higher change in entropy observed for the IDR may indicate that more significant conformational changes occur in the disordered STIL IDR compared to the structured STIL NTD. Other events coupled to the binding, such as proton exchange, may also contribute to this higher ΔS.
| Fragment | No. of sites | K d (μM) | ΔH (kcal mol−1) | ΔS (cal mol−1 K−1) | ΔG (kcal mol−1) |
|---|---|---|---|---|---|
| a The thermodynamic parameters represent the binding of EDTA to Zn2+ ions in each experiment. | |||||
| NTD | 0.74 ± 0.01 | 0.12 ± 0.02 | −9.7 ± 0.1 | −2.6 | −9.0 ± 0.1 |
| IDR | 0.91 ± 0.02 | 0.8 ± 0.1 | −10.3 ± 0.3 | −8.67 | −7.8 ± 0.3 |
Structural changes of STIL NTD and IDR induced by Zn2+ binding
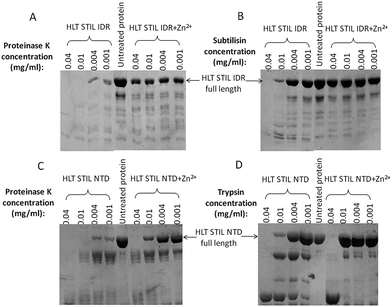
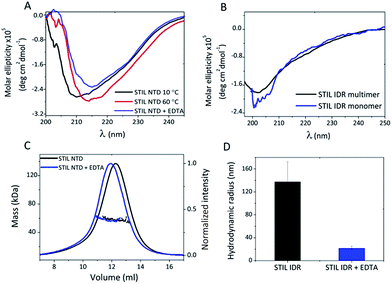
To study the possible conformational changes of the two STIL fragments upon Zn2+ binding we used limited proteolysis.32,33 HLT STIL NTD was first treated with EDTA to generate the apo-protein. Apo-HLT STIL NTD and monomeric HLT STIL IDR were incubated with subtilisin, chymotrypsin or proteinase K, in the presence or absence of ZnCl2 (Fig. 3). HLT STIL IDR was cleaved by most of the protease concentrations in the absence of ZnCl2 but was not cleaved in the presence of ZnCl2 (Fig. 3A and B). HLT STIL NTD was also cleaved by most of the protease concentrations in the absence of ZnCl2 but was not cleaved at the lower protease concentrations in the presence of ZnCl2 (Fig. 3C and D). ZnCl2 had no effect on the digestion of ovalbumin, indicating that its presence does not directly affect the activity of the proteases (Fig. S4†). Overall, the results indicate that HLT STIL NTD and HLT STIL IDR acquire a conformation that is more resistant to proteases in the presence of Zn2+ ions. This shielded conformation can be due to structural changes to a more compact form or due to oligomerization of the protein. Tryptophan fluorescence spectra of the STIL NTD (containing four Trp residues) and the STIL IDR (containing one Trp residue) in the presence or absence of Zn2+ ions also reveal structural changes between the apo and holo proteins, which can be due to structural rearrangements or oligomerization (Fig. S5†).To follow possible structural changes caused by Zn2+ binding we used CD spectroscopy. The CD spectrum of HLT STIL NTD after EDTA addition shows a shift of the major peak from 210 nm towards 215 nm, representing the increase in the β sheet content after EDTA addition. This indicates a change in the secondary structure between the apo and the holo STIL NTD (Fig. 4A). A similar structural change was observed after heating the protein to 60 °C, which is an alternative way of releasing Zn2+ ions from the binding Cys residues due to oxidation of the thiols.34 On the contrary, removal of Zn2+ ions did not change the secondary structure of the IDR fragment: the CD spectrum of the monomeric protein which was unbound to Zn2+ ions was similar to the CD spectrum of the Zn2+ pre-bound multimeric protein (Fig. 4B). Near-UV CD experiments also support structural changes in the STIL NTD in the presence of Zn2+ ions (Fig. S6†). Removing the Zn2+ ions by adding EDTA resulted in changes in the spectrum, mainly in the 270–280 nm region corresponding to the aromatic side chains. This reflects differences between the tertiary structures of the apo and holo STIL NTD.
To study oligomerization changes that may be induced by Zn2+ binding we used static and dynamic light scattering. SEC-MALS measurements of HLT STIL NTD revealed that the observed mass of the protein before and after EDTA treatment was the same (58 ± 2 kDa), in agreement with the calculated mass of the monomer (55 kDa). This indicates that the STIL NTD is monomeric and oligomerization did not occur (Fig. 4C). The elution volume of HLT STIL NTD with EDTA was slightly lower than that of free STIL NTD, indicating a minor change in the structure of the protein to a more extended conformation. We conclude that Zn2+ binding induces structural changes in the STIL NTD to a more compact form. We used DLS to measure the hydrodynamic radius of multimeric STIL IDR (which is pre-bound to Zn2+ ions) before and after the addition of EDTA (Fig. 4D). A significant decrease in the radius of HLT STIL IDR after EDTA addition was observed, from a radius of 137 ± 35 nm of the oligomer to a radius of 22 ± 4 nm of the EDTA treated protein. We conclude that the presence of Zn2+ ions induces oligomerization of the STIL IDR. Taken together, our results indicate that binding of Zn2+ ions induces structural changes in the structured STIL NTD and oligomerization of the STIL IDR.
Identification of the oligomerization sites within STIL IDR
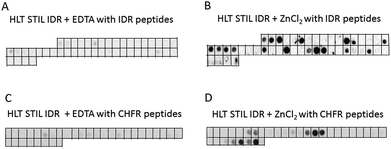
To characterize the oligomerization sites within the STIL IDR, we used peptide array screening. We designed an array of partly overlapping peptides derived from the STIL IDR (residues 370–700) and screened it for binding HLT STIL IDR itself in the presence of ZnCl2 or EDTA. HLT STIL IDR bound several peptides derived from the STIL IDR only in the presence of ZnCl2, while it did not bind the peptides in the presence of EDTA (Fig. 5A and B). The binding peptides may represent the oligomerization sites within the STIL IDR (Fig. S7†) or indicate intramolecular interactions within this domain. Most of the binding peptides contain a high proportion of Zn2+ binding residues (Cys, His, Glu or Asp), as detailed in ESI Table S1.† This observation further supports our conclusion that Zn2+ ions mediate STIL oligomerization. Alignment of the peptide sequences did not reveal known binding motifs. However, using the Block Maker tool35 we identified a new motif of 5 residues in most of the sequences. This motif contains Ser residues at the termini and a positive residue (His or Lys) or Leu residue in the middle: SxKxS/SxHxS or SxLxS (Table S1†). This motif as well as the Zn2+ binding residues are spread throughout the entire IDR sequence (Fig. S8†). HLT STIL NTD did not bind any peptides on an array derived from the STIL NTD, supporting the finding that this domain does not oligomerize (Fig. S9A and B†).Oligomerization of STIL IDR is required for the interaction with CHFR
We tested whether the Zn2+ binding is required for the interactions of STIL with its partner proteins, using the tumor suppressor CHFR as a model.24 An array of partly overlapping peptides derived from CHFR was designed and screened for binding with HLT STIL IDR and HLT STIL NTD in the presence of ZnCl2 or EDTA (Fig. 5C and D, S9C and S9D†). The results show that while the STIL IDR mediates the interaction of the protein with the CHFR peptides, the NTD does not bind CHFR peptides. Binding of HLT STIL IDR to the peptides was detected in the presence of Zn2+ ions while in the presence of EDTA almost no binding was observed. We conclude that oligomerization of STIL, induced by Zn2+ ions, is crucial for the STIL–CHFR interaction. The oligomerization may mediate the interaction with CHFR or other target proteins by exposing specific motifs required for the interaction. The Zn2+ ions may also serve as a direct mediator of the interaction between the STIL and CHFR peptides. It could be that both mechanisms take place in parallel. The HLT alone did not bind the peptide arrays in the presence of ZnCl2 (Fig. S9E and F†).Discussion
Zn2+ binding to disordered regions was shown to induce a disorder to order transition and gain of structure in many cases36–38 or lead to oligomerization, mainly aggregation, of IDPs.11,12,39 Here we show that binding of Zn2+ ions induces different effects on structured and disordered domains in the same protein: while binding of Zn2+ induced structural changes in the structured STIL NTD, it did not induce structural rearrangement or gain of structure of the disordered domain of STIL. Rather, it led to oligomerization of the disordered domain. The disordered region preserves its disordered features upon binding the Zn2+ ion ligand and oligomerizes to form an active protein. This oligomerization is reversible, as shown by the DLS studies (Fig. 4D) and may be a part of the regulation of the full length protein. Since the oligomerization of the STIL IDR is critical for its protein interactions, it may mediate the interaction with the target proteins by exposing specific motifs required for the interaction. Many centrosomal proteins act as oligomeric complexes that are crucial for centrosomal processes and functions.20,40,41 STIL may also act as an oligomeric complex in the centriole. Binding of Zn2+ ions can be one of the mechanisms to control its oligomerization.The STIL IDR oligomerization sites, as revealed by the peptide array screening, contain a high proportion of Zn2+ binding residues, Cys and His, at different positions in the IDR sequence (Fig. S8†). These together create the binding site of the Zn2+ ion. The Zn2+ binding site may be composed of residues from different IDR domains that are sequentially distant. Using this mechanism Zn2+ binding can bring these regions close together and, if they are from different monomers, directly mediate the oligomerization. Since the STIL IDR binds Zn2+ ions in a stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 1), it is likely that not all of the Cys and His residues participate in the Zn2+ binding. In addition, alignment of the peptide sequences revealed a new suggested motif for oligomerization: SxKxS/SxHxS or SxLxS (Table S1†). This motif resembles the SxxxS phosphorylation motif42 although here we used non-phosphorylated proteins or peptides. Since the STIL IDR contains several phosphorylation sites that mediate interactions of STIL,25 phosphorylation together with the oligomerization may play a part in the regulation of the protein. Binding of Zn2+ ions may induce conformational rearrangement of the STIL IDR, leading to exposure of this motif and enabling the oligomerization. The binding affinity of the STIL NTD and STIL IDR to Zn2+ ions, as revealed by the ITC experiments, is relatively strong compared to the binding affinities of Zn2+ ions to other Zn2+-binding proteins. Zn2+ binding to other proteins has a range of Kd between sub-micromolar to micromolar. BSA binds Zn2+ with a Kd of 1 μM,43 p25 binds Zn2+ with a Kd of 30 μM,9 α-crystallin binds Zn2+ ions with a Kd of 100 μM5 and the S100B protein binds Zn2+ with a Kd of 94 nM.44
1 (Table 1), it is likely that not all of the Cys and His residues participate in the Zn2+ binding. In addition, alignment of the peptide sequences revealed a new suggested motif for oligomerization: SxKxS/SxHxS or SxLxS (Table S1†). This motif resembles the SxxxS phosphorylation motif42 although here we used non-phosphorylated proteins or peptides. Since the STIL IDR contains several phosphorylation sites that mediate interactions of STIL,25 phosphorylation together with the oligomerization may play a part in the regulation of the protein. Binding of Zn2+ ions may induce conformational rearrangement of the STIL IDR, leading to exposure of this motif and enabling the oligomerization. The binding affinity of the STIL NTD and STIL IDR to Zn2+ ions, as revealed by the ITC experiments, is relatively strong compared to the binding affinities of Zn2+ ions to other Zn2+-binding proteins. Zn2+ binding to other proteins has a range of Kd between sub-micromolar to micromolar. BSA binds Zn2+ with a Kd of 1 μM,43 p25 binds Zn2+ with a Kd of 30 μM,9 α-crystallin binds Zn2+ ions with a Kd of 100 μM5 and the S100B protein binds Zn2+ with a Kd of 94 nM.44
The binding of Zn2+ ions to the STIL IDR (Kd = 50 nM) was stronger than the binding of Zn2+ ions to the STIL NTD (Kd = 360 nM). This emphasizes the significance of Zn2+ ions in this region and highlights the generally important role that the IDR plays in the protein. The binding of the same metal ion through a disordered and a structured domain in the same protein is a property that may have implications in regulating the activity of the protein. By doing so, the protein achieves two parallel outcomes: structural changes and oligomerization that can take place together (Fig. 6).
Experimental
Expression and purification of the STIL domains
Preparation of DNA encoding fragments of STIL was performed as detailed before.24 The proteins were expressed fused to the HLT tag that contains six His residues, a Lipo-domain for enhanced solubility and a Tev protease cleavage site for removing the tag (Fig. S10†). HLT STIL 2–370 (NTD) and HLT STIL 450–700 (IDR) vectors were transformed into E. coli BL21(DE3) pLysS cells (Novagen). Expression and purification of HLT STIL IDR were performed as previously described.24 HLT STIL NTD transformed bacteria cells were grown at 37 °C in 2xYT medium. At an OD600 nm of 0.2 heat-shock treatment was performed by adding 0.1% glycerol and 0.1 mM potassium glutamate and the bacteria were incubated at 42 °C for 20 min. The temperature was reduced to 37 °C and the bacteria were grown until an OD600 nm of 0.7. 0.1 mM ZnCl2 and 0.1 mM IPTG were added to start the induction that was carried out overnight at 22 °C. The bacteria were harvested, sedimented as pellets and kept at −80 °C. The bacteria were lysed using a microfluidizer and the soluble fraction was purified using a nickel Sepharose column. Elution was performed using an imidazole gradient and the protein was eluted with 25 mM TrisHCl buffer, pH = 8.5, 0.5 M NaCl, 5 mM βMe, 10% glycerol and 250 mM imidazole. The eluted protein was further purified using size exclusion chromatography with a Sephacryl S100 500 ml column. Elution was performed with a buffer of 25 mM Hepes, pH = 7, 0.3 M NaCl, 5 mM βMe and 2% glycerol. This buffer was also used as the storage buffer. The protein purity was confirmed by Coomassie staining of an SDS-PAGE gel.SEC and SEC-MALS
45 μM HLT STIL NTD was dissolved in 20 mM Hepes buffer, pH = 7, 150 mM NaCl, 2% glycerol and 2 mM βMe and was loaded on a Superose12 analytical GF column. A Multi Angle Light Scattering (MALS) miniDAWN TREOS instrument of Wyatt technology was used to measure the molecular weight of the protein alone and after 10 mM EDTA addition and 4 hours of incubation on ice. HLT STIL IDR was loaded on a Superose12 analytical GF column and eluted with 20 mM Hepes buffer, pH = 7, 300 mM NaCl, 2% glycerol and 2 mM βMe.Isothermal titration calorimetry (ITC)
ITC measurements were carried out at 10 °C on an isothermal titration calorimeter (ITC 200, MicroCal). 23 μM HLT STIL NTD and 13 μM HLT STIL IDR were dissolved in 13 mM Hepes buffer, pH = 7, 150 mM NaCl, 2% glycerol and 2 mM βMe. 100–200 μM EDTA was dissolved in the same buffer and titrated into the protein samples. 2 μl of EDTA solution was injected into the protein sample in 2 s titrations. A 180 s delay between injections was allowed for equilibration.45 Titration of EDTA into the buffer solution, titration of EDTA into the HLT solution and titration of the buffer into the protein solutions were performed as controls. The ITC data were analyzed with Origin 7.0 software.K d of Zn2+–EDTA binding in a free Zn2+ solution was measured using ITC and is described by equation (1):
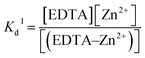 | (1) |
K d of Zn2+–EDTA binding in the STIL fragment solution was measured by ITC and is described by equation (2):
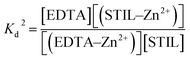 | (2) |
The Kd of Zn2+–STIL fragment binding was calculated using eqn (3):
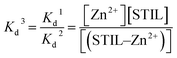 | (3) |
These calculations are under the assumption that EDTA binds only Zn2+ and not the protein and that STIL binds Zn2+ in a stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Atomic absorption spectroscopy
HLT STIL NTD, HLT STIL IDR multimeric fraction and monomeric fraction, and HLT samples were analyzed in an atomic absorption spectrophotometer 3110 or AAnalyst 400 (Perkin Elmer), with a Zn lamp at a wavelength of 213.9 nm. Samples were dissolved in 25 mM Hepes, pH = 7, 300 mM NaCl, 2 mM βMe and 2% glycerol.Circular dichroism (CD)
CD spectra of HLT STIL NTD and HLT STIL IDR were recorded using a J-810 spectropolarimeter (Jasco) in a 0.1 cm quartz cuvette for far-UV CD spectroscopy, in a spectral range of 195 nm to 260 nm. 16 μM HLT STIL NTD was incubated with 10 mM EDTA for 8 hours on ice, then dialyzed using 15 mM Hepes buffer, pH = 7, 150 mM NaCl, 2 mM βMe and 2% glycerol. CD spectra were recorded at 10 °C for 10 μM protein without EDTA addition and for the sample after EDTA treatment. A CD spectrum was also recorded for 10 μM protein at 60 °C. 3 μM monomeric HLT STIL IDR was dissolved in PBS buffer containing 2% glycerol and 2 mM βMe, and CD spectra of the protein alone and with the addition of ZnCl2 were recorded. A CD spectrum of 3 μM multimeric HLT STIL IDR dissolved in 20 mM TrisHCL, pH = 7.5, 50 mM Na2SO4, 0.5 mM βMe and 2% glycerol was also recorded. Near-UV CD spectra, in a spectral range of 250 nm to 340 nm, were recorded for 145 μM HLT STIL NTD alone or with addition of 10 mM EDTA. The measurements were performed in 25 mM Hepes buffer, pH = 7, 300 mM NaCl, 5 mM βMe and 5% glycerol.Dynamic light scattering (DLS)
Multimeric HLT STIL IDR was incubated with 10 mM EDTA for 2 days at 4 °C. DLS measurements were performed using a Zetasizer Nano-ZS (Malvern instruments) at 10 °C and the average hydrodynamic radius was calculated by the intensity for the protein alone and with EDTA addition. The calculation was performed using the Stokes–Einstein equation:| RH = kT/6πηD, |
Partially limited proteolysis
20 μl of 20 μM HLT STIL NTD was incubated with 10 mM EDTA for 5 hours on ice followed by overnight dialysis in 20 mM Hepes buffer, pH = 7, 150 mM NaCl, 2 mM βMe and 2% glycerol to remove the EDTA excess. 20 μl of 9 μM monomeric HLT STIL IDR was dissolved in 20 mM Hepes buffer, pH = 7, 300 mM NaCl, 2 mM βMe and 2% glycerol. The proteins were incubated with 5 μl of subtilisin, chymotrypsin or proteinase K at increasing concentrations with the presence or absence of 0.3 mM ZnCl2. After 30 min at 22 °C the proteolytic reaction was stopped by adding 6.5 μl of SDS solution, 5.2 mM PMSF and 5.2 mM EDTA. Ovalbumin was also treated with the above proteases in the presence or absence of 0.3 mM ZnCl2, as a control for ensuring that Zn2+ ions do not affect the activity of the proteases. The cleaved products were run on 12% SDS-PAGE gels.Fluorescence spectroscopy
Tryptophan fluorescence spectra were recorded using an LS45 luminescence spectrophotometer (Perkin Elmer). 2.5 μM monomeric HLT STIL IDR and 2.5 μM multimeric HLT STIL IDR were dissolved in 25 mM Hepes buffer, pH = 7, 150 mM NaCl, 2% glycerol and 2 mM βMe. Excitation was performed at 280 nm and the emission was screened between 300 nm to 430 nm. 0.25 μM HLT STIL NTD was dissolved in the same buffer and fluorescence measurements were performed using the same settings for the NTD sample alone and with addition of 10 mM EDTA.Peptide array screening
An array of 94 partly overlapping STIL derived peptides and an array of 32 partly overlapping CHFR derived peptides were synthesized by INTAVIS Bioanalytical Instruments AG, Koeln, Germany. The peptides were acetylated at their N-termini and attached to a cellulose membrane via their C-termini through an amide bond. The arrays were washed with TBST (50 mM TrisHCl, pH = 7.5, 150 mM NaCl, 0.1% Tween20) containing 2.5% milk. 3–5 μM HLT STIL NTD and monomeric HLT STIL IDR were dissolved in TrisHCl buffer, pH = 7.5, 150 mM NaCl, 5 mM βMe, 2% glycerol, 0.1% Tween20 and 2.5% milk, in the presence of 0.3 mM ZnCl2 or 10 mM EDTA, and incubated with the arrays at 4 °C with shaking overnight. After washing with TBST in the presence of 0.3 mM ZnCl2 or 10 mM EDTA, the arrays were incubated with an anti-His HRP conjugated antibody at room temperature for 1 hour and then washed again with TBST. Immunodetection was performed using chemiluminescence with ECL reagents.Acknowledgements
AF is supported by grants from the Israel Science Foundation (ISF), the Israel Cancer Research Foundation (ICRF) and by the Minerva Centre for Bio-Hybrid Complex Systems. HA is supported by the Dalia and Dan Meydan fellowship for Ph.D. students. AD is supported by an Eshkol grant from the Israel Science Ministry. SI is supported by grants from the Israel Science Foundation and Israel Science Ministry.References
- X. Arias-Moreno, O. Abian, S. Vega, J. Sancho and A. Velazquez-Campoy, Curr. Protein Pept. Sci., 2011, 12, 325–338 CrossRef CAS PubMed.
- H. S. Won, L. Y. Low, R. De Guzman, M. Martinez-Yamout, U. Jakob and H. Jane Dyson, J. Mol. Biol., 2004, 341, 893–899 CrossRef CAS PubMed.
- T. Yu, G. Wu, H. Yang, J. Wang and S. Yu, Int. J. Biol. Macromol., 2013, 56, 57–61 CrossRef CAS PubMed.
- M. Tourbez, C. Firanescu, A. Yang, L. Unipan, P. Duchambon, Y. Blouquit and C. T. Craescu, J. Biol. Chem., 2004, 279, 47672–47680 CrossRef CAS PubMed.
- S. Karmakar and K. P. Das, Biopolymers, 2011, 95, 105–116 CrossRef CAS PubMed.
- M. C. Mayer, D. Kaden, L. Schauenburg, M. A. Hancock, P. Voigt, D. Roeser, C. Barucker, M. E. Than, M. Schaefer and G. Multhaup, J. Biol. Chem., 2014, 289, 19019–19030 CrossRef CAS PubMed.
- A. Chenal, J. C. Karst, A. C. Sotomayor Pérez, A. K. Wozniak, B. Baron, P. England and D. Ladant, Biophys. J., 2010, 99, 3744–3753 CrossRef CAS PubMed.
- N. Selevsek, S. Rival, A. Tholey, E. Heinzle, U. Heinz, L. Hemmingsen and H. W. Adolph, J. Biol. Chem., 2009, 284, 16419–16431 CrossRef CAS PubMed.
- A. Zotter, J. Olah, E. Hlavanda, A. Bodor, A. Perczel, K. Szigeti, J. Fidy and J. Ovadi, Biochemistry, 2011, 50, 9568–9578 CrossRef CAS PubMed.
- A. C. Sotomayor-Pérez, D. Ladant and A. Chenal, J. Biol. Chem., 2011, 286, 16997–17004 CrossRef PubMed.
- T. Bund, J. M. Boggs, G. Harauz, N. Hellmann and D. Hinderberger, Biophys. J., 2010, 99, 3020–3028 CrossRef CAS PubMed.
- R.-H. Li, G.-B. Liu, H. Wang and Y.-Z. Zheng, Biosci., Biotechnol., Biochem., 2013, 77, 475–481 CrossRef CAS PubMed.
- P. D. Aplan, D. P. Lombardi and I. R. Kirsch, Mol. Cell. Biol., 1991, 11, 5462–5469 CrossRef CAS PubMed.
- A. Erez, A. Castiel, L. Trakhtenbrot, M. Perelman, E. Rosenthal, I. Goldstein, N. Stettner, A. Harmelin, H. Eldar-Finkelman, S. Campaner, I. Kirsch and S. Izraeli, Cancer Res., 2007, 67, 4022–4027 CrossRef CAS PubMed.
- S. Izraeli, L. A. Lowe, V. L. Bertness, D. J. Good, D. W. Dorward, I. R. Kirsch and M. R. Kuehn, Nature, 1999, 399, 691–694 CrossRef CAS PubMed.
- K. L. Pfaff, C. T. Straub, K. Chiang, D. M. Bear, Y. Zhou and L. I. Zon, Mol. Cell. Biol., 2007, 27, 5887–5897 CrossRef CAS PubMed.
- J. Vulprecht, A. David, A. Tibelius, A. Castiel, G. Konotop, F. Liu, F. Bestvater, M. S. Raab, H. Zentgraf, S. Izraeli and A. Krämer, J. Cell Sci., 2012, 125, 1353–1362 CrossRef CAS PubMed.
- C. Arquint, K. F. Sonnen, Y.-D. Stierhof and E. A. Nigg, J. Cell Sci., 2012, 125, 1342–1352 CrossRef CAS PubMed.
- A. Castiel, M. M. Danieli, A. David, S. Moshkovitz, P. D. Aplan, I. R. Kirsch, M. Brandeis, A. Kramer and S. Izraeli, J. Cell Sci., 2011, 124, 532–539 CrossRef CAS PubMed.
- R. Qiao, G. Cabral, M. M. Lettman, A. Dammermann and G. Dong, EMBO J., 2012, 31, 4334–4347 CrossRef CAS PubMed.
- M. A. Cottee, N. Muschalik, S. Johnson, J. Leveson, J. W. Raff and S. M. Lea, eLife, 2015, 4, e07236 Search PubMed.
- S. Ramaswamy, K. N. Ross, E. S. Lander and T. R. Golub, Nat. Genet., 2003, 33, 49–54 CrossRef CAS PubMed.
- A. Kumar, S. C. Girimaji, M. R. Duvvari and S. H. Blanton, Am. J. Hum. Genet., 2009, 84, 286–290 CrossRef CAS PubMed.
- H. Amartely, A. David, M. Lebendiker, H. Benyamini, S. Izraeli and A. Friedler, Chem. Commun., 2014, 50, 5245–5247 RSC.
- S. Campaner, P. Kaldis, S. Izraeli and I. R. Kirsch, Mol. Cell. Biol., 2005, 25, 6660–6672 CrossRef CAS PubMed.
- C. J. Tang, S. Y. Lin, W. B. Hsu, Y. N. Lin, C. T. Wu, Y. C. Lin, C. W. Chang, K. S. Wu and T. K. Tang, EMBO J., 2011, 30, 4790–4804 CrossRef CAS PubMed.
- N. R. Stevens, J. Dobbelaere, K. Brunk, A. Franz and J. W. Raff, J. Cell Biol., 2010, 188, 313–323 CrossRef CAS PubMed.
- C. Arquint, A.-M. Gabryjonczyk, S. Imseng, R. Böhm, E. Sauer, S. Hiller, E. A. Nigg and T. Maier, eLife, 2015, 4, e07888 Search PubMed.
- E. Shimanovskaya, R. Qiao, J. Lesigang and G. Dong, Worm, 2013, 2, e25214 CrossRef PubMed.
- V. N. Uversky and A. K. Dunker, Biochim. Biophys. Acta, 2010, 1804, 1231–1264 CrossRef CAS PubMed.
- B. L. Vallee and D. S. Auld, Biochemistry, 1990, 29, 5647–5659 CrossRef CAS PubMed.
- A. Fontana, P. Polverino de Laureto, V. De Filippis, E. Scaramella and M. Zambonin, Folding Des., 1997, 2, R17–R26 CrossRef CAS PubMed.
- T. Moldoveanu, C. M. Hosfield, Z. Jia, J. S. Elce and P. L. Davies, Biochim. Biophys. Acta, 2001, 1545, 245–254 CrossRef CAS.
- U. Jakob, M. Eser and J. C. A. Bardwell, J. Biol. Chem., 2000, 275, 38302–38310 CrossRef CAS PubMed.
- S. Henikoff, S. Pietrokovski and J. G. Henikoff, Nucleic Acids Res., 1998, 26, 309–312 CrossRef CAS PubMed.
- F. Guillière, C. Danioux, C. Jaubert, N. Desnoues, M. Delepierre, D. Prangishvili, G. Sezonov and J. I. Guijarro, PLoS One, 2013, 8, e52908 Search PubMed.
- K. Banaszak, V. Martin-Diaconescu, M. Bellucci, B. Zambelli, W. Rypniewski, M. J. Maroney and S. Ciurli, Biochem. J., 2012, 441, 1017–1026 CrossRef CAS PubMed.
- L. Rahman, V. Bamm, J. M. Voyer, G. T. Smith, L. Chen, M. Yaish, B. Moffatt, J. Dutcher and G. Harauz, Amino Acids, 2011, 40, 1485–1502 CrossRef CAS PubMed.
- P. Faller, C. Hureau and G. La Penna, Acc. Chem. Res., 2014, 47, 2252–2259 CrossRef CAS PubMed.
- G. Lukinavičius, D. Lavogina, M. Orpinell, K. Umezawa, L. Reymond, N. Garin, P. Gönczy and K. Johnsson, Curr. Biol., 2013, 23, 265–270 CrossRef PubMed.
- K. Habermann and B. M. Lange, Cell Div., 2012, 7, 17 CrossRef CAS PubMed.
- S.-Y. Lu, Y.-J. Jiang, J.-W. Zou and T.-X. Wu, J. Chem. Inf. Model., 2011, 51, 1025–1036 CAS.
- J. Lu, A. J. Stewart, P. J. Sadler, T. J. T. Pinheiro and C. A. Blindauer, Biochem. Soc. Trans., 2008, 36, 1317–1321 CrossRef CAS PubMed.
- P. T. Wilder, D. M. Baldisseri, R. Udan, K. M. Vallely and D. J. Weber, Biochemistry, 2003, 42, 13410–13421 CrossRef CAS PubMed.
- M. J. Cliff and J. E. Ladbury, J. Mol. Recognit., 2003, 16, 383–391 CrossRef CAS PubMed.
- A. Labrie, A. Marshall, H. Bedi and E. Maurer-Spurej, Transfus. Med. Hemother., 2013, 40, 93–100 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6sc00115g |
| This journal is © The Royal Society of Chemistry 2016 |