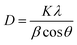Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Merits of photocatalytic and antimicrobial applications of gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite for pyridine removal and pathogenic bacteria/fungi disinfection: implication for wastewater treatment†
Gharieb S. El-Sayyad‡
 *a,
M. Abd Elkodous‡
*a,
M. Abd Elkodous‡ *bc,
Ahmed M. El-Khawagad,
Mohamed A. Elsayedd,
Ahmed I. El-Batal
*bc,
Ahmed M. El-Khawagad,
Mohamed A. Elsayedd,
Ahmed I. El-Batal a and
Mohamed Gobara
a and
Mohamed Gobara d
d
aDrug Microbiology Laboratory, Drug Radiation Research Department, National Center for Radiation Research and Technology (NCRRT), Egyptian Atomic Energy Authority, Cairo, Egypt. E-mail: Gharieb.Elsayyad@eaea.org.eg
bDepartment of Electrical and Electronic Information Engineering, Toyohashi University of Technology, Toyohashi, Aichi 441-8580, Japan. E-mail: mohamed.hamada.abdlekodous.xi@tut.jp
cCenter for Nanotechnology (CNT), School of Engineering and Applied Sciences, Nile University, Sheikh Zayed, Giza 16453, Egypt
dChemical Engineering Department, Military Technical College, Egyptian Armed Forces, Cairo, Egypt
First published on 3rd February 2020
Abstract
In this paper, we report a layer-by-layer approach for the preparation of a concentric recyclable composite (CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9) designed for wastewater treatment. The prepared composite was investigated by X-ray diffraction spectroscopy, high-resolution transmission electron microscopy and scanning electron microscopy (SEM) supported with energy dispersive X-ray (EDX) spectroscopy to analyze crystallinity, average particle size, morphology and elemental composition, respectively. The antimicrobial activities of the prepared composite have been investigated against multi-drug-resistant bacteria and pathogenic fungi using a variety of experiments, such as zone of inhibition, minimum inhibitory concentration, biofilm formation and SEM with EDX analysis of the treated bacterial cells. In addition, the effects of gamma irradiation (with different doses) and UV irradiation on the antibacterial abilities of the prepared composite have been evaluated. Moreover, the effect of gamma irradiation on the crystallite size of the prepared composite has been studied under varying doses of radiation (25 kGy, 50 kGy and 100 kGy). Finally, the photocatalytic efficiency of the prepared composite was tested for halogen-lamp-assisted removal of pyridine (artificial wastewater). Various parameters affecting the efficiency of the photocatalytic degradation, such as photocatalyst dose, pyridine concentration, pH, point of zero charge and the presence of hydrogen peroxide, have been studied. Our results show that the synthesized composite has a well-crystallized semi-spherical morphology with an average particle size of 125.84 nm. In addition, it possesses a high degree of purity, as revealed by EDX elemental analysis. Interestingly, the prepared composite showed promising antibacterial abilities against almost all the tested pathogenic bacteria and unicellular fungi, and this was further improved after gamma and UV irradiation. Finally, the prepared composite was very efficient in the light-assisted degradation of pyridine and its degradation efficiency can be tuned based on various experimental parameters. This work provides a revolutionary nanomaterial-based solution for the global water shortage and water contamination by offering a new wastewater treatment technique that is recyclable, cost effective and has an acceptable time and quality of water.
1 Introduction
Water contamination is one of the most serious factors affecting public health and terrestrial and aquatic environments.1 In addition, the percentage of available potable water on Earth, on which all living organisms rely, is tiny (only about 1%).2 Moreover, 6–8 million people die every year due to water-borne pathogens, which cause serious diseases, such as typhoid fever, diarrhea and hepatitis A.3–5 Wastewater contains different kinds of pollutants, such as heavy metals, dissolved and non-dissolved chemicals, phenols, dyes and other miscellaneous substances.6,7Among these pollutants, pyridine receives continuous attention due to the severity of its effects.8 Pyridine (C5H5N) is an organic compound with a basic heterocyclic structure.9,10 It is a colorless, volatile and flammable liquid possessing an unpleasant odor and high toxicity.11 Pyridine exposure has critical effects on the human immune system and may lead to carcinogenicity.12 In addition, pyridine derivatives exhibit toxicity to aquatic life and produce irritation due to their unpleasant smell.13 Currently, more than 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year of pyridine derivatives are produced worldwide because of their widespread use as herbicides and insecticides in cultivation and in some industrial activities, including textile manufacture, chemical and pharmaceutical synthesis.14
000 tons per year of pyridine derivatives are produced worldwide because of their widespread use as herbicides and insecticides in cultivation and in some industrial activities, including textile manufacture, chemical and pharmaceutical synthesis.14
Thus, wastewater treatment and strategies for water preservation should be a global consideration. Chemical and microbial removal of various kinds of pollutants are currently employed in traditional wastewater treatment plants.15 However, the efficiency of degradation of pollutants, the capacity of wastewater treatment plants and the treatment time all have serious limitations. To overcome these limitations more effective and satisfactory techniques for wastewater treatment are required. Advances in nanotechnology using nanomaterials provides a revolutionary solution for these issues and can also improve the efficacy of traditional wastewater treatment plants.16
Nanomaterials possess relatively higher degrees of chemical, physical and biological activities due to their large surface area with respect to their bulk counterparts.17–21 Among nanomaterials, titanium dioxide (TiO2) is still a very promising candidate for light-assisted photocatalysis and degradation of many kinds of pollutant present in wastewater.22
TiO2 nanoparticles (NPs) are chemically active, abundant, non-toxic and possess satisfactory photocatalytic activity.23 However, nanoparticles are too small to adsorb large quantities of pollutants and collection and reusability of the particles are very important considerations in terms of the overall cost. Thus, designing efficient and reusable photocatalytic systems for wastewater treatment based on nanomaterials is of critical importance.
Herein, we report the preparation of an improved recyclable nanocomposite (CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9) synthesized by a layer-by-layer approach. The CoxNi1−xFe2O4; x = 0.9 NPs are employed as a magnetic core for reusability (Fig. S1†) and easy collection of the material after successive wastewater treatment operations, while the SiO2 layer was used to separate the magnetic core from the photoactive TiO2 layer, so as not to reduce its quantum efficiency. Finally, the TiO2 layer was used as a photoactive catalyst for the removal of pollutants present in wastewater. The antimicrobial activities of the prepared composite were tested against pathogenic bacteria and unicellular fungi, such as Klebsiella pneumoniae, Proteus vulgaris, Proteus mirabilis, Salmonella typhi, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Candida tropicalis and Candida albicans. In addition, the effect of both gamma (with different doses) and UV irradiation on the antimicrobial activities of the prepared composite have been analyzed. The effects of different doses of gamma irradiation on the crystallite size of the prepared composite have also been studied. Moreover, the photocatalytic ability of the prepared composite was tested for the degradation of pyridine solution (as an artificial wastewater). Different parameters controlling the photocatalytic efficiency, such as photocatalyst dose, pH, pyridine concentration and the presence of H2O2 have been studied.
2 Materials and methods
2.1. Materials
Tetraethyl orthosilicate (TEOS) 98% [Si(OC2H5)4], titanium(IV) isopropoxide 97% (C12H28O4Ti), ammonium hydroxide (NH4OH), nickel chloride (NiCl2), ferric chloride (FeCl3·6H2O), absolute ethanol (C2H5OH), ∼80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MW hydroxypropyl cellulose, sodium hydroxide (NaOH), cobalt chloride (CoCl2), and pyridine (C5H5N) were purchased from Sigma-Aldrich (Germany). Precursors for this study were used as received without further purification. Materials are commercially available and of extra-pure grade.
000 MW hydroxypropyl cellulose, sodium hydroxide (NaOH), cobalt chloride (CoCl2), and pyridine (C5H5N) were purchased from Sigma-Aldrich (Germany). Precursors for this study were used as received without further purification. Materials are commercially available and of extra-pure grade.
2.2. Methods
The nanocomposite under investigation was prepared and fully characterized according to the methods reported in our previous paper.24 We briefly present the preparation steps below and list the new experiments performed, such as new characterization data, degradation of pyridine, study of the parameters affecting degradation efficiency (photocatalyst dose, contaminant concentration, pH, presence of H2O2), antimicrobial activities against multi-drug-resistant bacteria and pathogenic fungi and the effect of gamma and UV irradiation on the antimicrobial activity of the prepared composite.The degradation rate of pyridine was calculated by determining the variation in pyridine concentration versus irradiation time using a UV-vis spectrophotometer (Agilent Technologies Cary 60 UV-vis) at λmax = 256 nm. DI water was used as a ref. 25.
All prepared samples (each layer of the composite and the whole nanocomposite) were examined against distinct isolates of pathogenic bacteria, such as Staphylococcus aureus (methicillin-resistant S. aureus (MRSA)), Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa, Salmonella typhi, Klebsiella pneumoniae, Proteus vulgaris, and P. mirabilis. In addition, the antifungal activity was examined against unicellular pathogenic fungi such as Candida tropicalis and C. albicans. The tested microorganisms were obtained from the culture collection of the Drug Microbiology Laboratory, Drug Radiation Research Department, NCRRT, Cairo, Egypt. It is worth mentioning that the 0.5 McFarland standard of all tested inoculums was set at 2–5 × 108 CFU ml−1 (for bacteria) and 1–4 × 107 CFU ml−1 (for yeast). The repression of the tested bacteria and yeast was determined by the zone of inhibition (ZOI) method after 24 h of incubation.28
Standard antibiotic discs (diameter of 6.0 mm), such as amoxicillin/clavulanic acid (AMC) and nystatin (NS), were used for comparison of the action of the examined samples.29
The minimum inhibitory concentration (MIC) was determined using Luria–Bertani (LB) broth with appropriate serial dilution.30,31 The microorganism and the nutrient served as a positive control, and the nutrient alone was used as a negative control. The CoxNi1−xFe2O4; x = 0.9/SiO2/TiO2 nanocomposite (starting with a concentration of 100 mg ml; 100 ppm) was tested. MIC values were calculated after 24 h of incubation at 37 °C.32,33 The tested bacterial inoculums were set at 3–4 × 108 CFU ml−1, and at 2–4 × 107 CFU ml−1 for the tested yeasts. MIC values were determined through enzyme-linked immunosorbent assay (ELISA) after fixing the absorption wavelength at 600 nm.33,34
In addition, nutrient broth (5.0 ml) was added to the test tubes after setting the 0.5 McFarland standard at 1–2 × 107 CFU ml−1 (for the examined bacteria) and the tubes were then incubated for 24 h at 37 °C. The content of the control and treated tubes was discarded, and then all tubes were cleaned and rinsed with phosphate-buffered saline (PBS) with a pH of 7.5. Finally, all tubes were dried.36,37 The disciple bacterial and yeast films were fixed using sodium acetate (3%; 5 ml) for 10 min, and then tubes were rinsed with DI water. Bacterial and yeast biofilms were stained with crystal violet (CV; 0.1%) for 10 min and then soaked with DI water to remove excess CV.38 Subsequently, 4 ml of absolute ethanol was added to disintegrate the CV. The developed biofilms were recognized by the notable stained bands at the inner walls and the bottom of the test tubes.39 The bacterial and yeast biofilms were examined using a UV-vis spectrophotometer at 570 nm, and the biofilm repression percentage (%) was defined using eqn (1).37,40
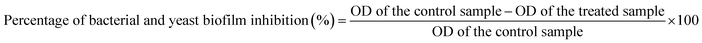 | (1) |
All test tubes contained nutrient broth and a fixed count of microorganisms (0.5 McFarland; CFU ml−1). The UV-emitting tube (10 W low-pressure mercury lamp; 90% emittance at 254.0 nm) was located horizontally and fixed in the laminar flow, and the tested tubes were exposed to UV irradiation for 1 h at a distance of 2 feet (60.96 cm).
It is worth mentioning that the bacterial and yeast count was calculated every 10 min by UV-vis spectrophotometry at 600 nm (for bacteria) and 630 nm (for Candida species) for about 1 h and the inhibition percentage was measured according to eqn (1).
3 Results and discussion
3.1. Characterization of the synthesized CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite
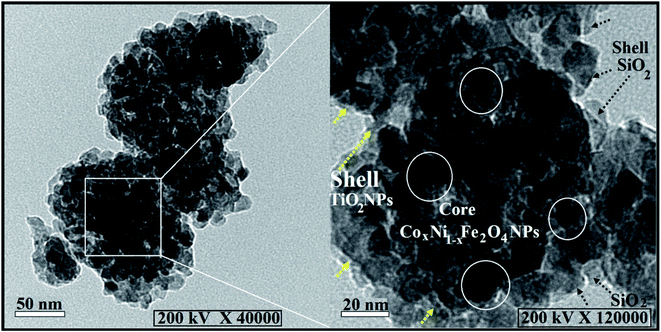
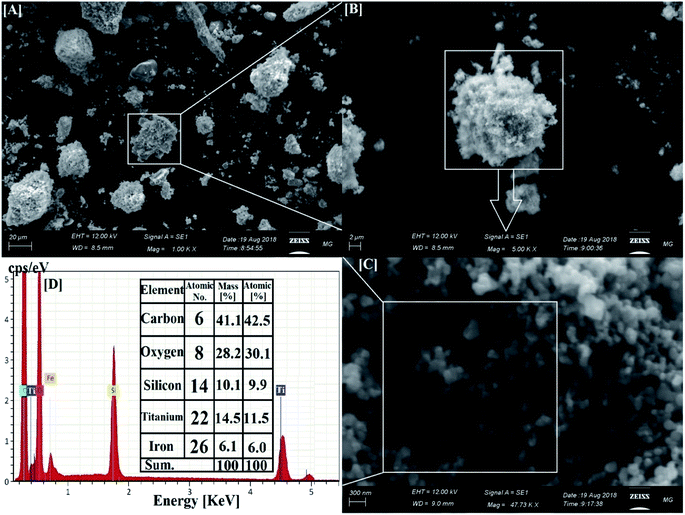
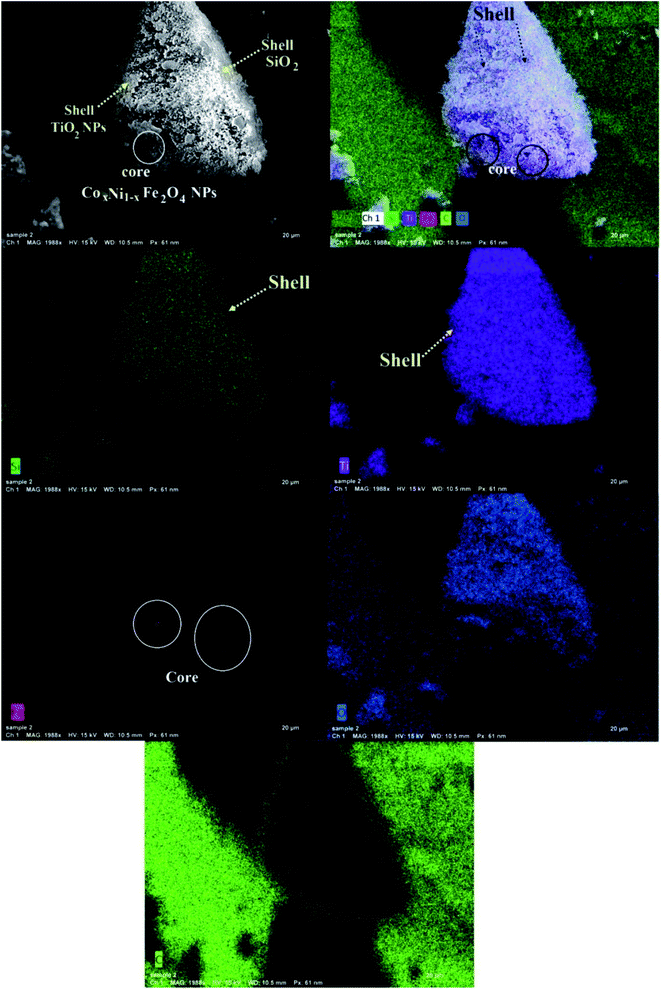
Fig. 2 shows SEM and EDX analysis of the synthesized CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite revealing its particle dispersion with high purity through the appearance of C, O, Si, Ti and Fe atoms and the lack of any foreign elements (impurities). The recorded carbon atoms are attributed to the sample holder used in the SEM imaging.47 The corresponding EDX elemental analysis confirmed the presence of all the constituent atoms of the nanocomposite. The absence of Ni and Co atoms is attributed to their location at the deep core of the composite and their smaller ratios.
This is one of the first times elemental mapping has been used as a tool to illustrate the development of a concentric structure, giving a promising explanation about the distribution and purity of the atoms and layers forming the nanocomposites.49–51
3.2. In vitro antimicrobial activity of the synthesized CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite
The disc agar spread method (which was performed as a screening step) revealed that the synthesized CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite displayed a relatively high antimicrobial activity against all examined bacteria and Candida species pathogens. Screening results confirmed that the CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite exhibited the highest antibacterial ability against E. coli (16.0 mm ZOI) and S. aureus (MRSA) (11.0 mm ZOI) as shown in Table 1.| Pathogenic microbes | ZOI of CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 NPs (mm) | MIC of CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 NPs (μg ml−1) | ZOI of CoxNi1−xFe2O4; x = 0.9 NPs; x = 0.9 (mm) | ZOI of SiO2 NPs (mm) | ZOI of TiO2NPs (mm) | ZOI of DMSO (mm) | AMC or NS |
|---|---|---|---|---|---|---|---|
| a Values are presented as mean ± SD (n = 3). a–eData within the groups were analyzed using one-way analysis of variance (ANOVA) followed by Duncan's multiple range test (DMRT). LSD, least significant difference. Nil, no ZOI measured. AMC, amoxicillin/clavulanic acid (standard antibacterial agent). NS, nystatin (standard antifungal agent). | |||||||
| Escherichia coli | 16.0e ± 0.5000 | 3.12 | 10.0e ± 0.1527 | 7.0a ± 0.3214 | 7.0a ± 0.2000 | Nil | Nil |
| Pseudomonas aeruginosa | 10.0c ± 0.2886 | 12.5 | 9.0d ± 0.2886 | 7.0a ± 0.4618 | 8.0b ± 0.3055 | Nil | Nil |
| Staphylococcus aureus; MRSA | 11.0d ± 0.2645 | 6.25 | 8.0c ± 0.4358 | Nil | 7.0a ± 0.2886 | Nil | Nil |
| Bacillus subtilus | 10.0c ± 0.5196 | 12.5 | 7.0a ± 0.3214 | Nil | Nil | Nil | Nil |
| Proteus mirabilis | 10.0c ± 0.2886 | 12.5 | 8.0bc ± 0.2886 | 7.0a ± 0.4163 | 7.0a ± 0.4725 | Nil | Nil |
| Salmonella typhi | 9.0b ± 0.2645 | 12.5 | 7.0abc ± 0.5507 | Nil | Nil | Nil | Nil |
| Proteus vulgaris | 8.0a ± 0.2645 | 25.0 | 8.0a ± 0.2886 | 8.0b ± 0.4509 | 8.0b ± 0.3055 | Nil | Nil |
| Klebsiella pneumoniae | 9.0b ± 0.0577 | 25.0 | 7.0 ab ± 0.4509 | 7.0a ± 0.4932 | 8.0b ± 0.2309 | Nil | Nil |
| Candida albicans | 10.0c ± 0.4041 | 12.5 | 8.0c ± 0.5196 | 8.0b ± 0.4000 | 7.0a ± 0.3214 | Nil | Nil |
| Candida tropicalis | 9.0b ± 0.4000 | 25.0 | 7.0 ab ± 0.4509 | 7.0a ± 0.3214 | Nil | Nil | Nil |
| LSD | 0.70000 | — | 1.33333 | 1.00000 | 0.76667 | — | — |
It is of interest to note that the CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite has more powerful potential in terms of antimicrobial abilities than its separate layers (CoxNi1−xFe2O4; x = 0.9 NPs, SiO2, TiO2 NPs), DMSO and standard antimicrobial agents (AMC). It is also worth noting that the synthesized CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite was more active against Gram-negative bacteria than against Gram-positive species. The reasons for this effect may be due to the fact that the cell walls of Gram-negative species essentially consist of layers of peptidoglycan, lipopolysaccharide, and lipid, while the cell walls of Gram-positive species just have thick peptidoglycan structures.52
In addition, the produced CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite was very promising as an antifungal agent, conferring great antifungal efficiency towards C. albicans (10.0 mm ZOI), as presented in Table 1.
The MIC results for the CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite against all examined pathogenic bacteria and Candida species were in the range 12.5–3.125 μg ml−1, as shown in Table 1. The CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite possesses a promising MIC value of 3.125 μg ml−1 against E. coli and 6.25 μg ml−1 against S. aureus.
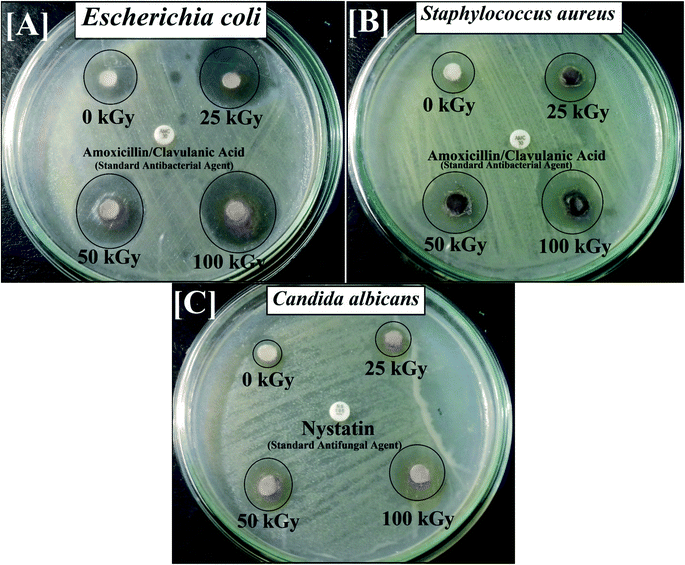
Subsequently, following gamma irradiation with doses of 25.0, 50.0 and 100.0 kGy, the antimicrobial activity of the synthesized CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite was evaluated. Gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite was more active against E. coli (30.0 mm ZOI; Fig. 4A), S. aureus (MRSA) (25.0 mm ZOI; Fig. 4B) and C. albicans (24.0 mm ZOI; Fig. 4C), as presented in Table 2.
| Pathogenic microbes | ZOI of non-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 NPs (mm) | ZOI of irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 NPs (25.0 kGy) (mm) | ZOI of irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 NPs (50.0 kGy) (mm) | ZOI of irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 NPs (100.0 kGy) (mm) | MIC of irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 NPs (100.0 kGy) (μg ml−1) | AMC or NS |
|---|---|---|---|---|---|---|
| a Values are presented as mean ± SD (n = 3). a–eData within the groups were analyzed using one-way analysis of variance (ANOVA) followed by Duncan's multiple range test (DMRT). LSD, least significant difference. Nil, no ZOI measured. AMC, amoxicillin/clavulanic acid (standard antibacterial agent). NS, nystatin (standard antifungal agent). | ||||||
| Escherichia coli | 17.0b ± 0.3214 | 25.0f ± 0.4509 | 27.0h ± 0.2645 | 30.0h ± 0.4163 | 0.024 | Nil |
| Pseudomonas aeruginosa | 11.0a ± 0.2645 | 18.0d ± 0.4509 | 19.0e ± 0.2516 | 20.0e ± 0.2516 | 0.390 | Nil |
| Staphylococcus aureus; MRSA | 12.0a ± 0.1808 | 19.0.0e ± 0.5507 | 22.0g ± 0.2081 | 25.0g ± 0.2645 | 0.097 | Nil |
| Bacillus subtilus | 10.0a ± 0.4725 | 12.0.0b ± 0.2645 | 14.0d ± 0.3214 | 16.0d ± 0.4582 | 1.562 | Nil |
| Proteus mirabilis | 11.0a ± 0.2000 | 13.0a ± 0.2516 | 13.0c ± 0.2081 | 15.0c ± 0.5000 | 3.125 | Nil |
| Salmonella typhi | 10.0a ± 0.4509 | 11.0a ± 0.2516 | 11.0a ± 0.1527 | 12.0a ± 0.2645 | 6.250 | Nil |
| Proteus vulgaris | 10.0a ± 0.4509 | 12.0b ± 0.2886 | 14.0d ± 0.4000 | 16.0d ± 0.3464 | 3.125 | Nil |
| Klebsiella pneumoniae | 11.0a ± 0.2516 | 11.0a ± 0.3055 | 12.0b ± 0.3214 | 13.0b ± 0.2516 | 6.250 | Nil |
| Candida albicans | 10.0a ± 0.4509 | 18.0d ± 0.4000 | 20.0f ± 0.4041 | 24.0f ± 0.2886 | 0.195 | Nil |
| Candida tropicalis | 10.0a ± 0.1527 | 12.0b ± 0.3214 | 14.0d ± 0.2886 | 15.0c ± 0.2516 | 6.250 | Nil |
| LSD | 6.06666 | 0.83333 | 0.90000 | 0.90000 | — | — |
Interestingly, the MIC values decreased with increasing dose of gamma rays and a superior MIC result was recorded at 0.024 μg ml−1 against E. coli for the CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite irradiated by 100.0 kGy.
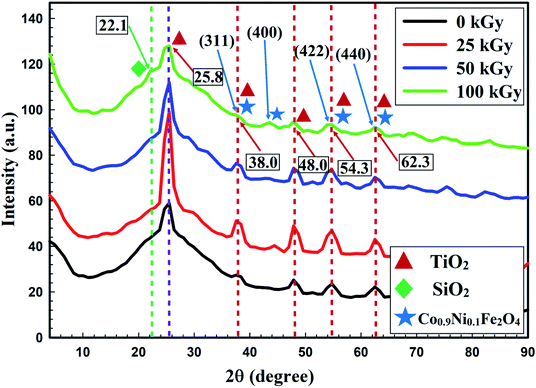
The enhanced activity of the prepared nanocomposite against all tested microorganisms after gamma irradiation was due to the reduction in crystallite size (from 44.2 nm to 17.4 nm) after 100 kGy irradiation.53,54 The XRD patterns of non-irradiated and gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite with different doses (25 kGy, 50 kGy and 100 kGy) are shown in Fig. 5.
The XRD patterns presented in Fig. 5 show the crystallinity of the non-irradiated and gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite. Characteristic peaks of TiO2 NPs, SiO2 NPs and CoxNi1−xFe2O4; x = 0.9 NPs were clearly recorded. Many sharp, strong and intense peaks were observed at 2θ values of 25.8° (reflection 101), 38.0° (reflection 112), 48.0° (reflection 200), 54.3° (reflection 105), and 60.3° (reflection 213), while the principal peak was observed at 2θ = 25.8°. Recorded peaks matched those of TiO2 NPs (JCPDS 04-0477).24
An amorphous extended peak was recorded at 2θ = 22.1°, which is associated with the interatomic lengths in SiO2 NPs.55–58 Finally, sharp and intense peaks were observed at 2θ = 38.0° (reflection 311), 45.2° (reflection 400), 54.3° (reflection 422), and 62.3° (reflection 440), while a promising peak appeared at 38.0°, registering the appearance of both Ni Fe2O4 (JCPDS 10-325) and Co Fe2O4 (JCPDS 1-1121), indicating the development of CoxNi1−xFe2O4; x = 0.9 NPs.59
The average crystallite size was calculated using the Debye–Scherrer equation as:
From Fig. 5, it can be seen that crystallite size was reduced by increasing the gamma radiation dose from 25 to 100 kGy. This influence may be due to the movement of gamma rays inside the materials, transporting its power in a compact time interval within flexible contact with particles,53 which leads to modification in the irradiated material by forcing the atoms from their initial conditions, and dividing particles.54
Additionally, with increase in irradiation dose the samples showed a tendency to become amorphous (Fig. 5) and this is because the crystallite size is independent of the crystallinity of the sample. Crystallization of a material means that there are long arrangements of atoms. This can be indicated by the intensity of the XRD lines. For XRD, the smallest crystallite size caused obvious broadening of the XRD peaks, but crystallite size gives information about the formed sample.60 A perfect crystal would extend infinitely in all directions; hence, no crystals are perfect because of their finite crystal size. This deviation from perfect crystallinity leads to broadening of the diffraction peaks.61 The two principal properties obtained from peak width analysis are the crystallite size and lattice strain.
Crystallite size is a measure of the size of coherently diffracting domains. The crystallite size of the particles is not usually the same as the particle size due to the production of polycrystalline aggregates.62 Lattice strain is a measure of the distribution of lattice constants resulting from crystal imperfections, such as lattice dislocations. Other sources of strain include the grain boundary triple junction, contact or sinter stresses, stacking faults, and coherency stresses.63 Crystallite size and lattice strain affect the Bragg peak in different ways. Both these effects extend the peak width and intensity and shift the 2θ peak position accordingly.
Interestingly, there is a relationship between the physical properties of the fabricated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite and its recorded antimicrobial abilities, with surface area playing a significant role in the antimicrobial action of the synthesized CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite against all tested pathogenic microbes.
The calculated surface area was 46.13 m2 g−1, with a broad spread of pore sizes of TiO2 NPs, with an average value of 3.71 nm and an average pore volume of about 0.19 cm3 g−1, as previously calculated in our recent paper,24 implying the appearance of two kinds of pores in the external layer of the composite TiO2 NPs: mesopores and macropores.64
The high surface area and broad pore size of the produced nanocomposite increased the contact area to the microbial cell and hence the antimicrobial activity of the CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite was increased.
The gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite had a more stable profile, with relatively lower crystal size (19.6 nm, 19.3 nm and 17.4 nm after gamma irradiation with 25 kGy, 50 kGy and 100 kGy, respectively; Fig. 5) compared to the non-irradiated composite. These physical properties were critical in improving its antimicrobial efficacy even at a low concentration (0.024 μg ml−1) against all examined pathogenic bacteria and Candida species.
3.3. Antimicrobial potential of UV-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite in liquid media
A comparative study of the inhibition percentage of E. coli, S. aureus, and C. albicans by non-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite and UV-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite was performed and is presented in Fig. 6.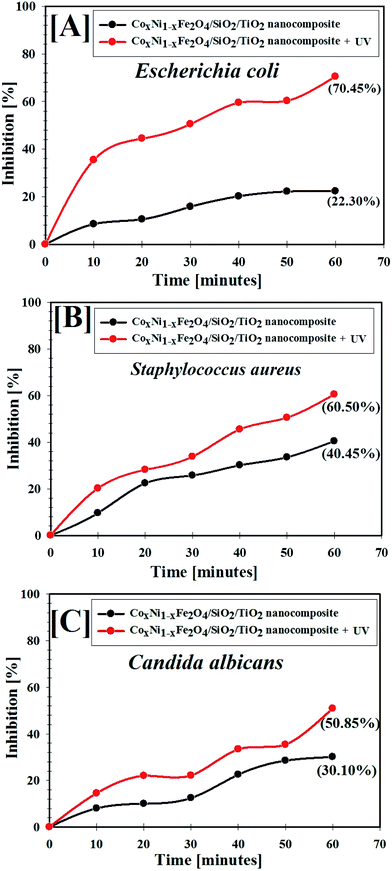 | ||
| Fig. 6 Antimicrobial abilities of UV-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite against different pathogens: [A] E. coli, [B] S. aureus and [C] C. albicans. | ||
As shown in Fig. 6A–C, the inhibition percentage of the tested pathogens increased with time upon treatment with the CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite, meaning that the CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite showed antimicrobial activities against colonies of E. coli, S. aureus and C. albicans. Interestingly, the UV-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite exhibited superior antimicrobial activities compared with the non-irradiated nanocomposite, as shown in Fig. 6. The maximum inhibition percentage of non-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite and UV-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite against E. coli was 22.30% and 70.45%, respectively, and the inhibition was 40.45% and 60.50% for S. aureus and 30.10% and 50.85% for C. albicans, after 60 min (experiment time).
It was found that the CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite was more active under UV irradiation, confirming the presence of photogenerated reactive oxygen species (ROS) that can decompose bacterial cells. Here, the CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite was shown to have good antimicrobial abilities attributed to its high UV absorption intensity. Hydroxyl (OH) radicals can also be generated by irradiating CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite with ultraviolet light, due to electron transfer between microbial cells and the nanocomposite. The OH radicals can destroy bacterial cells causing a reduction in cell coenzyme content.65
In addition, it has been reported that metal oxides (MO) display a positive charge in slightly acidic medium, while microbes possess a negative charge. This creates an electromagnetic affinity between microbes and the MO, leading to oxidization of microbial cells and their subsequent destruction.66 Additionally, nanomaterials can destroy cellular proteins and DNA by adhering to electron-donating structures, such as carbohydrates, thiols, indoles, amides, and hydroxyls. In addition, they can create holes in the bacterial cell walls, making them outwardly permeable and finally resulting in cell loss.67 We have previously reported that our composite possessed negative charge, but the media of the microbes is slightly acidic (pH = 6.0), which can change the surface charge of the composite to positive, and this is in a good agreement with our results (see Fig. 14B later).
3.4. Antibiofilm activity of CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite gamma-irradiated with 100 kGy
Biofilm creation has been recognized in several exopolysaccharide-forming microbes.36,50 Biofilm production by common pathogenic bacteria and yeast microorganisms in the absence and presence of gamma-irradiated (with 100 kGy) CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite was assessed using a test tube method.68Fig. 7A shows the antibiofilm activity of 100 kGy gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite against E. coli bacteria (as a model for susceptible bacteria).
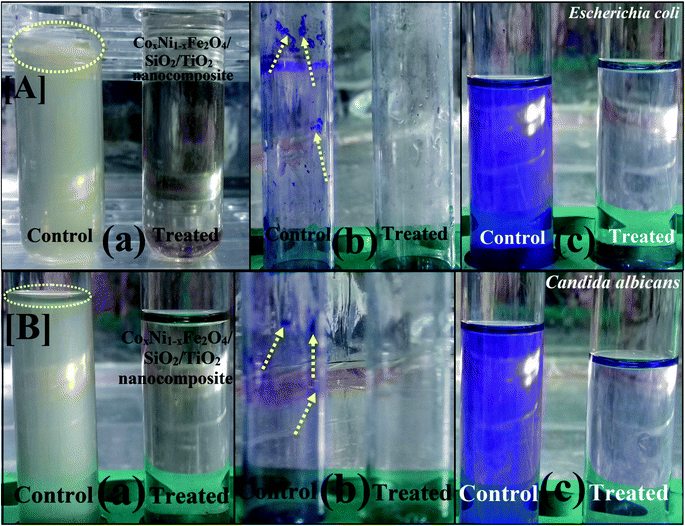 | ||
| Fig. 7 Antibiofilm activity of 100 kGy gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite using the test tube method against [A] E. coli and [B] C. albicans. The steps were reported as follows. (a) Growth of the bacterial and yeast cells and biofilm formation (rings) without treatment with the synthesized CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite and the inhibition of bacterial and yeast growth after treatment with CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite. (b) Staining of the adherent bacterial and yeast cells with crystal violet. (c) Removing and dissolving the adherent bacterial and yeast cells by ethanol for determination of semi-quantitative biofilm inhibition (%) (as shown in Table 3). | ||
E. coli inoculated in the absence of gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite exhibited a thick whitish-yellow mat throughout the air–liquid interface. This mat was totally adhered to the wall of the test tubes and appeared as a blue ring after CV staining. A blue suspension was developed after dissolving the CV-stained ring with absolute ethanol, as displayed in Fig. 7A.
On the other hand, E. coli-inoculated test tubes that were treated with gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite (10.0 μg ml−1) exhibited a suppressed effect where the development of bacterial rings was limited. In addition, the blue color representing CV-stained adherent bacterial cells was light and, after CV dissolution by ethanol, no blue color was observed, as shown in Fig. 7A. A similar effect was recorded for the biofilm repression of C. albicans (as an example of a sensitive yeast), as presented in Fig. 7B.
To determine the repression percentage (%) of bacterial and yeast biofilm, a UV-vis spectrophotometer was used (at 570.0 nm). The optical density was determined after separating CV-stained bacterial and yeast biofilms with ethanol. Table 3 shows the inhibition percentage of the biofilms formed by the tested bacteria and yeast strains. The highest percentage inhibition was observed against E. coli (92.82%; Fig. 7A and Table 3) followed by C. albicans (92.29%; Fig. 7B and Table 3) and S. aureus (90.69%; Table 3) after treatment with 10.0 μg ml−1 of gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite.
| Bacterial and yeast strains | OD of crystal violet stain at 570.0 nm (control) | OD of crystal violet stain at 570.0 nm (treated with 10.0 μg ml−1 CoxNi1−xFe2O4/SiO2/TiO2 nanocomposite) | Inhibition (%) |
|---|---|---|---|
| a Values are presented as mean ± SD (n = 3). a–eData within the groups were analyzed using one-way analysis of variance (ANOVA) followed by Duncan's multiple range test (DMRT). LSD, least significant difference. | |||
| Escherichia coli | 0.992j ± 0.0017 | 0.071d ± 0.0023 | 92.82% |
| Pseudomonas aeruginosa | 0.598e ± 0.0030 | 0.089d ± 0.0020 | 85.11% |
| Staphylococcus aureus (MRSA) | 0.645f ± 0.0026 | 0.060c ± 0.0026 | 90.69% |
| Bacillus subtilus | 0.574d ± 0.0023 | 0.098f ± 0.0010 | 82.92% |
| Proteus mirabilis | 0.411a ± 0.0045 | 0.088e ± 0.0047 | 78.58% |
| Salmonella typhi | 0.897i ± 0.0005 | 0.499b ± 0.0001 | 44.37% |
| Proteus vulgaris | 0.708h ± 0.0010 | 0.166h ± 0.0020 | 76.55% |
| Klebsiella pneumoniae | 0.666g ± 0.0030 | 0.313i ± 0.0015 | 53.00% |
| Candida albicans | 0.519c ± 0.0026 | 0.040a ± 0.0025 | 92.29% |
| Candida tropicalis | 0.501b ± 0.0026 | 0.111g ± 0.0040 | 77.84% |
| LSD | 0.01700 | 0.00767 | — |
The synthesized 100 kGy gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite was used to restrain the development of biofilm at its adhesion step (identified as the primary step).69
The variance in the percentage inhibition may be attributed to various circumstances, such as antimicrobial action, biosorption (because of the large surface area of the synthesized nanocomposite), physical characteristics (particle size of the nanocomposite), invasion capabilities and different chemical features controlling the interaction of the nanocomposite and the biofilms.68,70 It was also found that the 100 kGy gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite inhibited E. coli by greater than 98% at 0.024 μg ml−1 MIC (Table 2). When the exopolysaccharide assembly is restricted (the principal molecules for biofilm development), E. coli cannot make a biofilm.36,68
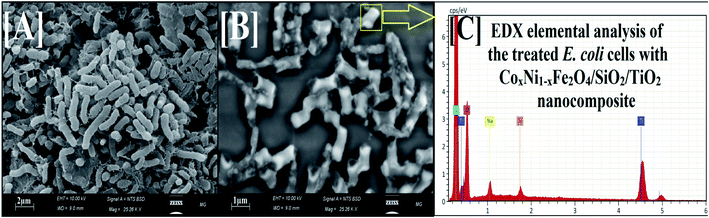
To further explain the antibiofilm abilities of the 100 kGy gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite, we propose a mechanism of action against biofilm production by E. coli from SEM and EDX studies.37,71 The SEM images show the bacterial cell morphologies before and after treatment with 100 kGy gamma irradiation of the prepared nanocomposite. In the control (non-treated bacterial cells), bacterial cultures were normally developed and had definite normal cellular morphology with a normal cell exterior and compressed biofilm production, as displayed in Fig. 8A.
After treatment, morphological modifications were recognized on E. coli cells (Fig. 8). There was an obvious change at the surface, followed by a deformation and decrease in the viable count of E. coli cells. Moreover, biofilm formation was arrested. SEM investigation also showed shrinkage of the bacterial cell walls71 (Fig. 8B), while the EDX elemental study showed the appearance of Ti and Si atoms (atoms of the outer shell of the prepared nanocomposite) at the shrinkage area and the external surface of the tested E. coli cells, confirming the reason for the recorded effect, namely nanocomposite treatment (Fig. 8C).
One probable cause for the reported activity against E. coli could be due to the large surface area (46.13 m2 g−1) enabling better static communication between the negatively charged cell walls of the examined bacteria,72,73 as exhibited in Fig. 8B.
This conclusion is in a reasonable agreement with several published reports confirming the interaction between MO NPs and pathogens through electrostatic power, leading to bacterial membrane separation.72,74,75 Recent research affirms that MO NPs increase the oxidative pressure against pathogens76 and rapidly destroy their cell membranes because of the high level of ROS generated. Additionally, detailed reaction mechanisms for MO NPs against pathogenic bacteria and yeast cells have been described in our previous studies.37,77
Herein, the prepared nanocomposite interacted externally with E. coli cells by means of electrostatic affinity and reduced the bacterial cell counts through membrane leakage.74 The proposed reaction mechanism begins with adhesion of the nanocomposite onto the surface of E. coli. After that, Ti2+ and Si2+ ions (from the outer shell) enter the tested bacterial cell and destroy its biological molecules, such as mitochondria and DNA. Then, cellular toxicity due to oxidative stress through the production of ROS develops. The nanocomposite can certainly block the signal transduction cycle of the examined bacterial cells.78
3.5. Photocatalytic activity of CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite
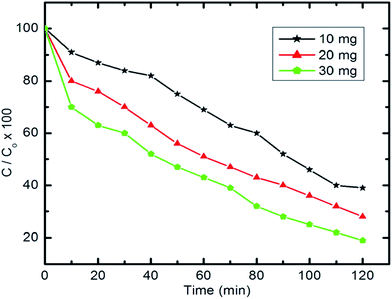
The photocatalytic activity of the synthesized CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite was determined using photocatalytic degradation of pyridine solution (Py) under visible light illumination.Fig. 9 illustrates the decline in absorption with irradiation time for the photocatalytic degradation of pyridine solution (50 ml, 100 mg l−1) using 10 mg of the prepared nanocomposite. With increasing irradiation period, the strong absorption bands of pyridine recorded at 256 nm (indicating the maximum wavelength, λmax, for the pyridine) are continuously reduced. The absorbance of the pyridine solution was reduced by 85% after 100 min of visible light irradiation at the specified experimental conditions.
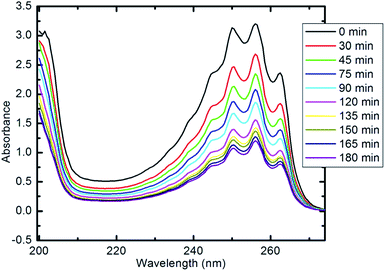 | ||
| Fig. 9 UV-visible spectra of pyridine showing its degradation with time (10 mg of nanocomposite, 50 ml Py solution (100 ppm), 25 °C and pH 7). | ||
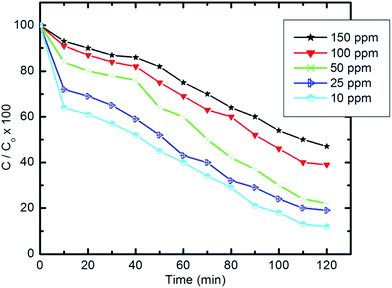 | ||
| Fig. 10 Effect of initial concentration of pyridine solution on the degradation efficiency (10 mg of composite, 50 ml Py solution, 25 °C and pH 7). | ||
Table 4 gives the percentage degradation of pyridine at a range of high initial concentrations (300–1000 ppm) and also indicates the effectiveness of pyridine degradation over this range. Upon increasing the initial concentration from 300 to 1000 ppm, a minor decrease in degradation efficiency was observed. The measured degradation efficiencies exceeded 86%. The pyridine concentration removed from wastewater increases upon increasing the initial concentration without affecting the degradation efficiency.
| Concentration (μg ml−1) | 300 | 500 | 800 | 1000 |
| Degradation percentage (%) | 88.2 | 87.4 | 87.1 | 86.3 |
The degradation rate of pyridine can be calculated using the following equation:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ct/C0 = −Kt Ct/C0 = −Kt
| (2) |
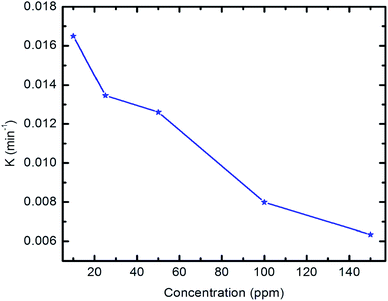
Fig. 11 shows the relationship of −ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ct/C0 vs. time. The results show that the kinetics of the degradation reaction follow the laws of a first-order rate (R2 > 98) at initial concentrations. Moreover, as revealed by Fig. 12, there is an inverse relationship between the apparent first-order rate constants and initial pyridine concentration, which indicates the non-elementary nature of the photocatalytic reaction. This recorded dependence of the reaction rate constants on initial concentration is in a good agreement with the literature.79,80
Ct/C0 vs. time. The results show that the kinetics of the degradation reaction follow the laws of a first-order rate (R2 > 98) at initial concentrations. Moreover, as revealed by Fig. 12, there is an inverse relationship between the apparent first-order rate constants and initial pyridine concentration, which indicates the non-elementary nature of the photocatalytic reaction. This recorded dependence of the reaction rate constants on initial concentration is in a good agreement with the literature.79,80
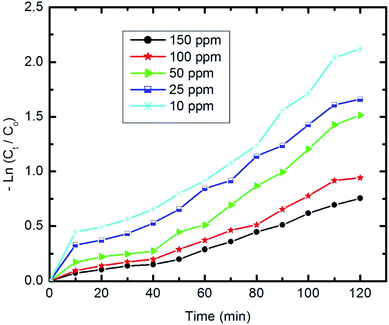 | ||
| Fig. 11 The first-order kinetics of pyridine degradation (10 mg of composite, 50 ml Py solution, 25 °C and pH 7). | ||
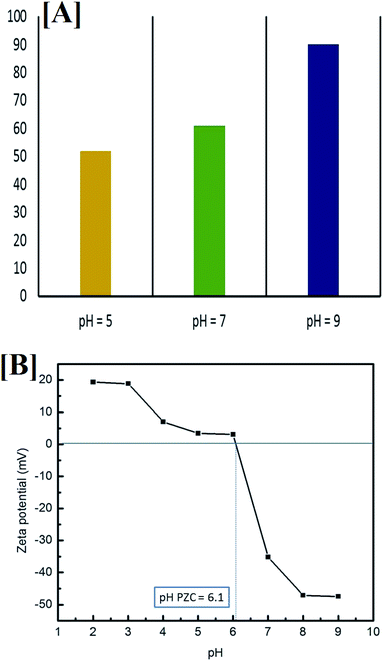
To better understand the relationship between pH and degradation efficiency, the point of zero charge was determined by measuring the zeta potential of the composite at different pH values, from 2 to 10, in the presence of 0.01 M NaCl (electrolyte), as shown in Fig. 14B.
It is clear that when the pH is equal to 7 (neutral media) and when it is more than 7 (alkaline media) the composite exhibited a negative charge (zeta potential = −35.19 mV at pH 7), which stabilized at pH 9 (zeta potential = −47.39). In acidic media (pH less than 7), the composite showed a positive charge, which stabilized at pH 2 (zeta potential = +19.37 mV).
Moreover, the point of zero charge (PZC) was been found to equal 6.1, which is in good agreement with the literature for TiO2 NPs (the outer layer of the composite).82 It was previously reported that at a pH of more than 8, TiO2 NPs are stabilized, with no agglomeration, which is very important for photocatalytic degradation, and this supports the enhanced photocatalytic activity of the prepared composite (with the TiO2 outer layer) at pH = 9.83 Pyridine is a heterocyclic compound containing nitrogen and, with degradation and upon mineralization, the N atom in the ring is released as ammonia, which can be detected easily by its unpleasant odor.84
Generally, it can be proposed that at high values of acidity for the reaction medium, and due to the higher electronegativity of the pyridine N atom compared with the sp2 hybridized C atoms in the ring, ammonium salt develops and is released into the solution, which is more stable and leads to a decrease in degradation efficiency. However, in alkaline medium, the anionic state of pyridine supports the absorption of visible light. In addition, the formation of more hydroxyl radicals from hydroxyl ions (OH− → OH˙) in the solution may lead to improvement in the degradation efficiency.14,85
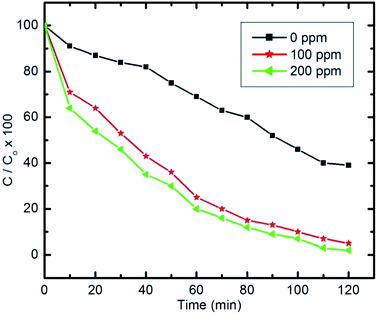 | ||
| Fig. 15 Effect of H2O2 on pyridine degradation (initial concentration of pyridine C0 = 100 ppm, 50 ml, 10 mg of nanocomposite and pH 9). | ||
Herein, experiments were repeated with pyridine solutions containing H2O2 to study its effect on the photocatalytic degradation of pyridine (100 mg l−1 and 50 ml) with H2O2 concentrations of 100 ppm and 200 ppm in the solution.
| H2O2 + visible light → 2˙OH | (3) |
| ˙H + H2O2 → ˙OH + H2O | (4) |
The amount of H2O2 that can be formed by the absorbed visible light is too small to dissociate into significant amounts of ˙OH. Therefore, further quantities of H2O2 are needed to foster the degradation process. Addition of H2O2 will increase and accelerate the generation of ˙OH in two ways; either through self-decomposition due to the absorption of visible light, or by H2O2 reduction at the conduction band, as illustrated in eqn (3) and (4), respectively.14,86
Generally, to improve the photocatalytic removal of organic compounds, several researchers have investigated the effect of adding external oxidants, such as H2O2, to improve the process. Under certain conditions, ˙OH is formed by the ready decomposition of H2O2, as the O–O bond dissociation energy in H2O2 is only 213 kJ mol−1, which is smaller than that of the O–H bond in H2O at 418 kJ mol−1.14 However, after analyzing various studies that suggest the use of H2O2 to improve the degradation rate of organic pollutants, it is clear that the optimal value for H2O2 is strongly dependent on the type of organic compound, the equipment configuration and the operating conditions, all of which were identified as having a direct effect on the production rate of ˙OH.
To sum up, it is worth noting that the H2O2 concentration should not exceed a certain optimal value as it could then recombine with the ˙OH formed, leading to a decrease in the total degradation rate.14,86,87
In the literature on degradation of pyridine and pyridine derivatives there are inconsistencies concerning the reaction mechanism by means of holes or hydroxyl radicals, as shown in Fig. 16. Agrios and Pichat88 suggest that pyridine reacts over TiO2 predominantly via formation of a radical centered on the pyridine ring. Some researchers report that free radicals would be generated by applying visible light simultaneously with oxidants (air bubbling).89,90 Wang et al.91 showed that hydroxyl radicals (˙OH) could be generated indirectly from the application of visible light. Therefore, the degradation relies on the generation of reactive free radicals, especially hydroxyl radicals (˙OH), which is a powerful oxidizing agent having an oxidation potential of 2.33 V, which can undergo rapid and non-selective reaction with most organic and many inorganic solutes.92
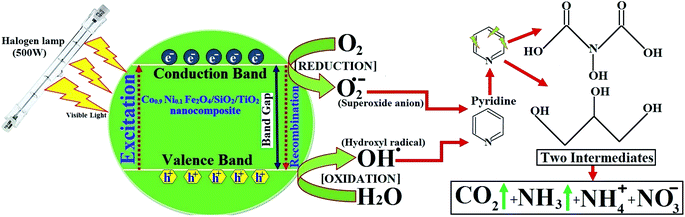 | ||
| Fig. 16 Photocatalyst mechanisms of pyridine using CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite and a possible degraded product. | ||
According to our previous work,14 detection of the pyridine intermediate decomposition product by gas chromatography-mass spectrometry (GC-MS) and high-performance liquid chromatography (HPLC) supports a mechanism by means of holes, as shown in Fig. 16, whereas the increase in the degradation rate on bubbling air (O2) through the solution evidences the important role played by hydroxyl and superoxide anion (˙O2−) radicals.
Pyridine wastewater consists of a large amount of molecular H2O and ammonia. In addition, N–H⋯O and O–H⋯N intermolecular hydrogen bonds can exist in the wastewater.93,94 Molecular H2O and ammonia are both polar molecules and can be polarized by visible light in the presence of H2O2, which causes the dipoles to rotate and line up rapidly (2450 million times per s). Therefore, the frequent pendulum vibration of molecular H2O and ammonia leads to breaking and weakening of the intermolecular hydrogen bond between ammonia and H2O, which is beneficial for escape of the pyridine decomposition product from the liquid phase to the gas phase (Fig. 16).
In order to confirm the degradation of pyridine, different samples were analyzed by HPLC (direct injection) as mentioned in our previous work.14 The results in ref. 14 show the HPLC response of the pyridine sample injection subjected to microwave (MW) radiation for 1 min. The pyridine retention time was 4.435 min. The HPLC results also show the response after 3 min irradiation time for the same sample. The chromatogram shows two other peaks which could be attributed to the pyridine degradation intermediate products according to the above discussion.
Finally, as can be seen in the GC-MS analysis,14 the N atom in the pyridine ring is released as NH3 upon mineralization and then stripped from solution by aeration.94 The ammonium nitrogen (NH3–N) is often monitored as a measure of pyridine degradation.94,95
4 Conclusion
In this work, a recyclable nanocomposite (CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9) was designed and fabricated using a layer-by-layer approach. The crystallinity and the effect of gamma radiation on the crystal size of the prepared nanocomposite were identified by XRD, while the average particle size was measured through HRTEM, which revealed the semi-spherical shape of the concentric nanocomposite. The synthesized CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite possesses good purity, as revealed by elemental EDX analysis. The core–multi-shell structure was evaluated using SEM-EDX mapping techniques. The antimicrobial abilities of the nanocomposite, gamma-irradiated nanocomposite and UV-irradiated nanocomposite were then studied. The whole composite was more powerful in terms of its antimicrobial abilities than its separated layers (separate core and two shells). It was active even at low concentrations (10.0 μg ml−1) against all tested pathogens. Our results show that the gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite was effective against all tested pathogenic microbes as a result of its high surface area and reduction in crystal size due to the gamma rays. The gamma-irradiated nanocomposite exhibited more enhanced antimicrobial abilities than the non-irradiated composite, and this ability increased proportionally with increasing radiation dose (the 100 kGy gamma-irradiated composite was the most active, for example, causing E. coli growth inhibition even at 0.024 μg ml−1 MIC; Table 2). Biofilm examination also revealed that 100 kGy gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite inhibited biofilm formation by 92.82%, 92.29%, and 90.69% against E. coli, C. albicans and S. aureus, respectively. Interestingly, the results for the antimicrobial abilities of UV-irradiated nanocomposite also revealed higher antimicrobial activity of the composite with time, confirming the generation of ROS that can decompose bacterial cells. In addition, SEM-EDX analysis of the treated bacterial cells confirmed the cellular internalization by the nanocomposite in E. coli cells and an observable external cell roughness, with subsequent deformation and a decrease in the viable count of E. coli cells being observed. Finally, the visible light-assisted photocatalytic degradation of pyridine by the prepared nanocomposite was studied taking into account many parameters that can affect its degradation abilities, such as pyridine initial concentration, photocatalyst dose (nanocomposite dose), different pH values and the presence of H2O2. The prepared composite showed promising visible light-assisted photodegradation abilities against pyridine. Our work provides a new nanocomposite-based solution for degradation of toxic pollutants and disinfection of water-borne pathogens, which cause serious diseases, and thus offers a new wastewater treatment technique to overcome the problems of global water shortage and water contamination.Funding
Not applicable.Research involving human participation and/or animals
Not applicable.Informed consent
Not applicable.Ethical approval
Not applicable.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
The authors would like to thank the Nanotechnology Research Unit (P. I. Prof. Dr Ahmed I. El-Batal), Drug Microbiology Laboratory, Drug Radiation Research Department, NCRRT, Egypt, for supporting this study under the project “Nutraceuticals and Functional Foods Production by using Nano/Biotechnological and Irradiation Processes”. The authors would also like to thank the Zeiss microscope team in Cairo for their invaluable advice during this study. Finally, the author M. Abd Elkodous would like to express his deep gratitude to Nile University's vice president for research (Prof. Dr Ahmed Radwan) for his continued support.References
- E. Hill and R. DiSalvo, Environmental Epidemiology, 2019, 3, 159 Search PubMed.
- B. Nath, Resonance, 2019, 24, 575–582 CrossRef.
- M. Forde, R. Izurieta and B. Ôrmeci, Water Quality in the Americas, 2019, vol. 27 Search PubMed.
- P. I. Ritter and R. Sanchez, Prenatal Exposure to Acute Diarrheal Diseases and Childhood Mortality, 2019 Search PubMed.
- K. Ravindra, S. Mor and V. L. Pinnaka, Environ. Sci. Pollut. Res., 2019, 1–11 Search PubMed.
- C. A. Martínez-Huitle and M. Panizza, Curr. Opin. Electrochem., 2018, 11, 62–71 CrossRef.
- M. A. Elkodous, A. Hassaan, A. Ghoneim and Z. Abdeen, Characterization and Application of Nanomaterials, 2018, 1, 1–9 CrossRef.
- R. A. Abramovitch, Pyridine and Its Derivatives, Supplement, John Wiley & Sons, 2009 Search PubMed.
- C. Xu, Q. Xiao, W. N. Leung, P. Wang and H. Lee, Chem. Eng. Commun., 2019, 206(9), 1199–1217 CrossRef.
- B. Bak, L. Hansen-Nygaard and J. Rastrup-Andersen, J. Mol. Spectrosc., 1958, 2, 361–368 CrossRef CAS.
- C. Patel, M. Mohnike, M. C. Hilton and A. McNally, Org. Lett., 2018, 20, 2607–2610 CrossRef CAS PubMed.
- Y. B. Poudel, L. He, S. Gangwar, S. L. Posy and P. Sivaprakasam, US Pat., US10494370B2, 2019.
- N. Gupta, E. J. O'Loughlin and G. K. Sims, in Microbial Metabolism of Xenobiotic Compounds, Springer, 2019, pp. 1–31 Search PubMed.
- O. Zalat and M. Elsayed, J. Environ. Chem. Eng., 2013, 1, 137–143 CrossRef CAS.
- A. K. Choubey and Y. Shukla, J. Water Pollut. Purif. Res., 2019, 6, 24–36 Search PubMed.
- M. Salar-García and V. Ortiz-Martínez, in Advanced Research in Nanosciences for Water Technology, Springer, 2019, pp. 341–362 Search PubMed.
- G. Govindasamy, K. Pal, M. Abd Elkodous, G. S. El-Sayyad, K. Gautam and P. Murugasan, J. Mater. Sci.: Mater. Electron., 2019, 30, 16463–16477 CrossRef CAS.
- C. W. Wong, Y. S. Chan, J. Jeevanandam, K. Pal, M. Bechelany, M. Abd Elkodous and G. S. El-Sayyad, J. Cluster Sci., 2019 DOI:10.1007/s10876-019-01651-3.
- M. Abd Elkodous, G. S. El-Sayyad, M. I. A. Abdel Maksoud, I. Y. Abdelrahman, F. M. Mosallam, M. Gobara and A. I. El-Batal, Biol. Trace Elem. Res., 2019 DOI:10.1007/s12011-019-01894-1.
- J. Jeevanandam, A. Sundaramurthy, V. Sharma, C. Murugan, K. Pal, M. H. Abdel Kodous and M. K. Danquah, in Sustainable Nanoscale Engineering, ed. G. Szekely and A. Livingston, Elsevier, 2020, pp. 83–113, DOI:10.1016/B978-0-12-814681-1.00004-7.
- M. A. Elkodous, G. S. El-Sayyad, H. A. Nasser, A. A. Elshamy, M. Morsi, I. Y. Abdelrahman, A. S. Kodous, F. M. Mosallam, M. Gobara and A. I. El-Batal, J. Cluster Sci., 2019, 30, 531–540 CrossRef.
- A. Meng, L. Zhang, B. Cheng and J. Yu, Adv. Mater., 2019, 1807660 CrossRef PubMed.
- K. Nakata and A. Fujishima, J. Photochem. Photobiol., C, 2012, 13, 169–189 CrossRef CAS.
- M. A. Elkodous, G. S. El-Sayyad, A. E. Mohamed, K. Pal, N. Asthana, F. G. de Souza Junior, F. M. Mosallam, M. Gobara and A. I. El-Batal, J. Mater. Sci.: Mater. Electron., 2019, 30, 8312–8328 CrossRef CAS.
- A. I. El-Batal, G. S. El-Sayyad, A. El-Ghamry, K. M. Agaypi, M. A. Elsayed and M. Gobara, J. Photochem. Photobiol., B, 2017, 173, 120–139 CrossRef CAS PubMed.
- M. S. Attia, G. S. El-Sayyad, S. S. Saleh, N. M. Balabel and A. I. El-Batal, J. Cluster Sci., 2019, 30, 919–935 CrossRef CAS.
- L. Boyanova, G. Gergova, R. Nikolov, S. Derejian, E. Lazarova, N. Katsarov, I. Mitov and Z. Krastev, J. Med. Microbiol., 2005, 54, 481–483 CrossRef PubMed.
- S. Rothenburger, D. Spangler, S. Bhende and D. Burkley, Surg. Infect., 2002, 3, s79–s87 CrossRef PubMed.
- A. El-Batal, B. M. Haroun, A. A. Farrag, A. Baraka and G. S. El-Sayyad, Br. J. Pharm. Res., 2014, 4, 1341 CrossRef.
- A. I. El-Batal, N. E. Al-Hazmi, F. M. Mosallam and G. S. El-Sayyad, Microb. Pathog., 2018, 118, 159–169 CrossRef CAS PubMed.
- A. I. El-Batal, G. S. El-Sayyad, A. El-Ghamery and M. Gobara, J. Cluster Sci., 2017, 28, 1083–1112 CrossRef CAS.
- G. S. El-Sayyad, F. M. Mosallam and A. I. El-Batal, Adv. Powder Technol., 2018, 29, 2616–2625 CrossRef CAS.
- A. I. El-Batal, G. S. El-Sayyad, F. M. Mosallam and R. M. Fathy, J. Cluster Sci., 2019, 31(1), 79–90 CrossRef.
- A. Baraka, S. Dickson, M. Gobara, G. S. El-Sayyad, M. Zorainy, M. I. Awaad, H. Hatem, M. M. Kotb and A. F. Tawfic, Chem. Pap., 2017, 71, 2271–2281 CrossRef CAS.
- G. D. Christensen, W. A. Simpson, A. L. Bisno and E. H. Beachey, Infect. Immun., 1982, 37, 318–326 CrossRef CAS PubMed.
- M. A. Ansari, H. M. Khan, A. A. Khan, S. S. Cameotra and R. Pal, Appl. Nanosci., 2014, 4, 859–868 CrossRef CAS.
- A. I. El-Batal, G. S. El-Sayyad, N. E. Al-Hazmi and M. Gobara, J. Cluster Sci., 2019, 30(4), 947–964 CrossRef CAS.
- G. S. El-Sayyad, H. S. El-Bastawisy, M. Gobara and A. I. El-Batal, Biol. Trace Elem. Res., 2019 DOI:10.1007/s12011-019-01842-z.
- S. H. Abidi, S. K. Sherwani, T. R. Siddiqui, A. Bashir and S. U. Kazmi, BMC Ophthalmol., 2013, 13, 57 CrossRef PubMed.
- T. Mathur, S. Singhal, S. Khan, D. Upadhyay, T. Fatma and A. Rattan, Indian J. Med. Microbiol., 2006, 24, 25 CrossRef CAS PubMed.
- K. F. El-Nemr, H. R. Mohamed, M. A. Ali, R. M. Fathy and A. S. Dhmees, Int. J. Environ. Anal. Chem., 2019, 1–25 Search PubMed.
- K. Brownlee, J. Am. Stat. Assoc., 1952, 47(260), 687–691 CrossRef.
- S. Elbasuney, Y. H. El-Sharkawy, G. S. El-Sayyad and M. Gobara, Talanta, 2020, 211, 120695 CrossRef.
- F. M. Mosallam, G. S. El-Sayyad, R. M. Fathy and A. I. El-Batal, Microb. Pathog., 2018, 122, 108–116 CrossRef CAS PubMed.
- M. A. Maksoud, G. S. El-Sayyad, A. Ashour, A. I. El-Batal, M. S. Abd-Elmonem, H. A. Hendawy, E. Abdel-Khalek, S. Labib, E. Abdeltwab and M. El-Okr, Mater. Sci. Eng., C, 2018, 92, 644–656 CrossRef PubMed.
- A. Ashour, A. I. El-Batal, M. A. Maksoud, G. S. El-Sayyad, S. Labib, E. Abdeltwab and M. El-Okr, Particuology, 2018, 40, 141–151 CrossRef CAS.
- M. A. Maksoud, A. El-ghandour, G. S. El-Sayyad, A. Awed, A. Ashour, A. I. El-Batal, M. Gobara, E. Abdel-Khalek and M. El-Okr, J. Sol-Gel Sci. Technol., 2019, 90, 631–642 CrossRef.
- P. Dobrowolska, A. Krajewska, M. Gajda-Rączka, B. Bartosewicz, P. Nyga and B. Jankiewicz, Materials, 2015, 8, 2849–2862 CrossRef CAS.
- M. A. Maksoud, A. El-ghandour, G. S. El-Sayyad, A. Awed, R. A. Fahim, M. Atta, A. Ashour, A. I. El-Batal, M. Gobara and E. Abdel-Khalek, J. Mater. Sci.: Mater. Electron., 2019, 1–12 Search PubMed.
- M. A. Maksoud, G. S. El-Sayyad, A. Ashour, A. I. El-Batal, M. A. Elsayed, M. Gobara, A. M. El-Khawaga, E. Abdel-Khalek and M. El-Okr, Microb. Pathog., 2019, 127, 144–158 CrossRef CAS PubMed.
- A. I. El-Batal, M. S. Attia, M. M. Nofel and G. S. El-Sayyad, J. Cluster Sci., 2019, 30, 687–705 CrossRef CAS.
- Z.-X. Tang and B.-F. Lv, Braz. J. Chem. Eng., 2014, 31, 591–601 CrossRef.
- M. B. Ali, K. El Maalam, H. El Moussaoui, O. Mounkachi, M. Hamedoun, R. Masrour, E. Hlil and A. Benyoussef, J. Magn. Magn. Mater., 2016, 398, 20–25 CrossRef.
- M. Veena, A. Somashekarappa, G. Shankaramurthy, H. Jayanna and H. Somashekarappa, J. Magn. Magn. Mater., 2016, 419, 375–385 CrossRef CAS.
- A. C. Wright, J. Non-Cryst. Solids, 1990, 123, 129–148 CrossRef CAS.
- R. Mozzi and B. Warren, J. Appl. Crystallogr., 1969, 2, 164–172 CrossRef CAS.
- Q. Mei, C. Benmore and J. Weber, Phys. Rev. Lett., 2007, 98, 057802 CrossRef CAS PubMed.
- Q. Mei, C. Benmore, S. Sen, R. Sharma and J. Yarger, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 144204 CrossRef.
- T. P. Almeida, M. W. Fay, Y. Zhu and P. D. Brown, Phys. E, 2012, 44(6), 1058–1061 CrossRef CAS.
- N. Patil, N. Dweltz and T. Radhakrishnan, Text. Res. J., 1962, 32, 460–471 CrossRef CAS.
- M. I. A. Abdel Maksoud, G. S. El-Sayyad, A. Abokhadra, L. I. Soliman, H. H. El-Bahnasawy and A. H. Ashour, J. Mater. Sci.: Mater. Electron., 2020, 31, 2598–2616 CrossRef.
- K. Ramakanth, Basics of X-ray Diffraction and its Application, IK, New Delhi, 2007 Search PubMed.
- J.-M. Zhang, Y. Zhang, K.-W. Xu and V. Ji, Solid State Commun., 2006, 139, 87–91 CrossRef CAS.
- W. Wang, J. Yu, Q. Xiang and B. Cheng, Appl. Catal., B, 2012, 119–120, 109–116 CrossRef CAS.
- G. Zhang, D. Wang, J. Yan, Y. Xiao, W. Gu and C. Zang, Materials, 2019, 12, 2010 CrossRef PubMed.
- M. Haghi, M. Hekmatafshar, M. B. Janipour, S. Gholizadeh, M. Faraz, F. Sayyadifar and M. Ghaedi, Int. J. Adv. Biotechnol. Res., 2012, 3, 621–624 CAS.
- H. A. Abdel-Rahman, E. H. Awad and R. M. Fathy, J. Compos. Mater., 2019, 54(3), 331–343 CrossRef.
- A. I. El-Batal, N. M. Balabel, M. S. Attia and G. S. El-Sayyad, J. Cluster Sci., 2019 DOI:10.1007/s10876-019-01707-4.
- C. Ashajyothi, K. H. Harish, N. Dubey and R. K. Chandrakanth, J. Nanostruct. Chem., 2016, 6, 329–341 CrossRef CAS.
- H.-J. Park, H. Y. Kim, S. Cha, C. H. Ahn, J. Roh, S. Park, S. Kim, K. Choi, J. Yi and Y. Kim, Chemosphere, 2013, 92, 524–528 CrossRef CAS PubMed.
- S. Priyadarshini, A. Mainal, F. Sonsudin, R. Yahya, A. A. Alyousef and A. Mohammed, Res. Chem. Intermed., 2019, 1–13 Search PubMed.
- A. I. El-Batal, F. M. Mosalam, M. Ghorab, A. Hanora and A. M. Elbarbary, Int. J. Biol. Macromol., 2018, 107, 2298–2311 CrossRef CAS PubMed.
- A. El-Batal, A. El-Baz, F. Abo Mosalam and A. Tayel, J. Chem. Pharm. Res., 2013, 5, 1–15 Search PubMed.
- P. K. Stoimenov, R. L. Klinger, G. L. Marchin and K. J. Klabunde, Langmuir, 2002, 18, 6679–6686 CrossRef CAS.
- A. I. El-Batal, F. M. Mosallam and G. S. El-Sayyad, J. Cluster Sci., 2018, 29(6), 1003–1015 CrossRef CAS.
- M. F. Khan, A. H. Ansari, M. Hameedullah, E. Ahmad, F. M. Husain, Q. Zia, U. Baig, M. R. Zaheer, M. M. Alam and A. M. Khan, Sci. Rep., 2016, 6, 27689 CrossRef CAS PubMed.
- M. Abd Elkodous, G. S. El-Sayyad, I. Y. Abdelrahman, H. S. El-Bastawisy, A. E. Mohamed, F. M. Mosallam, H. A. Nasser, M. Gobara, A. Baraka, M. A. Elsayed and A. I. El-Batal, Colloids Surf., B, 2019, 180, 411–428 CrossRef CAS PubMed.
- G. S. El-Sayyad, F. M. Mosallam, S. S. El-Sayed and A. I. El-Batal, J. Cluster Sci., 2019, 31(1), 147–159 CrossRef.
- D. L. Currell, G. Wilheim and S. Nagy, J. Am. Chem. Soc., 1963, 85, 127–130 CrossRef CAS.
- A. K. Mathur, C. B. Majumder, S. Chatterjee and P. Roy, J. Hazard. Mater., 2008, 157, 335–343 CrossRef CAS PubMed.
- A. G. Agrios and P. Pichat, J. Photochem. Photobiol., A, 2006, 180, 130–135 CrossRef CAS.
- G. A. Parks, Chem. Rev., 1965, 65, 177–198 CrossRef CAS.
- F. Loosli, P. Le Coustumer and S. Stoll, J. Nanopart. Res., 2015, 17, 44 CrossRef.
- K. V. Padoley, A. S. Rajvaidya, T. V. Subbarao and R. A. Pandey, Bioresour. Technol., 2006, 97, 1225–1236 CrossRef CAS PubMed.
- L. Zechmeister and E. F. Magoon, J. Am. Chem. Soc., 1956, 78, 2149–2150 CrossRef CAS.
- D. R. Stapleton, R. J. Emery, D. Mantzavinos and M. Papadaki, Process Saf. Environ. Prot., 2006, 84, 313–316 CrossRef CAS.
- D. R. Stapleton, D. Mantzavinos and M. Papadaki, J. Hazard. Mater., 2007, 146, 640–645 CrossRef CAS PubMed.
- A. G. Agrios and P. Pichat, J. Photochem. Photobiol., A, 2006, 180, 130–135 CrossRef CAS.
- X. Bi, P. Wang, C. Jiao and H. Cao, J. Hazard. Mater., 2009, 168, 895–900 CrossRef CAS PubMed.
- Y. Liu, L. Fang, H. Lu, Y. Li, C. Hu and H. Yu, Appl. Catal., B, 2012, 115, 245–252 CrossRef.
- X. Wang and M. Liu, Appl. Surf. Sci., 2019, 485, 353–360 CrossRef CAS.
- N. Remya and J.-G. Lin, Chem. Eng. J., 2011, 166, 797–813 CrossRef CAS.
- L. Perreux and A. Loupy, Tetrahedron, 2001, 57, 9199–9223 CrossRef CAS.
- M. Hamid, Heat Tran. Eng., 1992, 13, 73–84 CrossRef CAS.
- C. Eskicioglu, N. Terzian, K. J. Kennedy, R. L. Droste and M. Hamoda, Water Res., 2007, 41, 2457–2466 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra10505k |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2020 |