Themed collection FOCUS: Frontiers in Boron Chemistry

Recent advances and prospects for organoboron-based thermally activated delayed fluorescence emitters
The recent research progress of organic boron-containing thermally activated delayed fluorescence (TADF) materials is reviewed systematically with a focus on the molecular design, photophysical properties and performance of the corresponding OLEDs.

Mater. Chem. Front., 2023,7, 4420-4444
https://doi.org/10.1039/D3QM00498H
Recent advances in the chemistry of α-oxylboronate reagents
The synthesis and transformation of α-oxylboronates including their C–B bond and C–O bond functionalizations are reviewed.
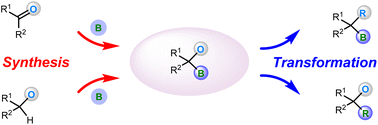
Org. Chem. Front., 2023,10, 3684-3700
https://doi.org/10.1039/D3QO00521F
Electrochemical synthesis and transformation of organoboron compounds
This review highlights the recent advances in both electrochemical borylation and hydroboration to synthesize organoboron compounds and electrochemical transformation of organoboron compounds to construct carbon–carbon and carbon–heteroatom bonds.
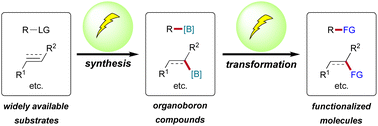
Org. Chem. Front., 2023,10, 3361-3377
https://doi.org/10.1039/D3QO00486D
Self-healing polymer design from dynamic B–O bonds to their emerging applications
In this review, the most recent development in healable polymers with dynamic B–O links is discussed, taking readers through the concept and features of dynamic B–O linkages to explore their advanced functionalities and emerging applications.

Mater. Chem. Front., 2023,7, 381-404
https://doi.org/10.1039/D2QM01128J
Triangularly shaped cyclic defects for the selective boron doping of π-extended systems
Entrapment of boron(III) in π-conjugated systems causes a strong modification of the observed properties, e.g. optical properties can be realized via post-synthetic reaction with 6.6.6 and 7.6.6 defects precisely incorporated into π-extended motifs.

Mater. Chem. Front., 2022,6, 3306-3317
https://doi.org/10.1039/D2QM00549B
Synthesis of (Z)-alkenyl boronates via a copper(I)-catalyzed linear-selective alkylboration of terminal allenes
A copper(I)-catalyzed intermolecular alkylboration of terminal allenes using unactivated alkyl iodides as the carbon electrophiles is reported.

Org. Chem. Front., 2023,10, 4786-4793
https://doi.org/10.1039/D3QO01095C
Copper-catalyzed Markovnikov hydroboration of aliphatic terminal alkenes using carbonyl as a weak directing group
A copper-catalyzed hydroboration of unactivated alkenes using carbonyl as a weak directing group was disclosed with exclusive Markovnikov regioselectivity.

Org. Chem. Front., 2023,10, 4692-4697
https://doi.org/10.1039/D3QO00988B
Visible light induced boryl radical and its application in reduction of unsaturated X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (X = C, N, S) bonds
O (X = C, N, S) bonds
A boryl radical is generated by 4-cyanopyridine and B2cat2 and illustrated a ketyl radical by visible light, furtherly reduced unsaturated double bond X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (X = C, N, S). The reaction mechanism is confirmed by spectroscopy and calculation studies.
O (X = C, N, S). The reaction mechanism is confirmed by spectroscopy and calculation studies.
![Graphical abstract: Visible light induced boryl radical and its application in reduction of unsaturated X [[double bond, length as m-dash]] O (X = C, N, S) bonds](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D3QO00808H&imageInfo.ImageIdentifier.Year=2023)
Org. Chem. Front., 2023,10, 4623-4630
https://doi.org/10.1039/D3QO00808H
Formal [4 + 1] cycloadditions of ketiminoboranes and isonitriles
A formal [4 + 1] cycloaddition reaction of ketiminoboranes with isonitriles is described. This sequential four-component reaction could successfully incorporate HB(C6F5)2, 2,5-dimethylhexa-2,4-diene, nitriles, and isonitriles, into new BN-cycles.
![Graphical abstract: Formal [4 + 1] cycloadditions of ketiminoboranes and isonitriles](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D3QO00695F&imageInfo.ImageIdentifier.Year=2023)
Org. Chem. Front., 2023,10, 3837-3842
https://doi.org/10.1039/D3QO00695F
Sequence-selective three-component reactions of alkyltrifluoroborates with α,β-unsaturated carbonyl compounds and vinylphosphonium salts
A photocatalytic three-component reaction of alkyltrifluoroborates with two electron-deficient alkenes proceeds in a sequence-selective manner, which can be followed by Wittig olefination to afford γ,δ-unsaturated ketones and esters.
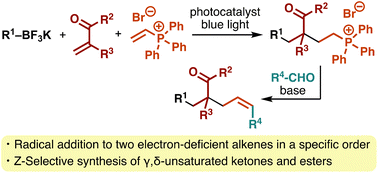
Org. Chem. Front., 2023,10, 3654-3661
https://doi.org/10.1039/D3QO00631J
Synthesis, properties and application of o-carborane-based π-conjugated macrocycles
Two o-carborane-based π-conjugated macrocycles were synthesized. Single-crystal structures revealed their micropore characteristics. These macrocycles exhibit aggregation-induced emission and selective identification.

Org. Chem. Front., 2023,10, 3293-3299
https://doi.org/10.1039/D3QO00487B
Stereoselective synthesis of five- and six-membered carbocycles via Matteson homologation/ring closing metathesis
The Matteson homologation is found to be a versatile tool for the stereoselective synthesis of polyunsaturated alkyl boronic esters, which can be used for the construction of five- and six-membered carbocycles via ring-closing metathesis.

Org. Chem. Front., 2023,10, 2963-2967
https://doi.org/10.1039/D3QO00457K
Crystalline radicals derived from boron-dipyrromethene and its heavier analogues
The first series of radicals derived from boron-dipyrromethene (BODIPY) and its heavier analogues have been isolated and fully characterized.

Org. Chem. Front., 2023,10, 2949-2954
https://doi.org/10.1039/D3QO00218G
Red-shift emission and rapid up-conversion of B,N-containing electroluminescent materials via tuning intramolecular charge transfer
The introduction of different donors at the para-carbon position of the BNCz core modulates the predominance of locally excited (LE)/charger transfer (CT) states of the TADF materials to achieve full-color emission.

Mater. Chem. Front., 2023,7, 2454-2463
https://doi.org/10.1039/D3QM00131H
Hydroxylation of organoborons via uranyl photocatalysis
Aerobic oxidation of organoboron, via uranyl photocatalysis using the principle of indirect single electron transfer, was established under ambient conditions, and afforded multifarious phenols and alcohols, including nine pharmaceuticals.

Org. Chem. Front., 2023,10, 2688-2694
https://doi.org/10.1039/D3QO00468F
Lamellar assembly and nanostructures of amphiphilic boron(III) diketonates through suitable non-covalent interactions
Cooperative assemblies of amphiphilic boron(III) diketonate compounds, which are found to be driven by the formation of non-covalent π–π and hydrophobic interactions in THF–water solution, result in the construction of nanosheet of lamellar packing.

Org. Chem. Front., 2023,10, 1694-1704
https://doi.org/10.1039/D3QO00031A
An alternative approach to triazatruxene synthesis and derivatization to a boron difluoride complex
An alternative method to access 5,10,15-triazatruxene has been developed. The 1,6,11-positions of triazatruxene can further be functionalized to construct a boron difluoride complex with a large Stokes shift.

Org. Chem. Front., 2023,10, 1811-1816
https://doi.org/10.1039/D3QO00144J
In situ pyrazolylborate ligand synthesis and coordination behaviours in aluminum oxo clusters
Presented herein is the discovery of the in situ synthesis of pyrazolylborates. They exhibit great adaptive coordination behaviors in aluminum oxo clusters and further provide a platform for the development of trans-chelating scorpionate analogues.

Inorg. Chem. Front., 2023,10, 1887-1893
https://doi.org/10.1039/D3QI00057E
Fused-ring molecules based on B ← N and imide units with low energy levels
We synthesized n-type polycyclic aromatic hydrocarbons incorporated with both B ← N and imide units for the first time and examined their optical and electrochemical properties.

Org. Chem. Front., 2023,10, 923-927
https://doi.org/10.1039/D2QO01900K
A metal-free protocol for the preparation of amines using ammonia borane under mild conditions
A mild and convenient reductive amination protocol for the synthesis of structurally diverse secondary and tertiary amines as well as N-methylated products under metal-free conditions is presented.
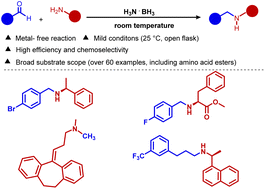
Org. Chem. Front., 2023,10, 970-976
https://doi.org/10.1039/D2QO01938H
Base-promoted synthesis of tetrasubstituted alkenylboronates from propargyl amines and B2pin2via dual 1,4-metallate shift and B–N elimination
An efficient approach to synthesize tetrasubstituted alkenylboronates via a cascade borylation/B–N elimination of propargyl amines and B2pin2 is disclosed.

Org. Chem. Front., 2023,10, 905-910
https://doi.org/10.1039/D2QO01576E
Highly diastereo- and enantioselective copper-catalyzed methylboration of 1,2-dihydroquinolines and 2H-chromenes
A Cu-catalyzed asymmetric methylboration of 1,2-dihydroquinolines, 2H-chromenes and 2H-thiochromenes providing boryl products bearing two adjacent chiral centers with excellent selectivities, up to 99 : 1 dr and 99.9% ee, has been realized for the first time.

Org. Chem. Front., 2023,10, 806-812
https://doi.org/10.1039/D2QO01620F
Highly oxidized U(VI) within the smallest fullerene: gas-phase synthesis and computational study of boron-doped U@C27B
The smallest borafullerene U@C27B has been synthesized using a laser vaporization cluster source. The U atom, placed in the middle of the cage and interacting with all the 28 atoms, is formally described as highly oxidized U(VI).

Inorg. Chem. Front., 2023,10, 908-914
https://doi.org/10.1039/D2QI02160A
Rb3B5O8F2 and K0.6Rb2.4B5O8F2: two new deep-ultraviolet transparent nonlinear optical fluorooxoborates designed by cation regulation
Two new alkali metal fluorooxoborates, Rb3B5O8F2 and K0.6Rb2.4B5O8F2, were successfully obtained by cation regulation, which exhibit a short UV cutoff edge and an appropriate SHG response.

Inorg. Chem. Front., 2023,10, 787-792
https://doi.org/10.1039/D2QI01466A
Photoinduced oxidation of benzylic boronic esters to ketones/aldehydes via α-borylalkyl radicals
The direct conversion from boronic esters to the carbonyl groups has been realized herein, via the bromine radical and α-borylalkyl radical under visible light.
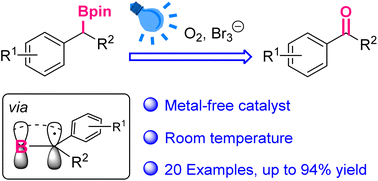
Org. Chem. Front., 2023,10, 104-108
https://doi.org/10.1039/D2QO01457B
Merging dual photoredox/cobalt catalysis and boronic acid (derivatives) activation for the Minisci reaction
Boronic acid (derivatives) activation for the Minisci reaction via dual photoredox/cobalt catalysis in batch and under continuous-flow.

Org. Chem. Front., 2022,9, 6958-6967
https://doi.org/10.1039/D2QO01441F
Amorphous CoB nanoarray as a high-efficiency electrocatalyst for nitrite reduction to ammonia
Amorphous CoB nanoarray is a high-efficiency catalyst for electrocatalytic NO2−-to-NH3 conversion, capable of attaining a large NH3 yield of 233.1 μmol h−1 cm−2 and a high faradaic efficiency of 95.2% at −0.7 V in 0.1 M Na2SO4 with 400 ppm NO2−.

Inorg. Chem. Front., 2022,9, 6075-6079
https://doi.org/10.1039/D2QI01363K
Sc2F2(B2O5): a deep ultraviolet scandium borate fluoride exhibiting large birefringence induced by the synergistic effect of B2O5 and ScOnF2 groups
A rare earth borate fluoride, Sc2F2(B2O5), displays a deep ultraviolet cutoff edge (<200 nm) and very large birefringence induced by the synergistic effect of B2O5 and ScOnF2 groups.

Inorg. Chem. Front., 2022,9, 5153-5160
https://doi.org/10.1039/D2QI01484J
Highly selective electrophilic B(9)-amination of o-carborane driven by HOTf and HFIP
An efficient B(9) electrophilic amination of o-carboranes with azodicarboxylates, promoted by a Brønsted acid and HFIP, was developed.

Org. Chem. Front., 2022,9, 4975-4980
https://doi.org/10.1039/D2QO00732K
Copper-catalyzed synthesis of cyclopropyl bis(boronates) from aryl olefins and carbon monoxide
A copper-catalyzed carbonylation process has been developed for the synthesis of diborylated cyclopropanes from both stable internal and terminal aryl olefins in moderate to good yields.

Org. Chem. Front., 2022,9, 4943-4948
https://doi.org/10.1039/D2QO00976E
Autocatalytic aerobic ipso-hydroxylation of arylboronic acid with Hantzsch ester and Hantzsch pyridine
ipso-Hydroxylation of arylboronic acids with Hantzsch ester has been developed. The by-product Hantzsch pyridine was found to promote the reaction in the presence of oxygen under ambient conditions.

Org. Chem. Front., 2022,9, 4091-4096
https://doi.org/10.1039/D2QO00618A
Coupling BODIPY with nitrogen-doped graphene quantum dots to address the water solubility of photosensitizers
Water-soluble photosensitizers based on covalently grafted nitrogen-doped graphene quantum dot–BODIPY for cellular imaging and photodynamic therapy.
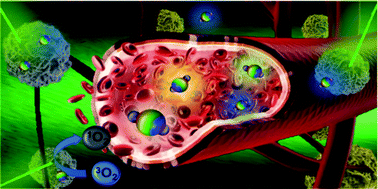
Mater. Chem. Front., 2022,6, 1719-1726
https://doi.org/10.1039/D2QM00200K
Regulation of solid-state dual-emission properties by switching luminescence processes based on a bis-o-carborane-modified anthracene triad
A combination of luminescence processes in dual-emission properties by selecting preparation protocols is designed but also temperature-driven switching of luminescence processes in dual-emission properties based on a bis-o-carborane-substituted anthracene triad in crystals.

Mater. Chem. Front., 2022,6, 1414-1420
https://doi.org/10.1039/D2QM00211F
Visible-light photoredox catalysis-enabled borocyclopropanation of alkenes
A convenient method for the synthesis of cyclopropyl boronates via visible-light photoredox-catalyzed borocyclopropanation of alkenes has been established. This protocol is characterized by its wide substrate scope and mild reaction conditions.
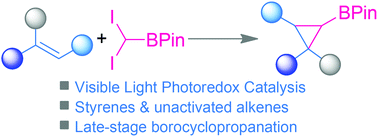
Org. Chem. Front., 2022,9, 2627-2633
https://doi.org/10.1039/D2QO00392A
The Mössbauer effect using 57Fe-ferrabisdicarbollide ([o-57FESAN]−): a glance into the potential of a low-dose approach for glioblastoma radiotherapy
Na[o-57FESAN] with potential for glioblastoma treatment by Mössbauer effect. Mössbauer spectrum and 2D Fe distribution maps indicate that [o-57FESAN]− is present inside U87 cells, a desired target for selective Mössbauer absorption energy deposition.
![Graphical abstract: The Mössbauer effect using 57Fe-ferrabisdicarbollide ([o-57FESAN]−): a glance into the potential of a low-dose approach for glioblastoma radiotherapy](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1QI01513C&imageInfo.ImageIdentifier.Year=2022)
Inorg. Chem. Front., 2022,9, 1490-1503
https://doi.org/10.1039/D1QI01513C
Regioselectivity of Pd-catalyzed o-carborane arylation: a theoretical view
B(3)-Arylation is unfavorable because the steric repulsion between the substituent group on C(2) and the metal moiety would lead to significant distortion of o-carborane and would result in a higher activation energy for reductive elimination.

Org. Chem. Front., 2022,9, 1686-1695
https://doi.org/10.1039/D2QO00046F
Highly dispersed Ru nanoclusters anchored on B,N co-doped carbon nanotubes for water splitting
Ultrafine dispersed ruthenium nanoparticles on B,N-co-doped carbon nanotubes with simultaneously optimized nanostructures and electronic property were developed to achieve a high catalytic activity for both HER and OER.

Inorg. Chem. Front., 2022,9, 968-976
https://doi.org/10.1039/D1QI01672E
Chiral tetracoordinate organoboranes based on double B←N bridged bipyridine with circularly polarized luminescence
Two chiral tetracoordinate organoboranes based on double B←N bridged bipyridine were synthesized via an Et2AlCl-mediated reaction, and they possess excellent circularly polarized luminescence performance.

Mater. Chem. Front., 2022,6, 600-606
https://doi.org/10.1039/D1QM01601F
Origins of regio- and stereoselectivity in Cu-catalyzed alkyne difunctionalization with CO2 and organoboranes
The anti-to-Cu 1,2-migration of alkynyl boronates is critical for the 1,1-E-selective difunctionalization of terminal alkynes with CO2 and organoboranes.

Org. Chem. Front., 2022,9, 1033-1039
https://doi.org/10.1039/D1QO01788H
A nickel/organoboron catalyzed metallaphotoredox platform for C(sp2)–P and C(sp2)–S bond construction
A boron-based organic photocatalyst has been applied in metallaphotoredox catalyzed C–P and C–S bond construction reactions.

Org. Chem. Front., 2022,9, 1070-1076
https://doi.org/10.1039/D1QO01778K
The B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond: some recent developments
C bond: some recent developments
The facile deprotonation of fluoroaryl-substituted alkylboranes or alkylboron cations readily leads to anionic boratalkenes or neutral boraalkenes, respectively. These B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond systems exhibit an increasing variety of chemical reactivities.
C double bond systems exhibit an increasing variety of chemical reactivities.
![Graphical abstract: The B [[double bond, length as m-dash]] C bond: some recent developments](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D3QO00796K&imageInfo.ImageIdentifier.Year=2023)
Org. Chem. Front., 2023,10, 4161-4166
https://doi.org/10.1039/D3QO00796K
A strategy for regioselective B–H functionalization of o-carboranes via base metal catalysis
This frontier article summarizes the recent developments in base metal-catalyzed regioselective cage B–H functionalization of carboranes and discusses the related challenging issues.
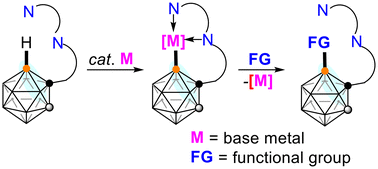
Org. Chem. Front., 2023,10, 3074-3079
https://doi.org/10.1039/D3QO00621B
About this collection
Welcome to the Boron Chemistry focus collection! With its unique electronic and structural properties, boron offers a plethora of opportunities for researchers to develop innovative applications in organometallics, catalysis, materials science, medicinal chemistry, etc. Its versatility has led to the discovery of numerous boron-based compounds with fascinating properties, making boron chemistry a vibrant and rapidly evolving discipline.
This collection highlights research breakthroughs, novel methodologies, and emerging applications in boron chemistry that were published in Frontiers Journals (Organic Chemistry Frontiers, Inorganic Chemistry Frontiers, and Materials Chemistry Frontiers). Articles featured in our focus collections are handpicked by Editors. We hope you find this collection of articles both informative and thought-provoking.