Yan Wang, Yan Gao, Wenjing Guo, Qianyi Zhao, Yan-Na Ma and Xuenian Chen
Org. Chem. Front., 2022,9, 4975-4980
DOI:
10.1039/D2QO00732K,
Research Article
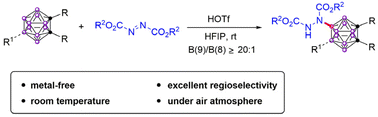
Despite the great importance of nitrogen-containing carboranes in organic and pharmaceutical syntheses, current methods to generate them are very limited and usually rely on the use of expensive transition metals. Herein, an efficient B(9) electrophilic amination of o-carboranes with azodicarboxylates, promoted by a Brønsted acid and HFIP, has been developed. The reaction proceeded smoothly under metal-free conditions and could be easily scaled up, giving B(9)-aminated o-carboranes in moderate to good yields and excellent selectivity under air.