Themed collection Synthetic Approaches to Natural Products via Catalytic Processes

Themed collection: Synthetic approaches to natural products via catalytic processes
Guest editors Louis Fensterbank, Shuanhu Gao and Armen Zakarian introduce this Organic Chemistry Frontiers themed collection entitled “Synthetic approaches to natural products via catalytic processes”.

Org. Chem. Front., 2018,5, 529-530
https://doi.org/10.1039/C8QO90009D
Catalytic approaches to assemble cyclobutane motifs in natural product synthesis
New strategies based on transition-metal catalysis or organocatalysis have provided new perspectives into the total synthesis of cyclobutane-containing natural products.
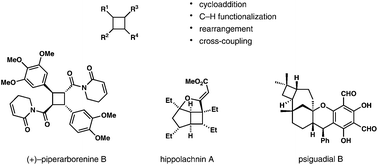
Org. Chem. Front., 2018,5, 254-259
https://doi.org/10.1039/C7QO00668C
Formation of tetrasubstituted C–C double bonds via olefin metathesis: challenges, catalysts, and applications in natural product synthesis
Among the many types of transition-metal-catalysed C–C bond forming reactions, olefin metathesis is without a doubt one of the most thriving fields in modern organic synthetic chemistry.
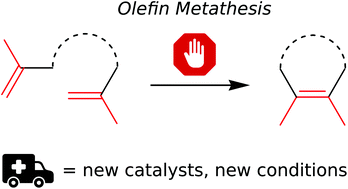
Org. Chem. Front., 2018,5, 494-516
https://doi.org/10.1039/C7QO00800G
Total syntheses of pyrroloazocine indole alkaloids: challenges and reaction discovery
Total syntheses of pyrroloazocine indole alkaloids (lapidilectines, grandilodines, lundurines and tenuisines) are discussed in terms of existing retrosynthetic solutions and novel reaction discoveries.
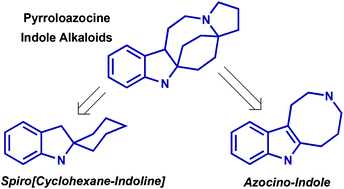
Org. Chem. Front., 2018,5, 273-287
https://doi.org/10.1039/C7QO00786H
Ir-Catalyzed reactions in natural product synthesis
This review highlights the recent applications of Ir-catalyzed reactions in the total synthesis of natural products.
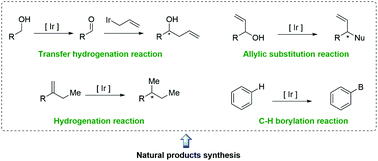
Org. Chem. Front., 2018,5, 132-150
https://doi.org/10.1039/C7QO00665A
Total synthesis of natural products via iridium catalysis
An overview of the highlights in total synthesis of natural products using iridium as a catalyst is given.
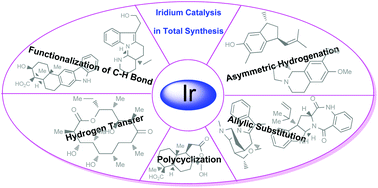
Org. Chem. Front., 2018,5, 106-131
https://doi.org/10.1039/C7QO00664K
Catalytic C–H amination at its limits: challenges and solutions
Pushing C–H amination to its limits fosters innovative synthetic solutions and offers a deeper understanding of the reaction mechanism and scope.
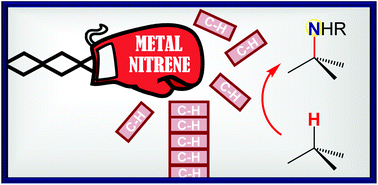
Org. Chem. Front., 2017,4, 2500-2521
https://doi.org/10.1039/C7QO00547D
Metal-mediated C–O bond forming reactions in natural product synthesis
Metal catalyzed reactions for the formation of C(sp2)–O bonds have had a dramatic impact in natural product synthesis. They have enabled the emergence of new bond disconnections, which notably resulted in remarkably efficient and short synthetic pathways. The use of these reactions for the formation of C–O bonds in natural product synthesis is overviewed in this critical review.

Org. Chem. Front., 2017,4, 2480-2499
https://doi.org/10.1039/C7QO00671C
Metal-catalyzed enyne cycloisomerization in natural product total synthesis
Enyne cycloisomerization has become a powerful and attractive strategy for the construction of cyclic compounds, thus possessing great potential for applications in total synthesis of natural products and pharmaceuticals.
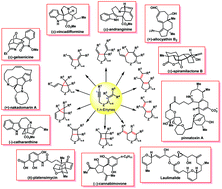
Org. Chem. Front., 2017,4, 2256-2275
https://doi.org/10.1039/C7QO00702G
Application of a rhodium-catalyzed cyclization cycloaddition cascade strategy to the total synthesis of (−)-curcumol
The first de novo total synthesis of (−)-curcumol was accomplished using a rhodium-catalyzed cyclization–cycloaddition cascade reaction as the key step.
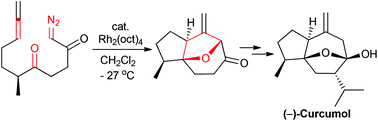
Org. Chem. Front., 2018,5, 1092-1095
https://doi.org/10.1039/C7QO01150D
Approach to pactamycin analogues using rhodium(II)-catalyzed alkene aziridination and C(sp3)–H amination reactions
Application of dirhodium(II)-catalyzed nitrene transfers allows for the preparation of a platform bearing the triamino moiety present in pactamycin.
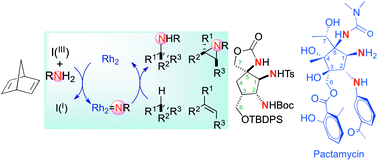
Org. Chem. Front., 2018,5, 948-953
https://doi.org/10.1039/C7QO00878C
Stereospecific C–H activation as a key step for the asymmetric synthesis of various biologically active cyclopropanes
A rapid and efficient synthesis of hoshinolatame, an enantiopure cyclopropane containing natural product, is described.
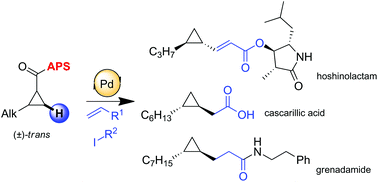
Org. Chem. Front., 2018,5, 409-414
https://doi.org/10.1039/C7QO00737J
Evolution of two routes for asymmetric total synthesis of tetrahydroprotoberberine alkaloids
Two routes were developed for the catalytic asymmetric total synthesis of tetrahydroprotoberberine alkaloids.
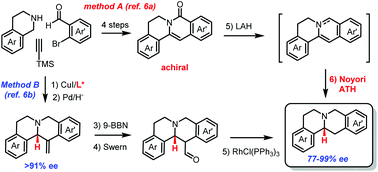
Org. Chem. Front., 2018,5, 242-246
https://doi.org/10.1039/C7QO00776K
Enantioselective synthesis of (−)-phaseic acid
A short, enantioselective synthesis of a newly identified ABA receptor agonist (−)-phaseic acid is described.
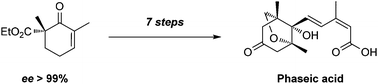
Org. Chem. Front., 2018,5, 29-31
https://doi.org/10.1039/C7QO00653E
Gold-catalyzed diversified synthesis of 3-aminosugar analogues of digitoxin and digoxin
A small library containing 3-aminosugar analogues of digitoxin and digoxin with potent anticancer activities was constructed by gold-catalyzed glycosylation.
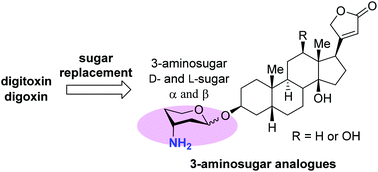
Org. Chem. Front., 2017,4, 2450-2454
https://doi.org/10.1039/C7QO00648A
Harnessing the potential diversity of resinic diterpenes through visible light-induced sensitized oxygenation coupled to Kornblum–DeLaMare and Hock reactions
A biomimetic procedure for the late functionalization of resinic acids is reported, implementing photooxygenation by singlet oxygen, using visible light and a photosensitized, combined to the Kornblum–DeLaMare reaction or the Hock rearrangement.
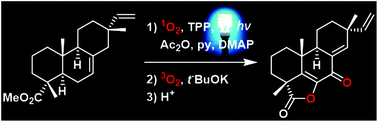
Org. Chem. Front., 2017,4, 2412-2416
https://doi.org/10.1039/C7QO00633K
Synthetic strategies towards mycolactone A/B, an exotoxin secreted by Mycobacterium ulcerans
Pitfalls and dead-ends pave the way to mycolactone A/B. This full account reports synthetic efforts towards this natural product that eventually culminated in a de novo total synthesis.
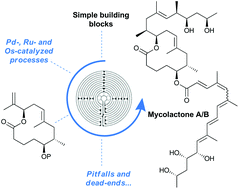
Org. Chem. Front., 2017,4, 2380-2386
https://doi.org/10.1039/C7QO00608J
Total synthesis of orientalol F via gold-catalyzed cycloisomerization of alkynediol
The total synthesis of orientalol F was accomplished in 13 steps using gold-catalyzed tandem cycloisomerization of alkynediol as a key step.
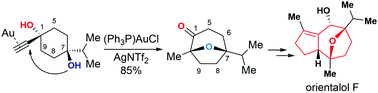
Org. Chem. Front., 2017,4, 2296-2300
https://doi.org/10.1039/C7QO00654C
Rapid construction of the 6/6/5 tricyclic framework via a tandem radical cyclization reaction and its application to the synthesis of 5-epi-7-deoxy-isoabietenin A
A tandem radical cyclization towards the 6/6/5 tricyclic skeleton, which exists in numerous natural products, was developed in modest to good yields.
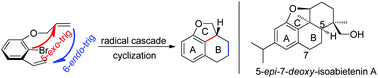
Org. Chem. Front., 2017,4, 2211-2215
https://doi.org/10.1039/C7QO00550D
Photoredox meets gold Lewis acid catalysis in the alkylative semipinacol rearrangement: a photocatalyst with a dark side
The alkylative semipinacol rearrangement of a variety of TMS protected α-styrenyl substituted cyclic alcohols with unactivated bromoalkanes that merge photoredox and Au(I)/Au(III) catalysis has been achieved.
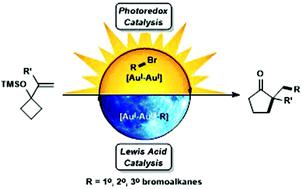
Org. Chem. Front., 2017,4, 2092-2096
https://doi.org/10.1039/C7QO00590C
Concise asymmetric total synthesis of (−)-patchouli alcohol
An asymmetric total synthesis of (−)-patchouli alcohol was realized featuring an enantioselective organocatalytic [4 + 2] approach to the [2.2.2] bicyclic core.
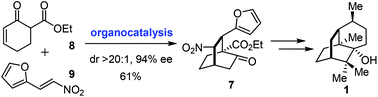
Org. Chem. Front., 2017,4, 2031-2033
https://doi.org/10.1039/C7QO00459A
Gold(I)-catalyzed access to neomerane skeletons
The gold(I) catalyzed cycloisomerization of an enynyl propargylic ester, featuring a 1,2-acyloxy migration/intramolecular cyclopropanation sequence, opens a straightforward access to the 5,7,3-tricyclic skeleton of neomerane sesquiterpenes. The first total synthesis of 5-epi-valeneomerin B in 12 steps with an overall yield of 5.3% is reported.
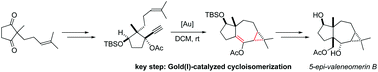
Org. Chem. Front., 2017,4, 1906-1916
https://doi.org/10.1039/C7QO00360A
A concise synthetic approach to parvistemin A and (±)-diperezone
Concise syntheses of parvistemin A and diperezone are achieved using ring expansion of cyclobutenones and oxidative phenolic coupling under basic conditions.
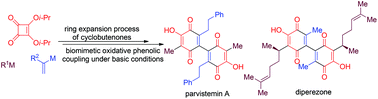
Org. Chem. Front., 2017,4, 1493-1498
https://doi.org/10.1039/C7QO00216E
About this collection
Welcome to Organic Chemistry Frontiers themed collection “Synthetic Approaches to Natural Products via Catalytic Processes”.
Catalysis serves as a powerful tool for chemical bonds transformation, asymmetric induction, and more importantly for achieving increased structural complexity in fewer synthetic steps. Over the past decade, stunning advances in catalytic methods like photoredox catalysis, organocatalysis and transition-metal mediated catalysis etc. have revolutionised the ways in preparing architecturally complex natural products and natural-product-like structures.
This web collection, guest edited by Louis Fensterbank (University Pierre et Marie Curie), Shuanhu Gao (East China Normal University) and Armen Zakarian (UC Santa Barbara), showcases the recent important development of catalytic methods and their successful utilization in natural products syntheses.