Photoredox meets gold Lewis acid catalysis in the alkylative semipinacol rearrangement: a photocatalyst with a dark side†
Abstract
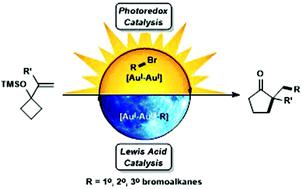
The alkylative semipinacol rearrangement of a variety of TMS protected α-styrenyl substituted cyclic alcohols with unactivated bromoalkanes that merge photoredox and Au(I)/Au(III) catalysis has been achieved. This redox neutral rearrangement is marked by a dimeric Au(I) photocatalyst that plays two roles; photoredox activation of bromoalkanes and Au(III)-mediated semipinacol rearrangement coupled with C(sp3)–C(sp3) reductive elimination; a reaction mode rarely accessed. This operationally simple methodology contains readily available starting materials that undergo reaction with [Au2(dppm)2]Cl2 upon irradiation with UVA LEDs, furnishing diversified ketone products. Primary, secondary, and tertiary bromoalkanes and a range of TMS protected α-styrenyl substituted alcohols were investigated in this transformation.
- This article is part of the themed collections: Synthetic Approaches to Natural Products via Catalytic Processes and Organic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...