Themed collection Mechanistic Aspects of Organic Synthesis

A reevaluation of the origin of the rate acceleration for enzyme-catalyzed hydride transfer
There is no consensus of opinion on the origin of the large rate accelerations observed for enzyme-catalyzed hydride transfer.
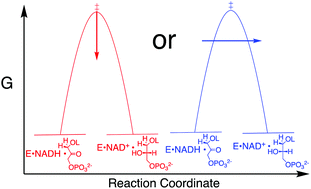
Org. Biomol. Chem., 2017,15, 8856-8866
https://doi.org/10.1039/C7OB01652B
Photochemical generation and trapping of 3-oxacyclohexyne
The strained heterocyclic alkyne, 3-oxacyclohexyne, has been generated for the first time by a photochemical route.
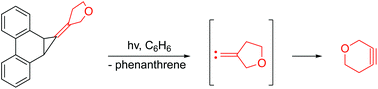
Org. Biomol. Chem., 2017,15, 8270-8275
https://doi.org/10.1039/C7OB01697B
Indole synthesis by palladium-catalyzed tandem allylic isomerization – furan Diels–Alder reaction
4-(Furan-2-ylamino)but-2-en-1-yl acetates are converted to substituted indoles under microwave heating in the presence of catalytic tetrakis(triphenylphosphine)palladium(0) and triisopropylphosphite.
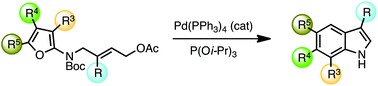
Org. Biomol. Chem., 2017,15, 7093-7096
https://doi.org/10.1039/C7OB01654A
Nucleophile dependent formation of 6- and 7-membered N-heterocycles by platinum-catalysed cyclisation of 1,5-bisallenes
Divergencies unravelled in new Pt-catalysed alkoxy- and hydroxycyclisation of N-tethered 1,5-bisallenes to give vinyl-tetrahydropyridines with alcohols and hydro-1H-azepines with water.
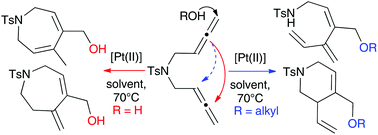
Org. Biomol. Chem., 2017,15, 6731-6737
https://doi.org/10.1039/C7OB01469D
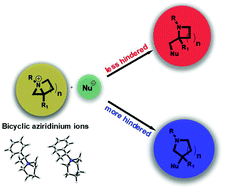
Theoretical insight into the regioselective ring-expansions of bicyclic aziridinium ions
Unraveling the factors affecting the regioselectivity in the ring-expansions of bicyclic aziridiniums.
Org. Biomol. Chem., 2018,16, 796-806
https://doi.org/10.1039/C7OB02253K
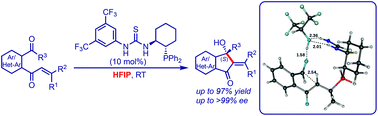
A computational investigation of the solvent-dependent enantioselective intramolecular Morita–Baylis–Hillman reaction of enones
A DFT study of the enantioselective organocatalytic intramolecular Morita–Baylis–Hillman (IMBH) reaction of enones under the influence of 1,1,1,3,3,3-hexafluoroisopropanol is reported. This study establishes an excellent fit between the experiment and theory.
Org. Biomol. Chem., 2017,15, 10212-10220
https://doi.org/10.1039/C7OB02025B
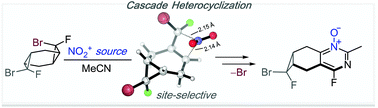
Substituent effects on stereoselectivity of dihalocarbene reactions with cyclohexadiene and on the reactivity of bis-dihalocyclopropanes in electrophilic nitrations en route to pyrimidine N-oxides
Bis-adducts of cyclohexa-1,4-diene with dihalocarbenes were synthesized and investigated in heterocyclization.
Org. Biomol. Chem., 2017,15, 9433-9441
https://doi.org/10.1039/C7OB02463K
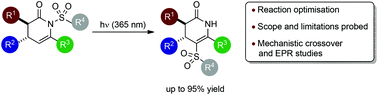
N- to C-sulfonyl photoisomerisation of dihydropyridinones: a synthetic and mechanistic study
The scope and limitations of a photoinitiated N- to C-sulfonyl migration process within a range of dihydropyridinones is assessed.
Org. Biomol. Chem., 2017,15, 8914-8922
https://doi.org/10.1039/C7OB01699A
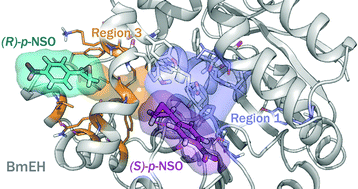
Exploring the origins of selectivity in soluble epoxide hydrolase from Bacillus megaterium
Epoxide hydrolase (EH) enzymes catalyze the hydration of racemic epoxides to yield their corresponding vicinal diols. In this work, the Bacillus megaterium epoxide hydrolase (BmEH)-mediated hydrolysis of racemic styrene oxide (rac-SO) and its para-nitro styrene oxide (rac-p-NSO) derivative are computationally investigated using density functional theory (DFT).
Org. Biomol. Chem., 2017,15, 8827-8835
https://doi.org/10.1039/C7OB01847A
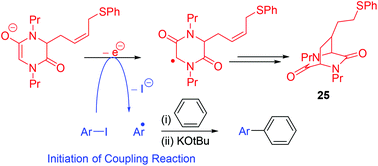
Evidence of single electron transfer from the enolate anion of an N,N′-dialkyldiketopiperazine additive in BHAS coupling reactions
A designed N,N′-dialkyldiketopiperazine (DKP) provides evidence for the role of DKP additives as initiators that act by electron transfer in base-induced homolytic aromatic substitution reactions, involving coupling of haloarenes to arenes.
Org. Biomol. Chem., 2017,15, 8810-8819
https://doi.org/10.1039/C7OB02209C
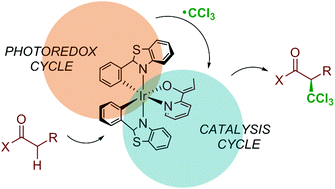
Computational characterization of the mechanism for the light-driven catalytic trichloromethylation of acylpyridines
DFT and DFT/MM calculations are applied to a photocatalytic enantioselective reaction and shown to be able to characterize the mechanism.
Org. Biomol. Chem., 2017,15, 8641-8647
https://doi.org/10.1039/C7OB01826F
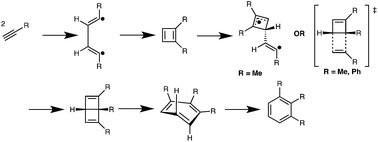
The trimerization of acetylenes involves a cascade of biradical and pericyclic processes
Thorough computational studies were performed on mechanisms and energies for the thermal trimerizations of neutral or electron-rich acetylenes used as cross-linkers in organic hard-masks for lithography applications.
Org. Biomol. Chem., 2017,15, 8326-8333
https://doi.org/10.1039/C7OB01885A
Mechanisms for C(sp2)–Si activation of aryltrimethylsilyl groups in palladium-catalysed couplings
DFT reveals why arenes with an amide or anilide directing group react faster by C–Si than by C–H cleavage in Pd coupling reactions.
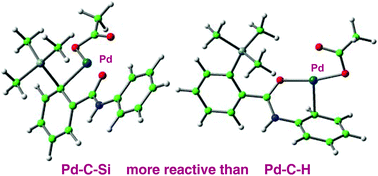
Org. Biomol. Chem., 2017,15, 8179-8185
https://doi.org/10.1039/C7OB01675A
The potential of pnicogen bonding for catalysis – a computational study
Computational investigations reveal that pnicogen bonding, a noncovalent interaction between the electrophilic region of a phosphorus atom and a Lewis base, can activate electrophiles in catalytic reactions.
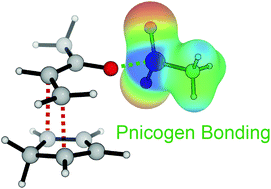
Org. Biomol. Chem., 2017,15, 8037-8045
https://doi.org/10.1039/C7OB01599B
Claisen rearrangements of benzyl vinyl ethers: theoretical investigation of mechanism, substituent effects, and regioselectivity
Theoretical calculations are reported which examine the mechanisms of Claisen rearrangements of benzyl vinyl ethers and the ways in which substituents influence reactivity and regioselectivity.

Org. Biomol. Chem., 2017,15, 7887-7893
https://doi.org/10.1039/C7OB01666B
The role of spin states in the catalytic mechanism of the intra- and extradiol cleavage of catechols by O2
Intradiol vs. extradiol selectivity of Fe(III)-complexes explained by spin-state consistent density functionals.
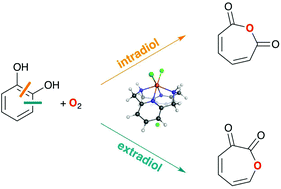
Org. Biomol. Chem., 2017,15, 7860-7868
https://doi.org/10.1039/C7OB01814B
Why can a gold salt react as a base?
A new mass-spectrometry method allows monitoring the key role of the gold–gold interaction in the transformation of gold salts to bases.
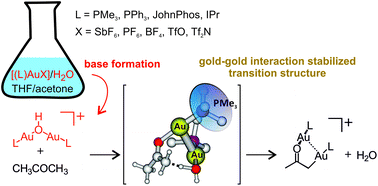
Org. Biomol. Chem., 2017,15, 7841-7852
https://doi.org/10.1039/C7OB01905J
Intramolecular hydrogen bonding in conformationally semi-rigid α-acylmethane derivatives: a theoretical NMR study
Experimental and computational evidence for unusual intramolecular hydrogen-bonding interactions is presented and discussed.
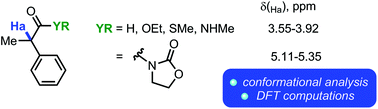
Org. Biomol. Chem., 2017,15, 7572-7579
https://doi.org/10.1039/C7OB01834G
Laser flash photolysis of nanocrystalline α-azido-p-methoxy-acetophenone
Irradiation of nanocrystals of azide 1 results in a solid-to-solid photoreaction that forms imine 2 in high chemical yield.
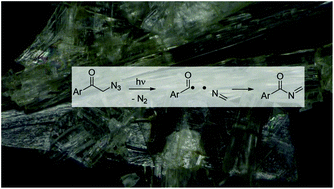
Org. Biomol. Chem., 2017,15, 7380-7386
https://doi.org/10.1039/C7OB01731F
Simulating the reactions of substituted pyridinio-N-phosphonates with pyridine as a model for biological phosphoryl transfer
This work provides a comprehensive model for non-enzymatic phosphoryl transfer, as a baseline for understanding biological phosphoryl transfer reactions.
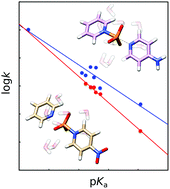
Org. Biomol. Chem., 2017,15, 7308-7316
https://doi.org/10.1039/C7OB01734K
Sigmatropic proton shifts: a quantum chemical study
Insights into [1,j] sigmatropic proton shifts in polyenyl anions and related conjugated systems have been revealed by quantum chemical calculations.
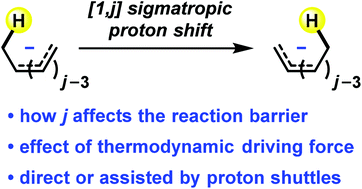
Org. Biomol. Chem., 2017,15, 7439-7446
https://doi.org/10.1039/C7OB01628J
Nitrile-assisted oxidation over oxidative-annulation: Pd-catalyzed α,β-dehydrogenation of α-cinnamyl β-keto nitriles
A palladium-catalyzed oxidation reaction is disclosed where the nitrile functionality on the substrate simply changes the course of the reaction. α-Cinnamyl β-keto nitriles provide α,β,γ,δ-diene containing β-keto nitriles with good substrate scope.
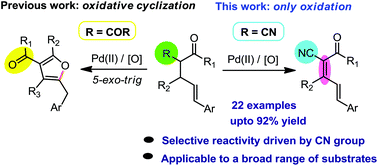
Org. Biomol. Chem., 2017,15, 7317-7320
https://doi.org/10.1039/C7OB00912G
A computational study of the influence of methyl substituents on competitive ring closure to α- and β-lactones
gem-Dimethyl substitution at C3 enhances the preference for β-lactone formation from 2-chlorosuccinate but methylation at C2 almost favours α-lactone.

Org. Biomol. Chem., 2017,15, 7235-7240
https://doi.org/10.1039/C7OB01653K
The mechanism and regioselectivities of (NHC)nickel(II)hydride-catalyzed cycloisomerization of dienes: a computational study
Computation elucidates the mechanism and origins of regioselectivity of (NHC)Ni(II)hydride-catalyzed cycloisomerization of dienes.
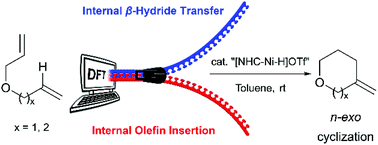
Org. Biomol. Chem., 2017,15, 7131-7139
https://doi.org/10.1039/C7OB01494E
DFT studies on reactions of boroles with carbon monoxide
DFT calculations were performed to study systematically the influence of borole substituents on the reaction of boroles with carbon monoxide.
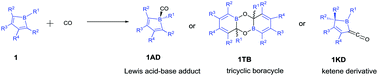
Org. Biomol. Chem., 2017,15, 7019-7027
https://doi.org/10.1039/C7OB01475A
A bis(pyridyl)-N-alkylamine/Cu(I) catalyst system for aerobic alcohol oxidation
Herein a bis(pyridyl)-N-alkylamine/CuI/TEMPO/NMI catalyst system is reported for aerobic oxidation of a variety of primary alcohols to the corresponding aldehydes using readily available reagents, at room temperature and ambient air as the oxidant.
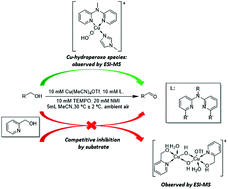
Org. Biomol. Chem., 2017,15, 6926-6933
https://doi.org/10.1039/C7OB01383C
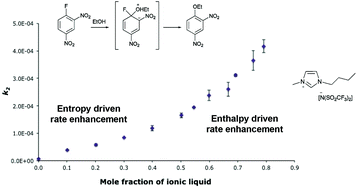
Rationalising the effects of ionic liquids on a nucleophilic aromatic substitution reaction
The nucleophilic aromatic substitution reaction between 1-fluoro-2,4-dinitrobenzene and ethanol was examined in a series of ionic liquids across a range of mole fractions.
Org. Biomol. Chem., 2017,15, 6433-6440
https://doi.org/10.1039/C7OB01476G
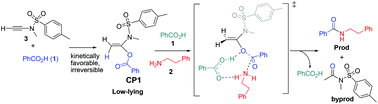
A computational study on the mechanism of ynamide-mediated amide bond formation from carboxylic acids and amines
A detailed computational study is presented on the reaction mechanism of ynamide-mediated condensation of carboxylic acids with amines to produce amides, which elucidates the reactivity pattern of the coupling reagent ynamide and discloses crucial bifunctional catalytic effects of the carboxylic acid substrate during aminolysis.
Org. Biomol. Chem., 2017,15, 6367-6374
https://doi.org/10.1039/C7OB01378G
About this collection
This collection, guest edited by Professor Franziska Schoenebeck (RWT Aachen University) and Dr AnnMarie C O'Donoghue (Durham University) covers the mechanistic aspects of transformations in relation to organic synthesis. This includes metal-based, purely organic, theoretical and catalysis aspects as well as studies in relation to enabling technologies.
Articles in this themed collecton will be added below as soon as possible after they are published.
Please return to this page frequently to see the collection grow.