Indole synthesis by palladium-catalyzed tandem allylic isomerization – furan Diels–Alder reaction†
Abstract
A Pd(0)-catalyzed elimination of an allylic acetate generates a π-allyl complex that is postulated to initiate a novel intramolecular Diels–Alder cycloaddition to a tethered furan (IMDAF). Under the reaction conditions, this convergent, microwave-accelerated cascade process provides substituted indoles in moderate to good yields after Pd-hydride elimination, aromatization by dehydration, and in situ N-Boc cleavage.
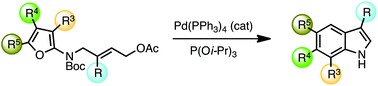
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...