DFT studies on reactions of boroles with carbon monoxide†
Abstract
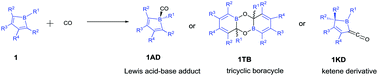
Boroles can react with CO to give Lewis acid–base adduct 1AD, tricyclic boracycle 1TB or ketene derivative 1KD depending on the substituents on the borole. DFT calculations at the M06-2X level of theory were performed to study systematically the influence of borole substituents on these reactions. It was found that the Lewis acid–base adduct 1AD is a kinetic product, which can further transform to the tricyclic boracycle 1TB or ketene derivative 1KD. The computational results show that strong electron-withdrawing perfluorophenyl substituents significantly stabilise the Lewis acid–base adduct 1AD, allowing its successful isolation. In most cases, the tricyclic boracycle 1TB is both kinetically and thermodynamically more favourable than the ketene derivative 1KD. However, a –B(C6F5)2 substituent at the 4-position and a silyl substituent at the 5-position together are able to lower the barrier leading to the formation of the ketene derivative 1KD.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...