A computational study on the mechanism of ynamide-mediated amide bond formation from carboxylic acids and amines†
Abstract
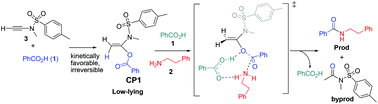
This paper reports a computational study elucidating the reaction mechanism for ynamide-mediated amide bond formation from carboxylic acids and amines. The mechanisms have been studied in detail for ynamide hydrocarboxylation and the subsequent aminolysis of the resulting adduct by an amine. Ynamide hydrocarboxylation is kinetically favorable and thermodynamically irreversible, resulting in the formation of a key low-lying intermediate CP1 featuring geminal vinylic acyloxy and sulfonamide groups. The aminolysis of CP1 by the amine is proposed to be catalyzed by the carboxylic acid itself that imparts favourable bifunctional effects. In the proposed key transition state TSaminolysis-acid-iso2, the amine undergoes direct nucleophilic substitution at the acyl of CP1 to replace the enolate group in a concerted way, which is promoted by secondary hydrogen bonding of carboxylic acid with both the amine and CP1. These secondary interactions are suggested to increase the nucleophilicity of the amine and to activate the Cacyl–O bond to be cleaved, thereby stabilizing the aminolysis transition state. The concerted aminolysis mechanism is competitive with the classic stepwise nucleophilic acyl substitution mechanism that features sequential amine addition to acyl/intramolecular proton transfer/C–O bond cleavage and a key tetrahedral intermediate. Based on the mechanistic model, the carboxylic acid substrate effect and studies of more acidic CF3SO3H as the catalyst are in good agreement with the experimental observations, lending further support for the mechanistic model. The bifunctional catalytic effect of the carboxylic acid substrate may widely play a role in related amide bond-forming reactions and peptide formation chemistry.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...