Themed collection Site-selective molecular transformations

Enzymatic site-selectivity enabled by structure-guided directed evolution
This review covers recent advances in the directed evolution of enzymes for controlling site-selectivity of hydroxylation, amination and chlorination.
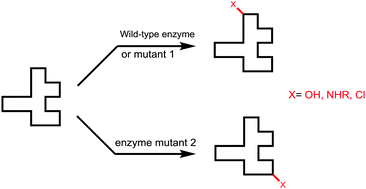
Chem. Commun., 2017,53, 3916-3928
https://doi.org/10.1039/C7CC00368D
Borylated oximes: versatile building blocks for organic synthesis
α-Boryl aldoximes – versatile synthetic intermediates that can be obtained from the corresponding α-boryl aldehydes – give rise to a range of previously inaccessible borylated products.
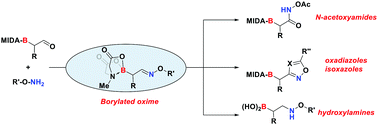
Chem. Commun., 2017,53, 11237-11240
https://doi.org/10.1039/C7CC06579E
Catalyst-controlled site-selective asymmetric epoxidation of nerylamine and geranylamine derivatives
Novel catalysts for site- and enantioselective epoxidation of dienyl amine derivatives have been developed.
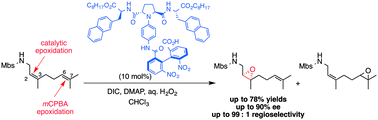
Chem. Commun., 2017,53, 9320-9323
https://doi.org/10.1039/C7CC04809B
Chiral phosphoric acid-catalyzed desymmetrizative glycosylation of 2-deoxystreptamine and its application to aminoglycoside synthesis
This work describes chiral phosphoric acid (CPA)-catalyzed desymmetrizative glycosylation of meso-diol derived from 2-deoxystreptamine.
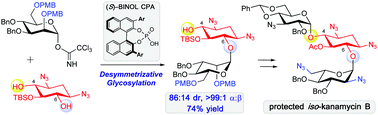
Chem. Commun., 2017,53, 8976-8979
https://doi.org/10.1039/C7CC05052F
Multinuclear zinc bisamidinate catalyzed asymmetric alkylation of α-ketoesters and its unique chemoselectivity
The multinuclear Zn-bisamidinate catalyst exhibited the preferential alkylation of α-ketoesters even in the presence of aldehydes.
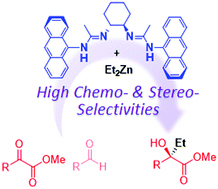
Chem. Commun., 2017,53, 7513-7516
https://doi.org/10.1039/C7CC01736G
Borinic acid-catalyzed stereo- and site-selective synthesis of β-glycosylceramides
Diphenylborinic acid catalysis enables the direct, stereo- and site-selective coupling of glycosyl donors with ceramide lipids. The β-1,1′-linkages accessed through this method are characteristic of mammalian glycosphingolipids that play diverse roles in physiology, human health and disease.
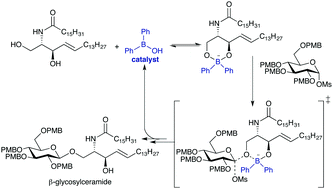
Chem. Commun., 2017,53, 5978-5980
https://doi.org/10.1039/C7CC01673E
Cp*CoIII-catalyzed directed C–H trifluoromethylthiolation of 2-phenylpyridines and 6-arylpurines
Cp*CoIII-catalyzed directed C–H trifluoromethylthiolation using N-trifluoromethylthiodibenzenesulfonimide as an electrophilic SCF3 source is described.
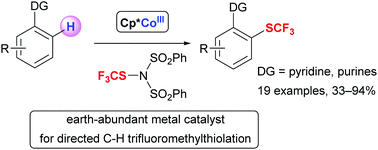
Chem. Commun., 2017,53, 5974-5977
https://doi.org/10.1039/C7CC03072J
Unique site-selectivity control in asymmetric Michael addition of azlactone to alkenyl dienyl ketones enabled by P-spiro chiral iminophosphorane catalysis
Simultaneous recognition of distance and direction of conjugation from carbonyl in alkenyl dienyl ketones enables a highly site-selective asymmetric 1,6-addition.
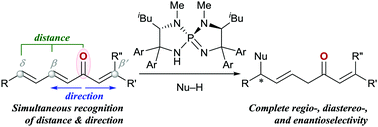
Chem. Commun., 2017,53, 5495-5498
https://doi.org/10.1039/C7CC01715D
Site-selective carbon–carbon bond formation in unprotected monosaccharides using photoredox catalysis
One-step site-selective, protection group-free synthesis of branched monosaccharides.
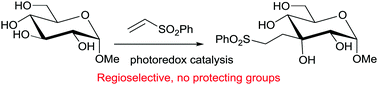
Chem. Commun., 2017,53, 4926-4929
https://doi.org/10.1039/C7CC01416C
Phase-transfer catalyzed asymmetric synthesis of α,β-unsaturated γ,γ-disubstituted γ-lactams
The direct, γ-selective vinylogous Michael addition of unsaturated γ-monosubstituted γ-lactams was realized by using chiral phase-transfer catalysis.
Chem. Commun., 2017,53, 4779-4782
https://doi.org/10.1039/C7CC01058C
Site-selective benzoin-type cyclization of unsymmetrical dialdoses catalyzed by N-heterocyclic carbenes for divergent cyclitol synthesis
A highly site-selective NHC-catalyzed benzoin-type cyclization of unsymmetrical dialdoses was achieved.
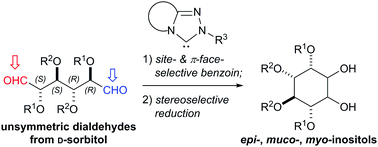
Chem. Commun., 2017,53, 4469-4472
https://doi.org/10.1039/C7CC01191A
Tunable differentiation of tertiary C–H bonds in intramolecular transition metal-catalyzed nitrene transfer reactions
Metal-catalyzed nitrene transfer reactions are an appealing and efficient strategy for accessing tetrasubstituted amines through the direct amination of tertiary C–H bonds.
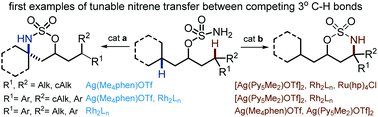
Chem. Commun., 2017,53, 4346-4349
https://doi.org/10.1039/C7CC01235G
Regioselective SN2 reactions for rapid syntheses of azido-inositols by one-pot sequence-specific nucleophilysis
Sequential nucleophilysis of myo-inositol-disulfonate provides easy access to azido-inositols.
Chem. Commun., 2017,53, 3971-3973
https://doi.org/10.1039/C7CC01219E
Scandium(III) triflate-promoted serine/threonine-selective peptide bond cleavage
The site-selective hydrolysis of peptide bonds at Ser and Thr positions was promoted by scandium(III) triflate with a high conversion yield.

Chem. Commun., 2017,53, 3311-3314
https://doi.org/10.1039/C6CC10300F
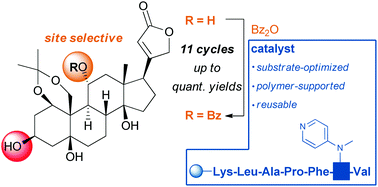
Enhanced site-selectivity in acylation reactions with substrate-optimized catalysts on solid supports
A concept for site-selective acylation is presented, using substrate-optimized DMAP–peptide conjugates on a solid support.
Chem. Commun., 2017,53, 3086-3089
https://doi.org/10.1039/C7CC00655A
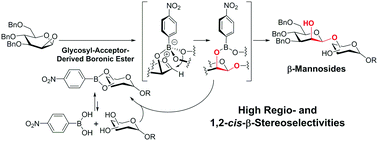
Regio- and stereoselective β-mannosylation using a boronic acid catalyst and its application in the synthesis of a tetrasaccharide repeating unit of lipopolysaccharide derived from E. coli O75
A novel regio- and stereoselective β-mannosylation using 1,2-anhydromannose and a diol sugar acceptor in the presence of a boronic acid catalyst and its application are reported.
Chem. Commun., 2017,53, 3018-3021
https://doi.org/10.1039/C7CC00269F
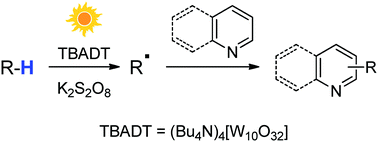
Versatile cross-dehydrogenative coupling of heteroaromatics and hydrogen donors via decatungstate photocatalysis
A facile sunlight-induced derivatization of heteroaromatics via TBADT photocatalyzed C–H functionalization in amides, ethers, alkanes and aldehydes is described.
Chem. Commun., 2017,53, 2335-2338
https://doi.org/10.1039/C6CC09725A
Ascorbate as a pro-oxidant: mild N-terminal modification with vinylboronic acids
The common biocompatible reductant, sodium ascorbate, serves as a “pro-oxidant,” facilitating N-terminal modification with vinylboronic acids in air, with divergent chemoselectivity from copper-catalyzed reactions.
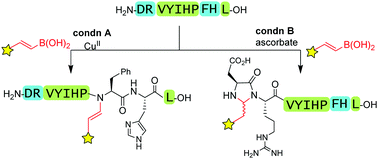
Chem. Commun., 2017,53, 1622-1625
https://doi.org/10.1039/C6CC09955F
Synthesis of a fluorescent photoaffinity probe of OSW-1 by site-selective acylation of an inactive congener and biological evaluation
A novel fluorescent photoaffinity probe of OSW-1 was prepared in two steps by a sequential site-selective acylation strategy using Me2SnCl2.
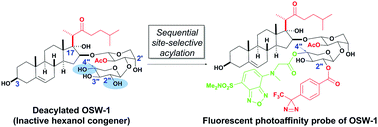
Chem. Commun., 2017,53, 517-520
https://doi.org/10.1039/C6CC08955K
Palladium catalyzed selective distal C–H olefination of biaryl systems
In this report, we disclose the distal C–H olefination of biphenyl systems with high regio- and stereo-selectivity and useful synthetic yields.
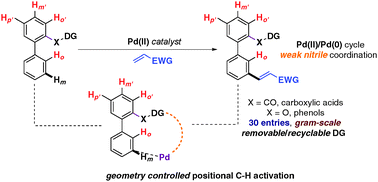
Chem. Commun., 2016,52, 14003-14006
https://doi.org/10.1039/C6CC07861C
Dehydrative condensation of carbonyls with non-acidic methylenes enabled by light: synthesis of benzofurans
Condensation of carbonyls with non-acidic methylenes such as those adjacent to heteroatoms and allylic types to generate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds is challenging but highly desirable.
C bonds is challenging but highly desirable.
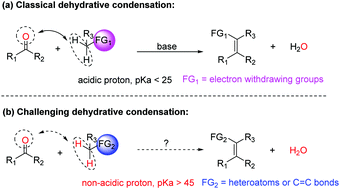
Chem. Commun., 2016,52, 13120-13123
https://doi.org/10.1039/C6CC07626B
About this collection
This web themed collection, guest edited by Takeo Kawabata (Kyoto University) and Mark S. Taylor (University of Toronto) highlights the breadth of ongoing research into the discovery and application of catalytic site-selective molecular transformations illustrating the diverse methods that can be used to achieve site-selectivity in chemical synthesis.
Chemoselectivity and stereoselectivity have been key goals for the development of new synthetic methods. Recently, control of site-selectivity has emerged as an exciting direction for organic chemistry research.