Tunable differentiation of tertiary C–H bonds in intramolecular transition metal-catalyzed nitrene transfer reactions†
Abstract
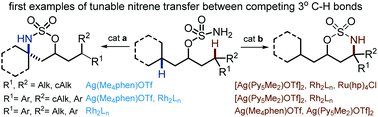
Metal-catalyzed nitrene transfer reactions are an appealing and efficient strategy for accessing tetrasubstituted amines through the direct amination of tertiary C–H bonds. Traditional catalysts for these reactions rely on substrate control to achieve site-selectivity in the C–H amination event; thus, tunability is challenging when competing C–H bonds have similar steric or electronic features. One consequence of this fact is that the impact of catalyst identity on the selectivity in the competitive amination of tertiary C–H bonds has not been well-explored, despite the potential for progress towards predictable and catalyst-controlled C–N bond formation. In this communication, we report investigations into tunable and site-selective nitrene transfers between tertiary C(sp3)–H bonds using a combination of transition metal catalysts, including complexes based on Ag, Mn, Rh and Ru. Particularly striking was the ability to reverse the selectivity of nitrene transfer by a simple change in the identity of the N-donor ligand supporting the Ag(I) complex. The combination of our Ag(I) catalysts with known Rh2(II) complexes expands the scope of successful catalyst-controlled intramolecular nitrene transfer and represents a promising springboard for the future development of intermolecular C–H N-group transfer methods.
- This article is part of the themed collection: Site-selective molecular transformations


 Please wait while we load your content...
Please wait while we load your content...