Site-selective benzoin-type cyclization of unsymmetrical dialdoses catalyzed by N-heterocyclic carbenes for divergent cyclitol synthesis†
Abstract
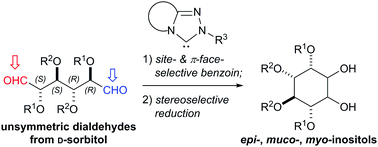
A highly site-selective N-heterocyclic carbene (NHC)-catalyzed benzoin-type cyclization of unsymmetrical dialdoses is developed to enable a divergent cyclitol synthesis. The choice of chiral NHCs and protecting groups affects the site-selectivity. The resulting inososes are converted into epi-, muco- and myo-inositols, and their chiral protected derivatives are formed in good yields.
- This article is part of the themed collection: Site-selective molecular transformations


 Please wait while we load your content...
Please wait while we load your content...