Themed collection Frontiers in Proton Coupled Electron Transfer (PCET)

Electron bifurcation: progress and grand challenges
Electron bifurcation moves electrons from a two-electron donor to reduce two spatially separated one-electron acceptors.
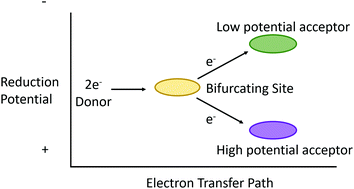
Chem. Commun., 2019,55, 11823-11832
https://doi.org/10.1039/C9CC05611D
Promoting proton coupled electron transfer in redox catalysts through molecular design
Mini-review on using the secondary coordination sphere to facilitate multi-electron, multi-proton catalysis.
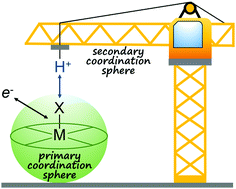
Chem. Commun., 2019,55, 10342-10358
https://doi.org/10.1039/C9CC05139B
Proton-coupled multi-electron transfer and its relevance for artificial photosynthesis and photoredox catalysis
Photoinduced PCET meets catalysis, and the accumulation of multiple redox equivalents is of key importance.
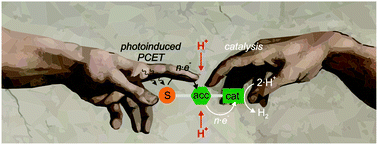
Chem. Commun., 2019,55, 4004-4014
https://doi.org/10.1039/C9CC00821G
Tyrosine, cysteine, and proton coupled electron transfer in a ribonucleotide reductase-inspired beta hairpin maquette
Tyrosine residues act as intermediates in proton coupled electron transfer reactions (PCET) in proteins.
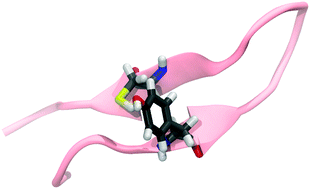
Chem. Commun., 2019,55, 9399-9402
https://doi.org/10.1039/C9CC04067F
Electric field modulated redox-driven protonation and hydration energetics in energy converting enzymes
Biological energy conversion is catalysed by proton-coupled electron transfer (PCET) reactions that form the chemical basis of respiratory and photosynthetic enzymes.
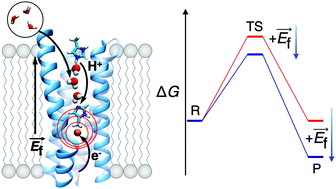
Chem. Commun., 2019,55, 6078-6081
https://doi.org/10.1039/C9CC01135H
Solvent-slaved protein motions accompany proton coupled electron transfer reactions catalysed by copper nitrite reductase
A novel approach to study PCET reactions illustrates the importance of solvent-slaved protein motions in copper nitrite reductase catalysis.
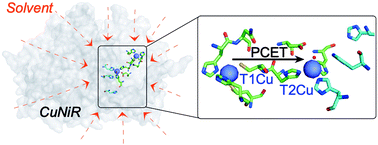
Chem. Commun., 2019,55, 5863-5866
https://doi.org/10.1039/C9CC01026B
Anthracene-based azo dyes for photo-induced proton-coupled electron transfer
Herein, we report a new donor–acceptor system for photo-induced proton-coupled electron transfer (PCET) that leverages an azo linkage as the proton-sensitive component and anthracene as a photo-trigger.
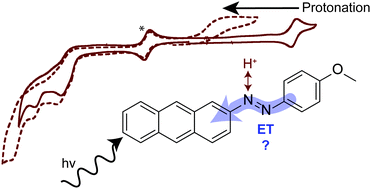
Chem. Commun., 2019,55, 5874-5877
https://doi.org/10.1039/C9CC01206K
Hydrogen bonding between hydroxylic donors and MLCT-excited Ru(bpy)2(bpz)2+ complex: implications for photoinduced electron–proton transfer
Rates of electron–proton transfer within the H-bonded exciplexes are evaluated using the free energy correlation with donor's H-bonding acidity.
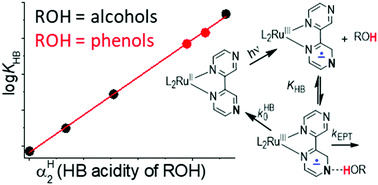
Chem. Commun., 2019,55, 5870-5873
https://doi.org/10.1039/C9CC01896D
Evaluation of excited state bond weakening for ammonia synthesis from a manganese nitride: stepwise proton coupled electron transfer is preferred over hydrogen atom transfer
Concepts for the thermodynamically challenging synthesis of weak N–H bonds by photoinduced proton coupled electron transfer are explored. By harvesting visible light as driving force, ammonia synthesis was achieved and mechanistically elucidated.
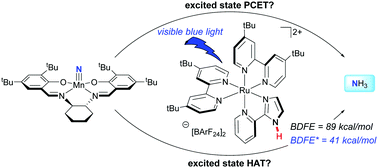
Chem. Commun., 2019,55, 5595-5598
https://doi.org/10.1039/C9CC01046G
Optimizing electron transfer from CdSe QDs to hydrogenase for photocatalytic H2 production
A series of viologen related redox mediators of varying reduction potential has been characterized and their utility as electron shuttles between CdSe quantum dots and hydrogenase enzyme has been demonstrated.
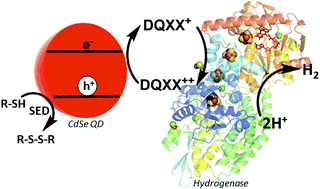
Chem. Commun., 2019,55, 5579-5582
https://doi.org/10.1039/C9CC01150A
Hydrogen atom abstraction by synthetic heme ferric superoxide and hydroperoxide species
To date, artificial dioxygen adducts of heme have not been demonstrated to be able to oxidize organic substrates in sharp contrast to their non-heme analogues and naturally occurring enzymes like heme dioxygenases.
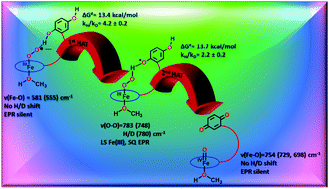
Chem. Commun., 2019,55, 5591-5594
https://doi.org/10.1039/C9CC01423C
Importance of proton-coupled electron transfer in cathodic regeneration of organic hydrides
This communication reports a combined experimental and computational study of mechanisms by which biomimetic NADH analogs can be electrochemically regenerated.
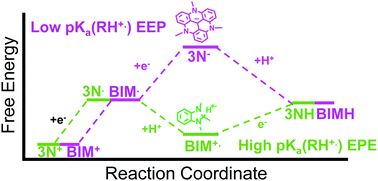
Chem. Commun., 2019,55, 5583-5586
https://doi.org/10.1039/C9CC00928K
pH-Dependent structure of water-exposed surfaces of CdSe quantum dots
Weakly-bound ligands in dynamic exchange expose the surface of CdSe quantum dots to pH-dependent modification.
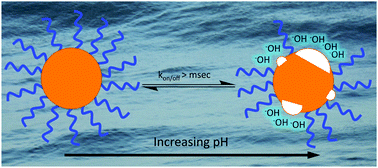
Chem. Commun., 2019,55, 5435-5438
https://doi.org/10.1039/C9CC01339C
Hydrogen atom transfer from 1,2- and 1,3-diols to the cumyloxyl radical. The role of structural effects on metal-ion induced C–H bond deactivation
Strong C–H bond deactivation toward HAT has been observed in the reactions of the cumyloxyl radical with 1,2- and 1,3-diols, following addition of Li+ and Ca2+. Weaker effects have been observed with Mg2+. The role of substrate structure and of the metal ion is discussed.

Chem. Commun., 2019,55, 5227-5230
https://doi.org/10.1039/C9CC01879D
Design and reactivity of pentapyridyl metal complexes for ammonia oxidation
Computational and experimental work shows that Mo pentapyridal complexes can oxidize ammonia in the presence of a chemical mediator and evolve N2.
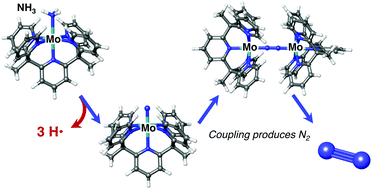
Chem. Commun., 2019,55, 5083-5086
https://doi.org/10.1039/C9CC01249D
Efficient photocatalytic proton-coupled electron-transfer reduction of O2 using a saddle-distorted porphyrin as a photocatalyst
Photocatalytic O2 reduction reactions proceeded efficiently to produce H2O2 using a diprotonated saddle-distorted dodecaphenylporphyrin as a photocatalyst.
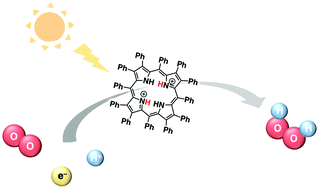
Chem. Commun., 2019,55, 4925-4928
https://doi.org/10.1039/C9CC01547G
Isotopic substitution affects excited state branching in a DNA duplex in aqueous solution
Branching to a multi-site PCET state in a photoexcited DNA duplex is dramatically reduced in H2O compared to D2O.
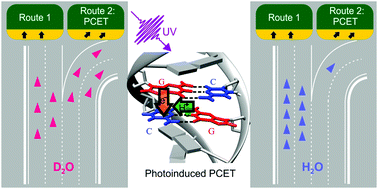
Chem. Commun., 2019,55, 4174-4177
https://doi.org/10.1039/C9CC01105F
Proton-coupled electron transfer oxidation of O–H bond by the N-radical cation of Wurster's blue salt (TMPDA˙+)
Beyond a single electron oxidant, the N-radical cation is also a good PCET reagent.

Chem. Commun., 2019,55, 3465-3468
https://doi.org/10.1039/C9CC00354A
Tuning the reduction potential of quinones by controlling the effects of hydrogen bonding, protonation and proton-coupled electron transfer reactions
An all-organic cell consisting of modified forms of vitamin E and vitamin K exhibited a large cell voltage, which was optimized via the use of diethyl malonate that served as a weak acid and hydrogen bond donor.
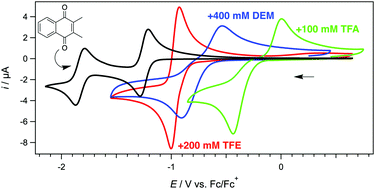
Chem. Commun., 2019,55, 2277-2280
https://doi.org/10.1039/C8CC09188A
Enzyme-like substrate-selectivity in C–H oxidation enabled by recognition
Substrate-selective C–H oxidation: supramolecular recognition enhances the reactivity of the bound substrate and enables its substrate-selective hydroxylation.
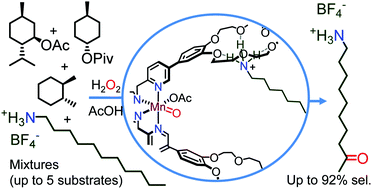
Chem. Commun., 2019,55, 917-920
https://doi.org/10.1039/C8CC09328H
A family of molecular nickel hydrogen evolution catalysts providing tunable overpotentials using ligand-centered proton-coupled electron transfer paths
The overpotential for H2 evolution from water can be rationally controlled by tuning the ligand-centered proton-coupled electron transfer (PCET) processes.
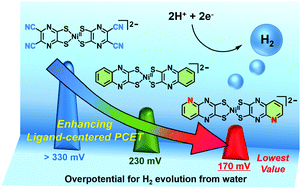
Chem. Commun., 2018,54, 12820-12823
https://doi.org/10.1039/C8CC07467D
About this collection
Proton-coupled electron transfer (PCET) is a class of elementary reactions involving protons and electrons, the wide-spread importance of which has been increasingly recognized during the past two decades. Examples of areas where PCET is intensively discussed include radical proteins, respiration, photosynthesis, inorganic and organic reactions, and solar fuels catalysis.
Our aim in publishing this special issue is to highlight the breadth of ongoing research into the understanding and control of PCET reactions. We anticipate that it will illustrate the diverse areas and problems in chemistry, biology and physics where PCET is central to the discussion.