Hydrogen atom abstraction by synthetic heme ferric superoxide and hydroperoxide species†
Abstract
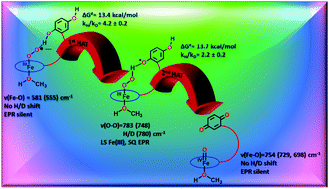
To date, artificial dioxygen adducts of heme have not been demonstrated to be able to oxidize organic substrates in sharp contrast to their non-heme analogues and naturally occurring enzymes like heme dioxygenases. To address this apparent anomaly, an iron porphyrin complex is synthesized which includes a pendant quinol group. The corresponding dioxygen bound iron porphyrin species is demonstrated to perform hydrogen atom transfer (HAT) from the quinol group appended to the porphyrin ligand. The resultant ferric peroxide, formed by the first HAT, performs a 2nd HAT generating a ferryl species (FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and resulting in the 2e−/2H+ oxidation of the pendant hydroquinone to quinone.
O) and resulting in the 2e−/2H+ oxidation of the pendant hydroquinone to quinone.
- This article is part of the themed collection: Frontiers in Proton Coupled Electron Transfer (PCET)


 Please wait while we load your content...
Please wait while we load your content...