Open Access Article
Open Access ArticleAxially chiral thiophene scaffolds: configurational stability and circularly polarized luminescence
Xingyang Li,
Shuai Qiu,
Wan Xu*,
Jia Tang,
Zhiying Ma and
Hua Wang
and
Hua Wang *
*
Institute of Nanoscience and Engineering, Henan University, Kaifeng, 475004, P. R. China. E-mail: 40070005@henu.edu.cn; hwang@henu.edu.cn
First published on 19th January 2026
Abstract
A new type of axially chiral compound, 3,3′-bidithieno[2,3-b:3′,2′-d]thiophene (BDTT), bearing aryl groups (BDTT-Ars) was synthesized and enantiomerically resolved. The racemization of enantiomers showed that their inversion barriers (ΔG) are in the range of 17.32–29.73 kcal mol−1. Among them, BDTT-9An exhibited exceptional circularly polarized luminescence (CPL) properties, achieving a luminescence dissymmetry factor ∣glum∣ up to 10−2. This work provides critical theoretical insights into non-benzenoid axial chirality in novel optoelectronic materials.
Introduction
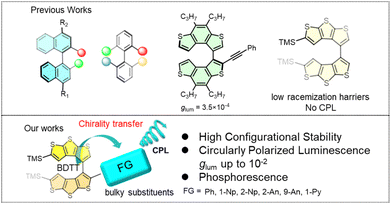
Axially chiral molecules with circularly polarized luminescence (CPL) have attracted significant research interest in the fields of organic chemistry, materials chemistry, polymer chemistry, supramolecular chemistry and bioscience due to their wide use in 3D information displays, biological probes, anti-counterfeiting and so forth.1–5 In recent decades, axially chiral CPL systems have mainly focused on hexacyclic aromatic rings as the chiral source, such as binaphthyl derivatives,6–10 peri-xanthenoxanthene derivatives,11 biphenyl derivatives,12–15, [N–I–N]+-type halogen bonded dimers,16 and C–N axial chirality systems.17 However, the structural diversity and functional extensibility remain limited owing to a heavy reliance on conventional scaffolds.18–24 This limitation has hampered the discovery of novel axially chiral skeletons and the advancement of CPL materials, consequently creating an urgent demand for rational molecular engineering to develop axially chiral systems that combine high-performance CPL with excellent configurational stability.Thiophene derivatives (Fig. 1) are versatile building blocks in materials chemistry and organic synthesis,25,26 yet few axially chiral systems based on them have been realized. A rare example—3,3′-bi(benzo[b]thiophene-S,S-dioxide) derivatives—exhibits optical properties and configurational stability.27 The scarcity of such scaffolds stems from the difficulty in constructing configurationally stable axially chiral structures from five-membered heteroaromatic rings. This challenge is further illustrated by recent axially chiral systems based on azoles,28,29 thiophene,30 and related analogues,31 which show CPL activity but suffer from low dissymmetry factors and low racemization barriers, undermining their long-term stability.
 | ||
| Fig. 1 The reported axial chirality molecules with CPL, and the designed BDTT-based axial chiral compounds (BDTT-Ars): molecular structures with CPL properties. | ||
Thieno[2,3-b:3′,2′-d]thiophene (DTT) is a key building block for thiophene-based [n]helicenes32–34 and organic electronics such as OLEDs35,36 and OFETs.37 Its dimer, BDTT, was previously identified by us as a novel axially chiral scaffold,38,39 but its low racemization barrier has precluded the development of its chiroptical properties, particularly circularly polarized luminescence (CPL). To address this issue, we leveraged the well-defined α-sites of BDTT to install aryl substituents of varying sizes (Fig. 1). This molecular design serves a dual purpose: sterically enhancing the configurational stability by modulating the atropisomerism energy barrier, and introducing high-fluorescence quantum yield fluorophores to transfer the axial chirality into CPL emission. Herein, we report the design and synthesis of this BDTT series, successfully granting them attractive CPL properties.
Results and discussion
Construction of BDTT-Ars
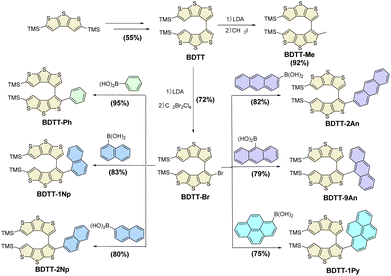
The synthetic route to the target BDTT-based axially chiral compounds (BDTT-Ars) is delineated in Scheme 1. The axially chiral precursor BDTT was synthesized via Li/Br exchange of (4-bromodithieno[2,3-b:3′,2′-d]thiophen-2-yl)trimethylsilane-(BDTT) and subsequent oxidation with CuCl2. BDTT was deprotonated by LDA and then quenched with C2Br2Cl4 to give (2-bromo-[3,3′-bidithieno[2,3-b:3′,2′-d]thiophene]-5,5′-diyl)bis(trimethylsilane) (BDTT-Br) in 85% yield. After intermolecular Suzuki coupling reactions for arylation between BDTT-Br and Ar-Bpin, the target products BDTT-Ph, BDTT-1Np, BDTT-2Np, BDTT-2An, BDTT-9An and BDTT-1Py were generated with yields of 95%, 83%, 80%, 82%, 73% and 75%, respectively. The relatively low yields of BDTT-9An and BDTT-1Py are attributed to the reduced coupling efficiency caused by their significant steric hindrance. The molecular structures of all target compounds, BDTT-Ars are confirmed by 1H NMR, 13C NMR, HRMS and IR spectra (see the SI).Spectroscopic features and electrochemical properties
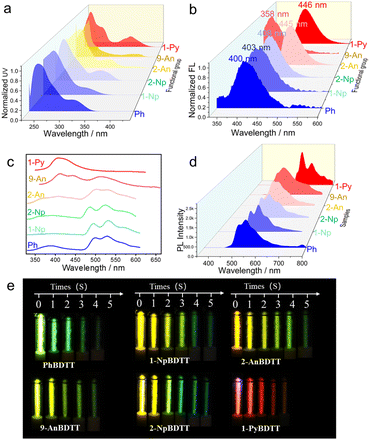
The UV-Vis absorption spectra of BDTT-Me and all BDTT-Ars exhibit two major absorption bands within 220–300 nm (band I) and 310–400 nm (band II). Gradual substitution of the α-H of BDTT with functional groups of different sizes in BDTT-Ars from methyl to pyrene leads to a modest shift in the main absorption peaks located at 313, 314, 319, 328, 329, 380 and 352 nm, respectively (Fig. 2a, and S40). This phenomenon is attributed to the enhanced conjugation of the axially chiral molecule with increasing size of the functional substituent, and the absorption peaks in band II are assigned to HOMO → LUMO transitions. Cyclic voltammetry (CV) behaviors of BDTT-Ars showed only one reversible oxidation wave at = +1.38 V for BDTT-Ph,
= +1.38 V for BDTT-Ph,  = +1.32 V for BDTT-9An and
= +1.32 V for BDTT-9An and  = +1.58 V for BDTT-1Py, while BDTT-1Np, BDTT-2Np and BDTT-2An showed two reversible oxidation waves at
= +1.58 V for BDTT-1Py, while BDTT-1Np, BDTT-2Np and BDTT-2An showed two reversible oxidation waves at  = +1.37 V and
= +1.37 V and  = +1.62 V for BDTT-1Np,
= +1.62 V for BDTT-1Np,  = +1.34 V and
= +1.34 V and  = +1.67 V for BDTT-2Np and
= +1.67 V for BDTT-2Np and  = +1.24 V and
= +1.24 V and  = +1.58 V for BDTT-2An (vs. Fc/Fc+, Fig. S15). Based on the first oxidation potentials, the HOMO energy levels were calculated to be −5.70 eV for BDTT-Ph, −5.68 eV for BDTT-1Np, −5.62 eV for BDTT-2Np, −5.53 eV for BDTT-2An, −5.50 eV for BDTT-9An, and −5.45 eV for BDTT-1Py (Table S15). With gradually increasing HOMO levels, the optical band gaps gradually narrow. Quantum chemistry calculations were employed to further predict the energy levels and electron-cloud distributions of the HOMO and LUMO orbitals for BDTT-Ars. As presented in Fig. S45, BDTT-Ars possess similar HOMO and LUMO distributions, with the HOMOs mainly distributed over the aromatic functional group and a small portion on the DTTs, while the LUMOs are distributed over the aromatic functional group. The calculated HOMO and LUMO levels are consistent with the experimental values.
= +1.58 V for BDTT-2An (vs. Fc/Fc+, Fig. S15). Based on the first oxidation potentials, the HOMO energy levels were calculated to be −5.70 eV for BDTT-Ph, −5.68 eV for BDTT-1Np, −5.62 eV for BDTT-2Np, −5.53 eV for BDTT-2An, −5.50 eV for BDTT-9An, and −5.45 eV for BDTT-1Py (Table S15). With gradually increasing HOMO levels, the optical band gaps gradually narrow. Quantum chemistry calculations were employed to further predict the energy levels and electron-cloud distributions of the HOMO and LUMO orbitals for BDTT-Ars. As presented in Fig. S45, BDTT-Ars possess similar HOMO and LUMO distributions, with the HOMOs mainly distributed over the aromatic functional group and a small portion on the DTTs, while the LUMOs are distributed over the aromatic functional group. The calculated HOMO and LUMO levels are consistent with the experimental values.
The FL spectra of BDTT-Ars are shown in Fig. 2b. As anticipated, BDTT, BDTT-Br, and BDTT-Me exhibit fluorescence emission that is too weak to be detected due to the heavy-atom effect of sulfur atoms. However, BDTT-Ars emit intense blue emission in degassed dilute dichloromethane (DCM) solution. Their emission peaks are located at 400 (Φ = 0.03%), 403 (Φ = 0.06%), 408 (Φ = 0.32%), 445 (Φ = 0.47%), 358 (Φ = 0.19%) and 446 nm (Φ = 0.59%) for BDTT-Ph, BDTT-1Np, BDTT-2Np, BDTT-2An, BDTT-9An and BDTT-1Py in DCM solution respectively, in line with the trend in λabs (Fig. 2a). In the solid state, with the gradual increase of the Ar size, the fluorescence quantum yields of BDTT-Ars increased due to the increase of intermolecular interactions among Ar moieties.
It is noteworthy that BDTT-9An exhibits the bluest emission, which can be attributed to the poor conjugation induced by the 9-substituted anthracene and the DTT moiety with a large dihedral angle between them. Moreover, the fluorescence quantum yield increases with the extension of substituent conjugation, demonstrating that BDTT-Ars can serve as excellent candidates for CPL materials. As previously reported in our work,32 the heavy atomic effect of sulfur atoms can promote the decay of intersystem crossing (ISC), resulting in more efficient phosphorescence intensity. However, no phosphorescence emission phenomena were observed in the solid powder of BDTT-Ars due to their high non-radiative rate (Table S15). Fortunately, we observed dual emission peaks of BDTT-Ars in 2-methyltetrahydrofuran (2-MTHF) at 77 K. The emission peaks in the short-wavelength region correspond to their local fluorescence emission, while the long-wavelength emission peaks (541, 543, 538, 546, 528, and 656 nm for BDTT-Ph, BDTT-1Np, BDTT-2Np, BDTT-2An, BDTT-9An and BDTT-1Py, respectively) were tentatively assigned to their phosphorescence emission (Fig. 2c). To verify this hypothesis, a 0.5 ms gated detection was applied in the detection of the samples. It was observed that the short-wavelength peaks disappeared, while the long-wavelength peaks remained (Fig. 2d), confirming their long-lived nature. Further lifetime measurements of the long-wavelength emission peak revealed lifetimes in the millisecond range (Fig. S47 in SI). Additionally, all samples exhibited an afterglow phenomenon lasting several seconds at 77 K (Fig. 2e). Theoretical calculations further confirm the existence of an efficient intersystem crossing (ISC) pathway between the singlet and triplet states in the BDTT-Ar molecules (Fig. S48). These experimental results demonstrate that the sulfur atoms in BDTT-Ars can facilitate the ISC process, showing significant potential as phosphorescent materials.
Resolution of BDTT-Ars and barrier for racemization
Racemates BDTT-Ars were successfully resolved into the corresponding enantiomers by using HPLC on a chiral stationary phase column (Fig. S22–S43). The absolute configurations of these enantiomers were determined through comparison of the experimental CD spectra (Fig. 4). We performed the racemization process of enantiomers BDTT-Ars in 1,2-dichlorobenzene by heating the enantiomers in a Schlenk tube at different temperatures. The process was monitored by chiral HPLC (CHIRALPAK®IB) to obtain the changes in ee values. The half-life times were measured from the plot of ln[(ee + 1)/2] vs. time, respectively. As shown in Fig. 3 and Table 1, clear linear relationships were observed in all of these plots, indicating that the racemization proceeds via a first order reaction.40 Based on these plots, the activation free energy (ΔG), heat content (ΔH) and entropy (ΔS) of racemization were calculated as shown in Table 2. Based on the above results, it can be observed that as the size of the functional groups gradually increases, the racemization barrier of BDTT-Ars progressively rises. Compared to other five-membered biaryl axially chiral compounds (ΔG = 23.18 kcal mol−1), namely 3,3′-bibenzo[1,2-b:4,3-b′]dithiophenes,27 BDTT-Ars exhibit superior conformational stability, comparable to that of BINOL (ΔG = 23.9 kcal mol−1). The above experimental results demonstrate that BDTT-Ars possess good chiral stability and can serve as a non-benzenoid axially chiral scaffold for constructing chiral materials.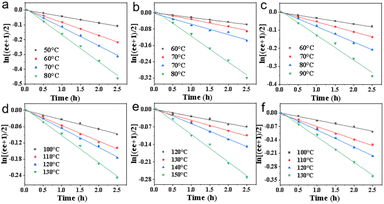 | ||
| Fig. 3 Kinetic measurements of the racemization of BDTT-Ars (a–f: BDTT-Ph, BDTT-1Np, BDTT-2Np, BDTT-2An, BDTT-9An and BDTT-1Py, respectively). | ||
| Compound | ΔH/kcal mol−1 | ΔS/J k−1 | ΔG/kcal mol−1 |
|---|---|---|---|
| BDTT-Me | 14.68 | 14.67 | 13.72 |
| BDTT-Ph | 17.47 | 2.31 | 17.32 |
| BDTT-1Np | 23.24 | 6.77 | 22.8 |
| BDTT-2Np | 21.26 | 6.9 | 20.9 |
| BDTT-2An | 24.96 | 1.92 | 24.83 |
| BDTT-9An | 30.26 | 8.18 | 29.73 |
| BDTT-1Py | 28.13 | 8.36 | 27.58 |
| Compound | λ FL (nm) | λ PL (nm) | ΦF (%) | τF (ns) | τP (ms) | [α]D25 | |gabs| | |glum| |
|---|---|---|---|---|---|---|---|---|
| BDTT-Me | — | — | — | — | — | −173.1° + 155.2° | 1.3 × 10−4 | — |
| BDTT-Ph | 401 | 504, 534 | 0.03 | 0.87 | 10 | −675.0° + 692.8° | 1.4 × 10−4 | 1.2 × 10−3 |
| BDTT-1Np | 421 | 506, 542 | 0.06 | 2.1 | 8 | −897.3° + 842.5° | 3.0 × 10−4 | 7.9 × 10−3 |
| BDTT-2Np | 416 | 512, 548 | 0.32 | 2.7 | 4 | −770.4° + 742.6° | 2.6 × 10−4 | 1.9 × 10−3 |
| BDTT-2An | 432 | 528, 552 | 0.47 | 6.3 | 110 | −1035.8° + 1002.2° | 4.4 × 10−4 | 2.7 × 10−3 |
| BDTT-9An | 447 | 544 | 0.19 | 1.7 | 26 | −1185.6° + 1180.2° | 2.3 × 10−3 | 1.1 × 10−2 |
| BDTT-1Py | 446 | 614, 672 | 0.59 | 7.2 | 75 | −1205.3° + 1242.1° | 9.7 × 10−4 | 6.0 × 10−3 |
CD and CPL properties
The CD and CPL spectra of the enantiomers BDTT-Ars are depicted in Fig. 4. Enantiomerically pure BDTT-Ars exhibit obvious Cotton effects in the range of 220 to 400 nm, with the maximum positive Cotton effects peaking at 250 nm for BDTT-Ph, 227 nm for BDTT-1Np, 273 nm for BDTT-2Np, 249 nm for BDTT-2An, 252 nm for BDTT-9An, and 231 nm for BDTT-1Py, respectively, with perfect mirror–image profiles in the CD spectra (Fig. 4a and Table 2). The CPL spectra of (+)-BDTT-Ars and (−)-BDTT-Ars showed perfect mirror image signals (Fig. 4b). As shown in Table 2, the luminescence dissymmetry factors (glum) of enantiomers BDTT-Ars are listed. From the data in Table 2, we found that different substitution positions of the same substituent can lead to varying asymmetric factors in the enantiomers, for instance, gBDTT-1Np > gBDTT-2Np and gBDTT-9An > gBDTT-2An. According to literature reports,41 the dihedral angle of axially chiral molecules critically influences the asymmetric factor. Therefore, we attribute the observed differences to changes in the dihedral angles of BDTT-Ars and the dipole moments of electric and magnetic transitions, caused by the steric effects of substituents at different positions. Among them, BDTT-9An, owing to its strongest steric hindrance, exhibits the highest asymmetric factor with glum up to 1.1 × 10−2 in BDTT-Ars. These results demonstrate that efficient chirality transfer occurred in the molecules of BDTT-Ars from the axially chiral BDTT moieties to the aryl moieties with high-fluorescence quantum yields and high performance CPL.42,43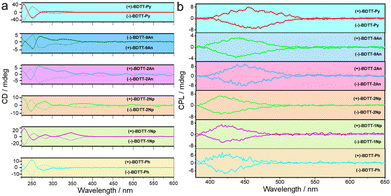 | ||
| Fig. 4 Chiroptical properties. (a) CD and (b) CPL spectra of enantiomers BDTT-Ars in DCM ([C] = 1 × 10−5 M). | ||
Conclusions
In summary, the 3,3′-bidithieno[2,3-b:3′,2′-d]thiophene (BDTT) based axially chiral compounds (BDTT-Ars) were successfully synthesized through oxidative coupling reaction and arylation. Incorporating size-varied aryl moieties into the axially chiral BDTT skeleton modulates their conformational stability, resulting in an increase in the racemization barrier with the increasing size of aryl substituents. Among them, BDTT-9An exhibits the highest racemization barrier of up to 29.73 kcal mol−1, which is higher than that of BINOL (23.9 kcal mol−1). Critically, the axially chiral BDTT scaffold enables efficient chirality transfer to functional substituents, conferring circularly polarized luminescence. And BDTT-9An exhibits the highest luminescence dissymmetry factor, reaching the order of 10−2 in solution. The chirality of BDTT-Ars may be amplified in chiral supramolecular assemblies44 or co-assemblies via fluorescence resonance energy transfer (FRET) processes.45 Simultaneously, BDTT-Ars demonstrate attractive phosphorescence emission at 77 K due to the heavy-atom effect of sulfur atoms in thiophene units. Collectively, this work represents the first systematic investigation of the chiroptical behaviors of BDTT-Ars as a new axially chiral scaffold, providing critical theoretical insights into axial chirality in novel optoelectronic materials. And it also opens up intriguing possibilities for exploring the chiral stability and circularly polarized luminescence properties of BDTT upon functionalization at the two α-positions. Relevant studies are currently underway.Author contributions
H. W. conceived the idea for this project. X. Y. L. performed the experiments. Z. Y. M., S. Q. and T. J. analyzed the data and theoretical calculations, and produced the artwork under the guidance of W. X. and H. W. All authors contributed to the manuscript preparation.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5sc07682j.Acknowledgements
We thank Professor Hegui Gong for helpful discussion. This work was financially supported by the National Natural Science Foundation of China (22471061 and U2004213) and the Natural Science Foundation of Henan (242300421607 and 252300420754).References
- F. Song, Z. Zhao, Z. Liu, J. W. Y. Lam and B. Z. Tang, J. Mater. Chem. C, 2020, 8, 3284–3301 RSC.
- S.-P. Wan, H.-Y. Lu, M. Li and C. F. Chen, J Photoch photobio C., 2022, 50, 100500 CrossRef CAS.
- Y. Sang, J. Han, T. Zhao, P. Duan and M. Liu, Adv. Mater., 2019, 32, e1900110 Search PubMed.
- Z. L. Gong, X. Zhu, Z. Zhou, S. W. Zhang, D. Yang, B. Zhao, Y. P. Zhang, J. Deng, Y. Cheng, Y. X. Zheng, S. Q. Zang, H. Kuang, P. Duan, M. Yuan, C. F. Chen, Y. S. Zhao, Y. W. Zhong, B. Z. Tang and M. Liu, Sci China Chem., 2021, 64, 2060–2104 CrossRef CAS.
- P. Zhao, H. Y. Lu and C. F. Chen, Chem. Soc. Rev., 2025, 54, 8534–8554 RSC.
- S. J. Brown, J. Zhao, E. Forehand, L. Dobrzycki, R. Roy, A. M. M. Hasan, W. Ding, C. Schaack and A. M. Evans, J. Am. Chem. Soc., 2025, 147, 3769–3775 CrossRef CAS PubMed.
- X. Mo, G. Chen, Y. Li, B. Xiao, X. Chen, X. Yin and C. Yang, Chem. Sci., 2024, 15, 17663–17670 RSC.
- K. Takaishi, S. Murakami, K. Iwachid and T. Ema, Chem. Sci., 2021, 12, 14570 RSC.
- L. Li, P. Jiang, X. Zhang and Y. Li, Angew. Chem., Int. Ed., 2025, 64, e202417149 CrossRef CAS PubMed.
- K. Wang, X. Ou, X. Niu, Z. Wang, F. Song, X. Dong, W. Guo, H.-Q. Peng, Z. Zhao, J. W. Y. Lam, J. Sun, H. Wu, S.-Y. Yu, F. Li and B. Z. Tang, Aggregate, 2025, 6, e667 CrossRef CAS.
- K. Takaishi, S. Hinoide, T. Matsumoto and T. Ema, J. Am. Chem. Soc., 2019, 141, 11852–11857 CrossRef CAS.
- X. Z. Wang, S. Xing, X. Xiao, Li Yuan, Z. Y. Hou and Y. X. Zheng, Adv. Funct. Mater., 2025, 35, 2412044 CrossRef CAS.
- M. Wang and G. Zhou, J. Org. Chem., 2025, 90, 11510–11518 CrossRef CAS PubMed.
- Q. Wang, A. Pietropaolo, M. Fortino, Z. Song, M. Bando, N. Naga and T. Nakano, Chirality, 2021, 34, 317–324 CrossRef PubMed.
- X. Zhang, H. Ma, Q. Yin, F. Bai and Y. H. Chaolumen, Org. Lett., 2025, 27, 8969–8973 Search PubMed.
- S. An, A. Y. Hao and P. Y. Xing, Chem. Sci., 2023, 14, 10194–10202 RSC.
- L. Zeng, C. H. Guo, C. Li, Z. W. Deng, Y. Lu, L. Lu, P. Meng, S. J. Sun, Z. J. Qiu, M. Li, Y. Xiong, Z. Zhao, C. F. Chen and B. Z. Tang, Aggregate, 2025, 6, e70069 Search PubMed.
- Y. Yu, Y. Tang, S. Xu, Y. Lv, X. Hu and W. Tang, Org. Lett., 2025, 27, 6251–6256 CrossRef PubMed.
- X. P. Wei, F. Gao, D. Li, Y. He, G. Li and X. J. Zhao, Org. Lett., 2025, 27, 1304–1309 Search PubMed.
- W. Ma, Z. Cao, N. Zhang, A. Hao and P. Xing, Chem. Sci., 2025, 16, 8405–8415 RSC.
- W. L. Zhao, K. K. Tan, W. C. Guo, C. H. Guo, M. Li and C. F. Chen, Adv. Sci., 2024, 11, 2309031 CrossRef CAS.
- S. P. Wan, H. Y. Lu, M. Li and C. F. Chen, J. Photochem. Photobiol., 2022, 50, 100500 CrossRef CAS.
- Y. F. Wang, X. Liu, Y. Zhu, M. Li and C. F. Chen, J. Mater. Chem. C, 2022, 10, 4805–4812 RSC.
- X. Z. Wang, S. Xing, X. Xiao, L. Yuan, Z. Y. Hou and Y. X. Zheng, Adv. Funct. Mater., 2024, 35, 2412044 CrossRef.
- S. Qiu, W. Li, S. Zhang, W. Xu, J. Tang, W. Tian and H. Wang, Chem.–Eur. J., 2025, 31, e202500554 Search PubMed.
- Z. Sun, W. Xu, S. Qiu, Z. Ma, C. Li, S. Zhang and H. Wang, Chem. Sci., 2024, 15, 1077–1087 RSC.
- V. Pelliccioli, R. Franzini, G. Mazzeo, C. Villani, S. Abbate, G. Longhi, E. Licandro and S. Cauteruccio, New J. Chem., 2021, 45, 16442–16451 RSC.
- K. Zhang, B. Zhang, L. Feng, L. He, C. F. Chen and M. Li, Angew. Chem., Int. Ed., 2025, 64, e202425094 CrossRef.
- Y. Wang, K. Chen, H. Zhou, X. Chen and J. Xu, Org. Lett., 2025, 27, 7064–7069 CrossRef CAS PubMed.
- X. Bao, J. Rodriguez and D. Bonne, Chem. Sci., 2020, 11, 403–408 Search PubMed.
- S. Shaaban, H. Li, F. Otte, C. Strohmann, A. P. Antonchick and H. Waldmann, Org. Lett., 2020, 22, 9199–9202 CrossRef CAS.
- T. Qi, S. Guo, D. Wang, L. Li, Y. Liu and Y. Liu, Dyes Pigments, 2024, 235, 112615 CrossRef.
- S. Topal, O. S. Ipek, E. Sezer and T. Ozturk, Chem.–Eng. J., 2022, 434, 133868 Search PubMed.
- Z. X. Zhang, Z. F. Yu, M. J. Li and S. J. Zhen, J. Mater. Chem. C, 2025, 13, 4480–4487 RSC.
- B. H. Jiang, S. N. Afraj, Y. Ezhumalai, C. Y. Chang, Y. H. Yang, Y. W. Su, A. L. Abdelhady, Y. Q. Li, Z. E. Shi, C. L. Liu, M. C. Chen, H. M. Kao and C. P. Chen, J. Mater. Chem. C, 2024, 12, 17966–17976 RSC.
- K. Yang, Y. Mao, Z. Zhang, J. Xu, H. Wang, Y. He, P. Yu and Q. Song, Nat. Commun., 2023, 14, 4438 Search PubMed.
- S. X. Zhang, Z. Wu, D. Liu, Y. Zhao, S. J. Chen, Y. Wang and Y. Q. Liu, Sci. China Mater., 2025, 68, 1777–1787 CrossRef.
- C. Li, J. Shi, L. Xu, Y. Wang, Y. Cheng and H. Wang, J. Org. Chem., 2009, 74, 408–411 Search PubMed.
- L. Dang, W. Xu, S. Qiu, Y. Yu, Z. Ma, L. Yue, H. Su, C. Li and H. Wang, Org. Lett., 2024, 266, 10141–10145 Search PubMed.
- M. Xu, S. Corio, J. Warnica, E. Kuker, A. Lu, J. Hirschi and V. Dong, J. Am. Chem. Soc., 2025, 147, 16270–16281 Search PubMed.
- K. Takaishi, S. Murakami, F. Yoshinami and T. Ema, Angew. Chem., Int. Ed., 2022, 61, e202204609 CrossRef CAS PubMed.
- D. Amsallem, A. Kumar, R. Naaman and O. Gidron, Chirality, 2023, 35, 562–568 Search PubMed.
- W. Liu, J. Chen and W. Dou, J. Phys. Chem. C, 2025, 129, 10181–10188 Search PubMed.
- H. Shang, Z. Ding, Y. Shen, B. Yang, M. Liu and S. Jiang, Chem. Sci., 2020, 11, 2169–2174 RSC.
- T. Zhao, J. Han, P. Duan and M. Liu, Acc. Chem. Res., 2020, 53, 1279–1292 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |