Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Heterocyclic scaffold-fused dimethoxy-dibenzocyclooctynes for photoactivatable click chemistry
N. Alfred
Larsson
 a,
Taegeun
Jo
a,
Taegeun
Jo
 c,
Ulf
Bremberg
b,
Stefano
Crespi
c,
Ulf
Bremberg
b,
Stefano
Crespi
 c,
Luke R.
Odell
c,
Luke R.
Odell
 b and
Daniel
Fürth
b and
Daniel
Fürth
 *ad
*ad
aDepartment of Immunology, Genetics & Pathology, Uppsala University, SE-751 85, Uppsala, Sweden. E-mail: furth@scilifelab.uu.se
bDepartment of Medicinal Chemistry, Uppsala University, Uppsala, Sweden
cDepartment of Chemistry, Uppsala University, Uppsala, Sweden
dScience for Life Laboratory (SciLifeLab), Uppsala University, Uppsala, Sweden
First published on 29th October 2025
Abstract
Dibenzocyclooctynes (DBCOs) are widely used in bioorthogonal chemistry for strain-promoted azide–alkyne cycloaddition (SPAAC), but generally lack reactivity toward inverse-electron-demand Diels–Alder cycloaddition (iEDDAC) with tetrazines. While photoactivatable DBCOs for SPAAC are well established and photoresponsive cyclooctynes for iEDDAC have recently been reported, photoactivatable DBCOs designed for dual reactivity remain scarce. Motivated by the possibility that heterocyclic fusions could tune reactivity and introduce new photochemical properties, we developed a modular synthetic route to a previously inaccessible heterocycle-fused DBCO. Upon photoactivation, the pyrrolidine fusions efficiently released the alkyne from the cyclopropenone cage, exhibiting strong SPAAC reactivity but, contrary to computational predictions, showing no detectable reactivity with tetrazines in iEDDAC. By contrast, triazole fusions, previously described as fluorogenic dibenzocyclooctyne (FL-DIBO), have not been evaluated in photo-click chemistry. We found that the cyclopropenone cage is essential for fluorescence, and its decarbonylation produced non-alkyne byproducts with no functional reactivity. This work expands the chemical space of DBCOs and establishes pyrrolidine-fused derivatives as the first functional heterocyclic-fused photoactivatable alkyne within the dimethoxy-DBCO scaffold class.
Introduction
The strain-promoted azide–alkyne cycloaddition (SPAAC)1,2 and the inverse electron-demand Diels–Alder cycloaddition (iEDDAC)3 are two of the most widely used copper-free click reactions in chemical biology. Dibenzocyclooctyne (DBCO) is a prominent SPAAC reagent, valued for its high reactivity with azides and broad utility in bioconjugation. In parallel, iEDDAC reactions commonly employ strained alkenes such as trans-cyclooctene (TCO), which displays exceptionally fast kinetics with tetrazines and has become a mainstay in bioorthogonal labeling. These two reactions are generally considered mutually orthogonal, forming the basis for many dual-labeling strategies.Expanding the structural diversity of strained alkynes has been a productive route to tuning reactivity and orthogonality. Bicyclononyne (BCN), which features a cyclopropane-fused cyclooctyne core, is notable for its ability to undergo both SPAAC with azides and iEDDAC with tetrazines.4,5 Inspired by BCN's dual reactivity, Mayer et al. introduced a cyclopropene fusion onto the dibenzocyclooctyne scaffold, which surprisingly endowed the resulting compound with tetrazine reactivity.6 This finding challenged the assumption that DBCO derivatives are inert toward tetrazines and highlighted how subtle ring modifications can dramatically reshape reactivity in bioorthogonal chemistry.
Building on these insights, Svatunek and colleagues recently used density functional theory (DFT) calculations to predict that certain cycloalkane-fused DBCO derivatives, such as cyclopentane-DMBO (5C-DMBO), could exhibit enhanced reactivity toward tetrazines while retaining SPAAC reactivity7 (Fig. 1a). Motivated by these computational predictions, we have developed a novel synthesis of a pyrrolidine-fused DMBO derivative closely resembling the predicted 5C-DMBO structure (Fig. 1c). A key limitation of the cyclopentane-fused DMBO (5C-DMBO) scaffold is the absence of a heteroatom handle for straightforward functionalization onto biomolecules. In contrast, the cyclopropane-fused DMBO (3C-DMBO) synthesized by Mayer et al. includes such a handle, but at the cost of a significantly more complex and lengthy synthetic route (see SI Fig. S2 for comparison). Our approach introduces a pyrrolidine ring fused to the DMBO core, providing a heterocyclic nitrogen atom that can, in principle, serve as a convenient handle for further derivatization. This modular strategy not only simplifies access to these heterocycle-fused DBCO derivatives but also opens new avenues for functionalization and conjugation in bioorthogonal applications. This route achieves a sixteen-fold higher yield compared to the original photo-DMBO synthesis, significantly improving access to this chemical space. Contrary to computational predictions, the synthesized compound retains efficient reactivity toward azides but shows no measurable reaction with tetrazines under conditions where DMBO undergoes rapid iEDDAC. This highlights how subtle differences in heterocyclic structures, compared to cycloalkanes, can significantly influence reactivity profiles. Understanding these nuances is crucial for the rational design of bioorthogonal reagents with predictable performance.
 | ||
| Fig. 1 Rational engineering of scaffold-fused DBCOs for iEDDAC reactivity. (a) Chemical structure of dibenzocyclooctynes illustrating the “edge” and “face” approach trajectories of an incoming reactant. A three-dimensional model of DBCO demonstrates that inverse electron-demand Diels–Alder (iEDDAC) reactivity requires a direct “face-on” approach, analogous to a punch to the face. However, this trajectory is sterically hindered in the native scaffold, preventing productive ligation. Incorporation of a cycloalkane fusion, as in 3C-DMBO,7 induces a more open, “tub-like” conformation that accommodates face-on approach and lowers the activation barrier, thereby enabling efficient tetrazine ligation. (b) Previously suggested hypothetical dibenzocyclooctyne derivatives.6 DFT-calculated activation energies for the reaction with tetrazine, taken from Svatunek and colleagues,7 are shown below each compound. ADIBO reacts exclusively with azides, while Mayer et al.6 showed 3C-DMBO reacts with both azides and tetrazines. Although untested experimentally, computational results predict that 4C-DMBO and 5-DMBO will also react with these partners. (c) Chemical structure of pyrrolidine-DMBO introduced in this work. | ||
Results
Feasibility of synthesizing DMBO derivatives
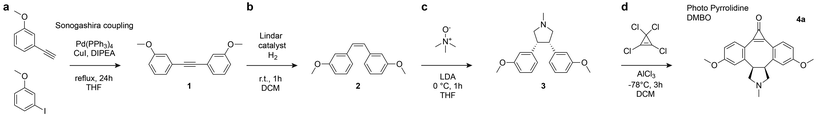
The recently reported photo-DMBO scaffold exhibits an exceptionally low overall yield of just 0.5%, highlighting a significant synthetic bottleneck despite its promising bioorthogonal reactivity.6 This challenge underscores the need for improved synthetic strategies to access similarly reactive dibenzocyclooctyne derivatives with more practical yields. Computational predictions have suggested that fusing carbocyclic rings to the DMBO core can enhance tetrazine reactivity by stabilizing more favorable geometries, identifying a series of four-, five-, and six-membered ring fused DMBO derivatives as promising candidates7 (Fig. 1b). Among these, the four-membered ring fused derivative (4C-DMBO) showed the lowest predicted activation barrier for the inverse-electron-demand Diels–Alder reaction (19.2 kcal mol−1 compared to 20.3 kcal mol−1 for DMBO).7 Such highly strained carbocycles are notoriously difficult to synthesize due to limited synthetic routes compared to flexible, strain-free propane linkers – making the most reactive predicted structure also the least accessible. The six-membered ring derivative (6C-DMBO) exhibited predicted activation energies comparable to ADIBO, a scaffold known to be unreactive towards tetrazines, leaving the five-membered ring derivative (5C-DMBO) as the most promising candidate for improved synthesis.To this end, we pursued the synthesis of a pyrrolidine-functionalized DMBO (pyrrolidine-DMBO), replacing the carbocyclic 5C-DMBO ring with a five-membered nitrogen heterocycle to both retain the strained geometry required for reactivity while also introducing a handle for convenient functionalization onto biomolecules of interest (Fig. 1c). This approach facilitates ring formation via a [3 + 2] cycloaddition between an alkene and trimethylamine N-oxide,8 providing a straightforward and modular approach to the fused system. Lastly, the incorporation of nitrogen offers additional sites for functional modification, while preserving the key structural features suggested by the original computational model.
Our suggested synthesis path can be broken down into four steps (Scheme 1). First, 1-ethynyl-3-methoxybenzene and 3-iodoanisole undergo an alkyne-aryl halide cross-coupling reaction (Scheme 1a). The internal alkyne is then selectively reduced to a cis-alkene using H2 and Lindlar catalyst in DCM (Scheme 1b). Next, the resulting stilbene derivative was reacted with an azomethine ylide formed from trimethylamine N-oxide using lithium diisopropylamide (LDA) in THF at 0 °C, forming the pyrrolidine-fused product8 (Scheme 1c). Finally, the key photoreactive cyclopropenone motif9,10 (Scheme 1d) was installed via an intramolecular Friedel–Crafts alkylation with tetrachlorocyclopropene.
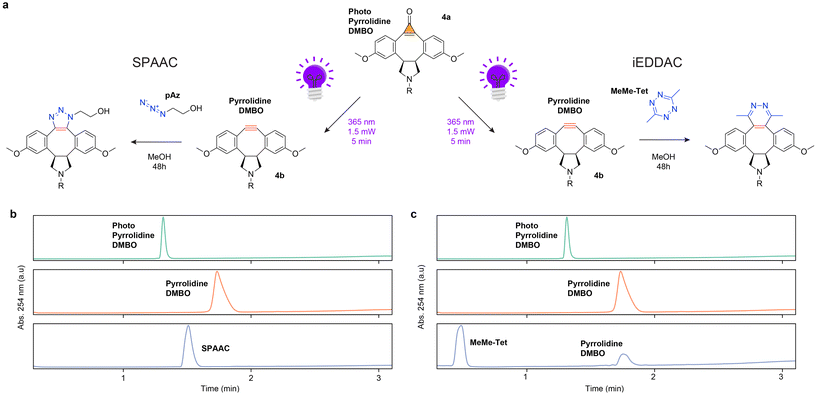
Pyrrolidine-DMBO retains azide reactivity but shows no reactivity towards tetrazines
To assess the orthogonal reactivity of the synthesized pyrrolidine-DMBO, we conducted LCMS experiments with 2-azidoethanol and 3,6-dimethyltetrazine (Fig. 2a). Pyrrolidine-DMBO reacted efficiently with 2-azidoethanol, yielding the expected triazole product and confirming efficient SPAAC performance (Fig. 2b). However, no iEDDAC reaction was observed when 3,6-dimethyltetrazine was treated with pyrrolidine-DMBO under identical conditions (Fig. 2c). This is in contrast with recent computational predictions of high tetrazine reactivity for fused five-membered DBMO derivatives.7This discrepancy may be attributable to problems encountered during synthesis. More specifically, one plausible explanation that reconciles the computational predictions with the observed lack of tetrazine reactivity (despite retained azide reactivity) is that epimerization occurred during or before pyrrolidine ring formation, resulting in the formation of a trans-fused rather than the intended cis-fused system. To evaluate this possibility, we analyzed the compound by proton nuclear magnetic resonance (1H NMR) spectroscopy. To assign the configuration of photo-pyrrolidine-DMBO, we performed conformational analysis and NMR simulations with Boltzmann averaging over all conformers (see SI for details). The predicted 1H–1H coupling constants between 2H and 6H are 0.4 Hz (trans) and 7.0 Hz (cis), compared to the experimental values of 4.3 Hz and 6.7 Hz (Fig. S5). Predicted chemical shifts also distinguish the isomers: the cis-isomer shows three symmetric signals, while the trans-isomer shows six asymmetric signals. The experimental spectrum displays symmetric shifts, confirming the cis-fused configuration (Fig. 3). These results indicate that the lack of tetrazine reactivity cannot be attributed to ring fusion geometry, as the cis isomer is present rather than the trans.
Lastly, we considered whether the lack of tetrazine reactivity could be attributed to solvent effects or other reaction conditions. Since the original experiments were carried out under conditions analogous to those reported previously7 (specifically in methanol) we repeated the reaction under different solvent conditions to evaluate the potential influence of solvent polarity, ionic strength, or protonation state of the amine. We performed the reaction in an aprotic solvent (DMSO) supplemented with a non-nucleophilic base (DIPEA, 1 eq.) to ensure that the secondary amine remains neutral and to minimize potentially unfavorable ionic interactions. Even under these conditions, no tetrazine reactivity was observed but SPAAC reactivity was retained (SI Fig. S3), reinforcing the conclusion that the absence of iEDDAC reactivity reflects an intrinsic limitation of the compound itself, rather than an artifact of solvent or reaction conditions.
Reduced SPAAC reaction rates for pyrrolidine-DMBO compared to azadibenzocyclooctyne
Azadibenzocyclooctyne (ADIBO) is the most widely used and commercially produced derivative of dibenzocyclooctyne (DBCO). The second-order rate constant for the ADIBO–azide cycloaddition in methanol has been consistently reported at approximately 0.3 to 0.4 M−1 s−1 by multiple independent groups.7,11 This reproducibility makes ADIBO a useful benchmark or “gold standard” for comparing the reactivity of newly developed cyclooctynes. The second-order kinetics of the reactions between pyrrolidine-DMBO or azadibenzocyclooctyne (ADIBO) and 2-azidoethanol were measured by monitoring the decrease in absorbance at 307 nm in methanol at room temperature. Time-resolved UV-Vis spectra were fitted to obtain second-order rate constants (Fig. 4 and Table 1).| Compound | k 2 (M−1 s−1) | Standard error | R 2 |
|---|---|---|---|
| Pyrrolidine-DMBO | 0.0444 | ±0.0011 | 0.998 |
| ADIBO-amine | 0.3387 | ±0.0108 | 0.996 |
Pyrrolidine-DMBO exhibited a significantly slower reaction rate (k2 = 0.04 M−1 s−1) compared to ADIBO (k2 = 0.34 M−1 s−1) under identical conditions, consistent with attenuated SPAAC kinetics (b = −0.29 M−1 s−1, SE = 0.02 M−1 s−1, t(7) = −13.10, p < 0.001).
These results indicate that incorporation of the pyrrolidine moiety decreases the cyclooctyne's reactivity towards azides relative to the standard ADIBO scaffold.
Fluorogenic triazole fusions undergo photodecarbonylation to radicals, preventing alkyne release
Boons and colleagues12 were the first to synthesize a heterocyclic compound via a strategy analogous to the one we used for pyrrolidine, resulting in a triazole-fused dimethoxy-DIBO scaffold. To our knowledge, it has not yet been systematically tested whether decarbonylation of the cyclopropenone in FL-DIBO can generate a functional alkyne, and whether such an alkyne retains reactivity toward azides and tetrazines. We applied a similar synthetic scheme as Boons and collegues,12 introducing a PEG1-acid linker through triazole fusion (Scheme 2). As previously reported, the resulting conjugate exhibits a strong absorption peak at 360 nm and a pronounced emission peak at 488 nm, effectively acting as a fluorophore (Fig. 5a). This stands in stark contrast to the pyrrolidine fusion, which is non-fluorescent. Next, we investigated whether the cyclopropenone photocage could be removed to generate a reactive alkyne and whether this alkyne would undergo cycloaddition with azides and tetrazines. Following the irradiation protocol previously used for the pyrrolidine fusion, we first exposed the molecule to 5 minutes of 365 nm LED light at 1.5 mW, but the molecule remained fluorescent, and the cyclopropenone cage was intact. Given that prior studies identified the cyclopropenone group as critical for fluorescence,12 this suggested that longer irradiation, sufficient to bleach the fluorophore, might be required. We therefore increased the irradiance to 3 mW and irradiated for 100 minutes, monitoring the reaction every 5 minutes by fluorescence and LC-MS, which revealed decarbonylation of the cyclopropenone cage. Unfortunately, immediately upon decarbonylation, we observed radical generation and detected no formation of the expected cyclooctyne, as indicated by both LC-MS and a subsequent SPAAC reaction. Unsurprisingly, no iEDDAC reaction was observed either (Fig. 5b). To minimize potential side reactions and radical quenching by oxygen or protic solvents, we repeated the experiment in degassed dichloromethane instead of methanol, as well as tested other wavelengths than 365 nm, such as 308 nm, which have previously been reported for decarbonylation of cyclopropenones in triazole-fused DBCOs.13 Unfortunately, the results remained similar. In summary, while the pyrrolidine-fused system underwent efficient photocleavage under brief irradiation, the triazole-fused cyclopropenone required prolonged irradiation to induce decarbonylation. However, we observed radical formation and no detectable cyclooctyne, as confirmed by LC-MS and SPAAC assays, and no iEDDAC reactivity was observed. In contrast, the pyrrolidine fusion reacts cleanly, highlighting a key difference in photochemical behavior and reactivity between the two heterocyclic-fusion systems.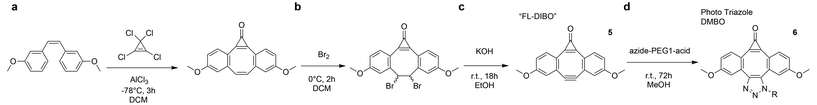 | ||
| Scheme 2 Synthesis of fluorescent triazole-DMBO, the initial step is identical to step (a) in Scheme 1. Steps a–c are adapted from Friscourt et al.12 resulting in what they refer to as fluorescent DIBO (FL-DIBO). R = PEG1-acid. | ||
 | ||
| Fig. 5 Photo-triazole-DMBO produces a fluorophore, while UV decarbonylation yields radical byproducts instead of a functional alkyne. (a) Normalized absorbance and emission spectra of photo-triazole-DMBO, showing excitation maxima at 360 nm (purple) and emission maxima at 488 nm (green). Inset: photograph of methanol solutions under UV illumination, showing fluorescent photo-triazole-DMBO and non-fluorescent/bleached decarbonylated triazole-DMBO. (b) LC-MS analysis of photo-triazole-DMBO. The black trace shows the original non-fluorescent construct, FL-DIBO, reported by Boons et al.,12 which converts to fluorescent photo-triazole-DMBO upon ligation with azide-PEG1-acid (turquoise trace). UV irradiation (3 mW, 100 min) produces multiple smaller LC peaks, with one dominant peak no longer corresponding to the original photo-triazole-DMBO. The associated mass spectrum reveals several new species (e.g., 438.1, 467.9, 514 m/z), consistent with radical byproducts from decarbonylation. Both SPAAC (with 2-azidoethanol) and iEDDAC (with 3,6-dimethyltetrazine) fail to generate the intended products (purple traces), instead showing a similar pattern of scattered peaks within the dominant LC peak (438, 468, 514 m/z), indicating analogous radical decomposition pathways. All reactions were performed sequentially; the azide or diene was added only after UV irradiation, and was not present during the photodecarbonylation step. | ||
Discussion
A punch to the face: does scaffold fusions give dibenzocyclooctynes an edge in tetrazine ligation?
In this study, we set out to experimentally investigate computational predictions7 on how fusion with a five-membered nitrogen-containing heterocycle affects cyclooctyne reactivity. Incorporating a nitrogen atom offers a useful functional handle for downstream bioconjugation while maintaining the critical geometric features imparted by the cycloalkane fusion described by Svatunek et al.7 Surprisingly, while azide reactivity is retained, the resulting pyrrolidine-fused DMBO shows no detectable reactivity toward tetrazines. This lack of tetrazine reactivity cannot be explained by common synthetic issues such as cis/trans isomerism or by protonation states influenced by the buffer conditions. The failure of the pyrrolidine-fused DMBO to react with tetrazines, despite DFT predictions to the contrary, highlights limitations in current computational models.Facing the future at the edge of reactivity
While the fused nitrogen heterocycle was expected to preserve, or even enhance tetrazine reactivity, based on favorable geometric strain and electronic alignment, the complete lack of iEDDAC reactivity in the synthesized compound suggests that nitrogen incorporation introduces subtler electronic or conformational effects that are not fully captured by computational methods. Looking forward, the computational predictions by Svatunek et al.7 indicate that fusion of a four-membered ring may provide the lowest activation barrier for tetrazine ligation among the designs tested (Fig. 1b). We propose that future studies focus on the synthesis and evaluation of this strained 4C-DMBO scaffold. Promising synthetic strategies for such four-membered ring-fused systems may include visible-light mediated [2 + 2] cycloadditions, such as those reported by Pagire et al.14 or cycloaddition of olefins by Liu et al.15Dimethoxy substitutions versus structural alternatives
The current work has focused exclusively on the dimethoxy-DIBO scaffold previously employed by Lang and colleagues,6 as well as by Boons and co-workers12 for their fluorogenic cyclooctyne. This scaffold was originally introduced by Rutjes and colleagues11 through a two-step synthetic route to aza-dibenzocyclooctynes (ADIBO), starting from 3-ethynylanisole and 3-iodoanisole to generate two methoxy substituents on the benzene rings of the DBCO scaffold. In contrast, Popik and colleagues13 reported a triazole fusion on a scaffold lacking methoxy groups, derived from a Sondheimer diyne in which the 5,10-dimethoxy substituents were replaced by hydrogen or butoxy groups.16 Unexpectedly, this substitution did not yield a fluorescent compound; instead, the absorption maximum shifted from 350 nm to 310 nm, rendering the cyclopropenone cage inert to 365 nm irradiation but responsive to 308 nm light. Importantly, the resulting decarbonylation produced an intact and functional alkyne with one of the fastest reported reaction rates for cyclooctynes (34 M−1 s−1).13 These findings suggest that future efforts should explore whether the 5,10-dibutoxy substituents can be harnessed in iEDDAC chemistry.Taken together, our findings highlight the utility of photoactivatable cyclooctynes as a model platform for dissecting the geometric and electronic factors governing SPAAC versus iEDDAC reactivity. These scaffolds offer a valuable testbed for assessing the predictive power of computational models and for systematically exploring how ring strain and heteroatom incorporation influence cycloaddition kinetics. While recent developments in photoreactive tetrazines17 open new avenues for light-controlled iEDDAC reactions with precise temporal resolution – potentially bypassing some of the structural limitations of strained alkynes – it is important to note that tetrazine-based systems may lose their fluorogenic properties when paired with certain dye classes, such as xanthenes,18 which are critical for no-wash in vivo imaging. In this context, photoreactive cyclooctynes remain a compelling and complementary strategy for expanding the bioorthogonal toolbox, especially in applications requiring both fluorogenicity and spatiotemporal control. Future efforts that integrate the distinct advantages of both cyclooctyne- and tetrazine-based systems may yield versatile, high-performance reagents optimized for diverse biological settings.
Author contributions
N. A. L. conceived the synthesis route, performed the chemical synthesis, conducted experiments, analyzed data, and co-wrote the manuscript. T. J. conducted computational analysis, and contributed to manuscript writing, U. B. contributed to synthesis route suggestions, and assisted with NMR interpretation, and contributed to manuscript writing. S. C. contributed to computational analysis and provided supervision. L. R. O. provided supervision, contributed to writing, and assisted with synthesis route suggestions. D. F. supervised the project, contributed to data analysis, and wrote the manuscript.Conflicts of interest
D. F. serves on the scientific advisory board of Navinci Diagnostics AB.Data availability
Data for this article, including analysis and plotting scripts to reproduce figures, chemical structure files (ChemDraw), as well as raw data (LC-MS, NMR) are available in an article-associated GitHub repository at https://github.com/furthlab/pyrrolidine-DMBO/.Supplementary information (SI) is available. Cartesian coordinates of cis isomer conformers obtained with r2SCAN-3c/CPCM(chloroform). Cartesian coordinates of trans isomer conformers obtained with r2SCAN-3c/CPCM(chloroform). See DOI: https://doi.org/10.1039/d5ob01314c.
Acknowledgements
D. F. is supported by SciLifeLab startup, SciLifeLab Technology Development Project, NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (29810), the Chan Zuckerberg Initiative (239942), the Swedish Research Council (2022-02706), Swedish Brain Foundation (FO2022-0054), and Åke Wibergs Foundation (M22-0217). S. C. is supported by Swedish Research Council (2021-05414). The computations were enabled by resources provided by the National Academic Infrastructure for Supercomputing in Sweden (NAISS) at the Tetralith cluster (NSC in Linköping, thanks to the NAISS 2024/5-570 medium and 2025/22-1003 small compute projects) partially funded by the Swedish Research Council through grant agreement no. 2022-06725. L. R. O. is supported by Swedish Research Council (2021-03293 and 2022-04831), the Swedish Brain Foundation (FO2024-0317-HK-70) and the Swedish Cancer Society (Cancerfonden 243889 Pj).References
- N. J. Agard, J. A. Prescher and C. R. Bertozzi, J. Am. Chem. Soc., 2004, 126, 15046–15047 CrossRef CAS PubMed.
- X. Ning, J. Guo, M. A. Wolfert and G. Boons, Angew. Chem., Int. Ed., 2008, 47, 2253–2255 CrossRef CAS PubMed.
- M. L. Blackman, M. Royzen and J. M. Fox, J. Am. Chem. Soc., 2008, 130, 13518–13519 CrossRef CAS PubMed.
- J. Dommerholt, S. Schmidt, R. Temming, L. J. A. Hendriks, F. P. J. T. Rutjes, J. C. M. van Hest, D. J. Lefeber, P. Friedl and F. L. van Delft, Angew. Chem., Int. Ed., 2010, 49, 9422–9425 CrossRef CAS PubMed.
- H. E. Murrey, J. C. Judkins, C. W. am Ende, T. E. Ballard, Y. Fang, K. Riccardi, L. Di, E. R. Guilmette, J. W. Schwartz, J. M. Fox and D. S. Johnson, J. Am. Chem. Soc., 2015, 137, 11461–11475 CrossRef CAS.
- S. V. Mayer, A. Murnauer, M. von Wrisberg, M. Jokisch and K. Lang, Angew. Chem., Int. Ed., 2019, 58, 15876–15882 CrossRef CAS PubMed.
- D. Svatunek, A. Murnauer, Z. Tan, K. N. Houk and K. Lang, Chem. Sci., 2024, 15, 2229–2235 RSC.
- R. Beugelmans, G. Negron and G. Roussi, J. Chem. Soc., Chem. Commun., 1983, 31 RSC.
- A. Poloukhtine and V. V. Popik, J. Org. Chem., 2003, 68, 7833–7840 CrossRef CAS PubMed.
- A. A. Poloukhtine, N. E. Mbua, M. A. Wolfert, G.-J. Boons and V. V. Popik, J. Am. Chem. Soc., 2009, 131, 15769–15776 CrossRef CAS PubMed.
- M. F. Debets, J. S. Prins, D. Merkx, S. S. van Berkel, F. L. van Delft, J. C. M. van Hest and F. P. J. T. Rutjes, Org. Biomol. Chem., 2014, 12, 5031–5037 RSC.
- F. Friscourt, C. J. Fahrni and G.-J. Boons, J. Am. Chem. Soc., 2012, 134, 18809–18815 CrossRef CAS.
- D. A. Sutton and V. V. Popik, J. Org. Chem., 2016, 81, 8850–8857 CrossRef CAS.
- S. K. Pagire, A. Hossain, L. Traub, S. Kerres and O. Reiser, Chem. Commun., 2017, 53, 12072–12075 RSC.
- Z. Liu, C. Zhou, T. Lei, X.-L. Nan, B. Chen, C.-H. Tung and L.-Z. Wu, CCS Chem., 2020, 2, 582–588 CrossRef CAS.
- D. A. Sutton, S.-H. Yu, R. Steet and V. V. Popik, Chem. Commun., 2015, 52, 553–556 RSC.
- L. Liu, D. Zhang, M. Johnson and N. K. Devaraj, Nat. Chem., 2022, 14, 1078–1085 CrossRef CAS PubMed.
- G. Beliu, A. J. Kurz, A. C. Kuhlemann, L. Behringer-Pliess, M. Meub, N. Wolf, J. Seibel, Z.-D. Shi, M. Schnermann, J. B. Grimm, L. D. Lavis, S. Doose and M. Sauer, Commun. Biol., 2019, 2, 261 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2026 |