Regioselective β-organoseleno/α-,N2-tetrazole addition to alkenes
Li-Li
Zhu
a,
Lifang
Tian
 b,
Zijian
Zhang
b,
Qinying
Li
a,
Yingying
Tian
a,
Xiaofeng
Han
a,
Jiawei
Li
a,
Guanglu
Liu
a,
Hui
Zhang
*a and
Yahui
Wang
b,
Zijian
Zhang
b,
Qinying
Li
a,
Yingying
Tian
a,
Xiaofeng
Han
a,
Jiawei
Li
a,
Guanglu
Liu
a,
Hui
Zhang
*a and
Yahui
Wang
 *b
*b
aSchool of Chemistry and Chemical Engineering, Zhoukou Normal University, Wenchang Road, Zhoukou, 466001, China. E-mail: hxzhanghui@zknu.edu.cn
bSchool of Chemistry and Molecular Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing, 211816, China. E-mail: ias_yhwang@njtech.edu.cn
First published on 2nd December 2025
Abstract
A method for the regioselective β-organoseleno/α-,N2-tetrazole addition to alkenes is reported. PhSeCl acts dually as a selenylating agent and a bridging agent for blocking the N1 position of tetrazoles, thereby directing selectivity towards the N2 position. The reactions show a broad substrate scope, accommodating various tetrazoles and alkenes, and are executed under mild conditions.
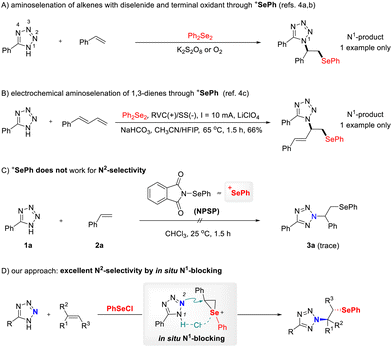
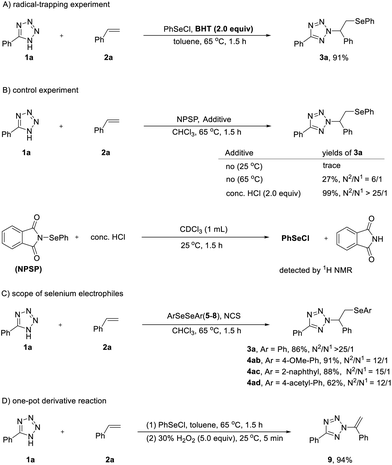
Both nitrogen- and selenium-containing molecules have found widespread applications in the fields of organic synthesis, materials science, and pharmaceutical chemistry.1 Consequently, continuous efforts have been dedicated to devising novel and efficient methods for incorporating both selenium and nitrogen moieties into organic compounds.2 Among established methods, the intermolecular aminoselenation of alkenes stands out as one of the most effective methods for the assembly of this ubiquitous framework, enabling the concurrent formation of vicinal C–N and C–Se bonds. Aminoselenation of alkenes has been extensively studied in recent decades, with several nitrogen-containing reagents employed as nitrogen nucleophiles, including amines, nitriles, phthalimide and sulfamides, sulfoximines, azides and azoles.3 Methods employing tetrazoles as the nitrogen sources for the aminoarylselenation have drawn significant interest.4 This stems from the broad utility of the tetrazole motif itself, which serves as an important synthetic scaffold in medicine, biochemistry, pharmacology, and materials science.5 For example, Sun and co-workers reported the aminoselenations of alkenes with tetrazole as the nitrogen source, mediated by K2S2O8 and O2, respectively (Scheme 1A).4a,b Additionally, the Meng group developed a three-component electrochemical aminoselenation reaction of 1,3-dienes, azoles, and diselenide derivatives, achieving selenium-containing allylazoles with exclusive 1,2-regioselectivity (Scheme 1B).4c Under the methodologies described above, the N1 position of tetrazoles acts as an exclusively nucleophilic site, thus affording N1-selective tetrazole functionalization. Controlling regioselectivity remains a fundamental challenge for tetrazole functionalization.6 The dynamic N1/N2 tautomerism presents a distinct obstacle to regiocontrol, as both N1- and N2-selective pathways are theoretically possible.7 Generally, diselenides represent preferable selenylation agents due to their bench stability, ready availability, and ease of handling. Mechanistically, external oxidants have been used for the in situ generation of a reactive electrophilic selenium species, e.g. phenylselenium ion (+SePh), that participates in the aminoselenation of alkenes. However, under the conditions reported previously,4 tetrazole functionalization predominantly occurs at the N1 position, likely due to the absence of directing interactions between the tetrazole and alkene substrates. This is again revealed by our initial experimental results. When N-(phenylselenenyl)phthalimide (NPSP) was introduced as the selenylation source, the desired N2-β-selenoalkylated tetrazole product 3a was nearly not formed (Scheme 1C). Therefore, new strategies other than through the phenylselenium ion (+SePh) have attracted our attention.
We recognized that the high density of nitrogens in tetrazoles offers significant potential for hydrogen bonding, analogous to carboxylic acids as hydrogen bond donors,5 and the inherent nature of tetrazoles results in a negative charge delocalized among N2, N3 and N4 atoms. Inspired by these features, we hypothesized that introducing a suitable selenylation source capable of bridging the N1–H position of tetrazoles could block nucleophilic attack from N1, thereby redirecting selectivity towards the N2 position (Scheme 1D). In our previous work,8 we have found that intermolecular hydrogen bonding critically governs N2-selectivity in triazole functionalization.8a,b We hypothesize herein that PhSeCl could serve as both selenylating agent and linking agent for blocking the N1 position of tetrazoles. If this works, the desired regioselective N2-tetrazole addition could occur smoothly (Scheme 1D). With these mechanistic considerations in mind, we started our studies on this regioselective β-organoseleno/α-,N2-tetrazole addition to alkenes.
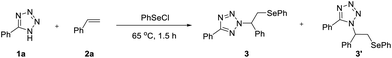
In our preliminary investigations, we examined the reaction among tetrazole (1a), styrene (2a), and PhSeCl in CHCl3 at room temperature (25 °C) for 24 h. The reaction proceeded smoothly to provide the desired N2-product 3a in 73% yield with high N2-selectivity (>20/1) (Table 1, entry 1). Increasing the temperature to 65 °C reduced the reaction time to 1.5 h while improving both yield (85%) and N2-selectivity (>25/1) (Table 1, entry 2). While diamines effectively promoted N2-alkylation of triazoles in prior work,8c their inclusion here in this reaction significantly diminished N2-selectivity, with various bases, including K2CO3, KOtBu, Cs2CO3, N,N′-dimethylpiperazine (DMP), N,N,N′,N′-tetramethylethylenediamine (TMEDA) and Et3N (Table 1, entries 3–8). This suggested that HCl, generated in situ from tetrazole and PhSeCl, might participate in governing the N2-selectivity. Solvent screening (DCE, ethyl acetate, 1,4-dioxane, toluene, DMF and CH3OH) identified toluene as optimal, providing excellent yield (Table 1, entries 9–14). When the reaction was carried out under an inert atmosphere (argon), both the yield and N2-selectivity remained comparable to those observed in air. These results indicated that the reaction was not sensitive to oxygen (SI, Table S1, entry 13). Moreover, the reaction tolerated aqueous conditions, and afforded 3a in 86% yield employing H2O as a solvent (Table 1, entry 15). In addition, lowering the temperature to 25 °C severely diminished the yield (33%) even after 36 h (Table 1, entry 16). Reducing the loading of 2a to 1.5 equivalents led to a dramatic decrease in the yield to 46%, while still maintaining a high N2-selectivity (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 1, entry 17).
1) (Table 1, entry 17).
| Entry | Deviation | Solvent | Yield of 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
3a/3a′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.4 mmol), PhSeCl (0.3 mmol), CHCl3 (1 mL), 1.5 h, air. ND implies that no product was detected by 1H NMR analysis. b Isolated yield of the N2-selective product 3a. c N2/N1 ratios were determined by 1H NMR analysis of the crude mixtures. d The reaction was carried out with 0.3 mmol of 2a. | ||||
| 1 | 25 °C, 24 h | CHCl3 | 73 | >20/1 |
| 2 | — | CHCl3 | 85 | >25/1 |
| 3 | K2CO3 | CHCl3 | 82 | 14/1 |
| 4 | KOtBu | CHCl3 | 75 | 7/1 |
| 5 | Cs2CO3 | CHCl3 | Trace | — |
| 6 | Et3N | CHCl3 | 40 | 2/1 |
| 7 | TMEDA | CHCl3 | 38 | 2/1 |
| 8 | DMP | CHCl3 | 55 | 2/1 |
| 9 | — | DCE | 90 | >25/1 |
| 10 | — | Ethyl acetate | 71 | 9/1 |
| 11 | — | 1,4-Dioxane | 65 | 20/1 |
| 12 | — | Toluene | 94 | >25/1 |
| 13 | — | DMF | 57 | 4/1 |
| 14 | — | CH3OH | ND | — |
| 15 | — | H2O | 86 | >25/1 |
| 16 | 25 °C, 36 h | Toluene | 33 | >20/1 |
| 17d | — | Toluene | 46 | >20/1 |
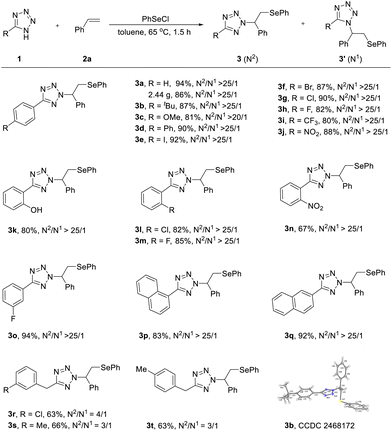
With the optimized conditions established, we explored the reaction scope primarily by varying tetrazole substrates (Scheme 2). Evaluation commenced with para-substituted aryl tetrazoles. Remarkably, these substrates exhibited excellent compatibility, affording exclusive N2-products (3a–3j) in yields up to 94%, highlighting a key advantage of this protocol. The structure of 3a was unequivocally confirmed by X-ray crystallography (CCDC 2468172). Iodo-, bromo-, chloro-, and fluoro-substituted substrates also proved viable, delivering 3e–3h in good yields with high N2-selectivities (>25/1). Furthermore, electron-donating (–OMe) or electron-withdrawing (–NO2 and –CF3) groups did not impede the reaction, providing products 3c, 3i and 3j in good yields. Substituents at the ortho- or meta-positions were equally tolerated, furnishing products 3k–3o in good to excellent yields (80–94%) with exceptional N2-selectivity. The free hydroxyl (3k) group was well tolerated. Both α- and β-naphthyl tetrazoles performed effectively under the standard conditions, with no significant electronic or positional effects observed on the aromatic moiety (3p and 3q). Aliphatic tetrazoles underwent smooth conversion to products 3r–3t in satisfactory yields (63–66%), albeit with reduced N2-selectivity (>3/1). This diminished selectivity likely stems from the absence of stabilizing π–π interactions between the tetrazole ring and aryl groups in 5-alkyl tetrazoles.5 Moreover, a gram-scale application further demonstrated synthetic utility; the reaction of 1a afforded 3a (2.44 g) in 86% yield without compromising N2-selectivity.
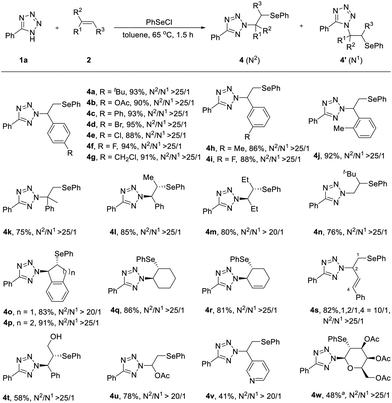
An excellent tolerance of substituents was also observed during the subsequent investigation of the alkene scope (Scheme 3). A broad range of substituted styrenes, featuring electron-donating or electron-withdrawing groups at the para position, underwent the transformation smoothly, leading to the desired N2-products (4a–4g) in excellent yields. A reactive benzylic chloromethyl (Ar-CH2Cl) group was found untouched under these standard conditions (4g). The electronic and steric effects on the ortho or meta position of styrenes did not affect the reactivity and N2-selectivity of the reaction (4h–4j). Substrates containing steric hindrance, such as α-methylstyrene and β-methylstyrene, smoothly delivered the desired N2-products 4k and 4l in yields of 75% and 85%, respectively. Moreover, this protocol could also be applied to inactive aliphatic alkenes, such as (E)-hex-3-ene, from which an anti-addition process was clearly seen (4m). In the case of tert-butylethene, product 4n with reverse regioselectivity was formed due to the steric hindrance of the bulky tBu group. Other cyclic alkenes, such as indene, 1,2-dihydronaphthalene and cyclohexene, were also efficiently converted to 4o–4q. 1,3-Dienes are often used as versatile feedstocks to assemble structurally diverse, complex molecular architectures.3j,4c Considering the conjugated nature of 1,3-dienes, two different reaction sites would occur simultaneously; the regioselective alkene functionalisation of 1,3-dienes is a challenging research topic. However, this protocol was expanded to cyclohexadiene and 1-aryl-1,3-diene under the standard conditions with high 1,2-regioselectivities and excellent N2-selectivities (4r and 4s). Furthermore, alkenes with various functional groups, including cinnamic alcohol, vinyl acetate and 3-vinyl pyridine, were also effective to give the desired N2-products 4t–4v in good yields. Moreover, N-glycosylation9 has been recognized as a powerful tool for the synthesis of essential nucleosides and has also been applied for the modification of peptide-based drugs to improve their pharmacological properties. This protocol was also applicable to sugar chemistry. When commercially available triacetyl-D-galactal and tetrazole were subjected to the standard reaction conditions, the N-glycosylated product 4w was obtained with excellent β- and N2-selectivity. The structure of 4w was assigned based on a series of 2D NMR experiments (HSQC, HMBC, COSY, and NOESY).
To further elucidate the reaction mechanism, we performed a series of controlled experiments (Scheme 4). Under the optimized reaction conditions, addition of the radical scavenger 2,6-di-tert-butyl-4-methylphenol (BHT, 2.0 equiv.) afforded 3a in 91% yield without compromising N2-selectivity. This result suggested that radical intermediates were not likely involved in the reaction (Scheme 4A). Moreover, using N-(phenylselenenyl)phthalimide (NPSP) as the selenylation source, in the presence of 2.0 equivalents of concentrated HCl, the yield remarkably reached up to a quantitative level with excellent N2-selectivity. When NPSP was treated with concentrated HCl in CDCl3 at room temperature for 1.5 hours, PhSeCl was generated and was detected by 1H NMR analysis of the crude product. These results clearly suggested that PhSeCl apart from +SePh served as an effective selenylation agent for the desired N2-selectivities.
Moreover, we explored the scope of selenium electrophiles (Scheme 4C). Owing to their high moisture sensitivity, these species are difficult to isolate as pure compounds. As an alternative, we opted to generate ArSeCl reagents in situ from diaryl diselenides and N-chlorosuccinimide (NCS) following a literature procedure.10 Employing this protocol, we conducted the model reaction in CHCl3 with diphenyl diselenide (5), affording product 3a in 86% yield with excellent N2-selectivity (>25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)—a result comparable to that obtained using PhSeCl (Table 1, entry 2). Selenium reagents bearing electron-donating groups (e.g., OMe, 6) enhanced the reactivity, presumably by improving the stability of the selenium species. In contrast, those with electron-withdrawing groups (e.g., acetyl, 7) led to diminished yields. The presence of a bulky substituent (naphthyl, 8) did not significantly impact the reactivity; however, a slight reduction in N2-selectivity was observed in these cases.
1)—a result comparable to that obtained using PhSeCl (Table 1, entry 2). Selenium reagents bearing electron-donating groups (e.g., OMe, 6) enhanced the reactivity, presumably by improving the stability of the selenium species. In contrast, those with electron-withdrawing groups (e.g., acetyl, 7) led to diminished yields. The presence of a bulky substituent (naphthyl, 8) did not significantly impact the reactivity; however, a slight reduction in N2-selectivity was observed in these cases.
Finally, transformation of selenides was achieved via a one-pot reaction of 1a and 2a, affording the N2-olefinated tetrazole 9 in 94% yield without adjustment of the standard reaction conditions.11
In summary, we have developed an efficient intermolecular regioselective β-organoseleno/α-,N2-tetrazole addition to alkenes. The in situ blocking of the N1 position of tetrazoles has been proposed to play important roles for N2-selectivities. We expect that this new strategy for controlling N2-selectivity in tetrazole functionalization will find potential synthetic applications in the future.
Author contributions
L.-L. Zhu, H. Zhang and Y. Wang conceived this work and designed the experiments. L.-L. Zhu, L. Tian, Z. Zhang, Q. Li, Y. Tian, X. Han, J. Li, and G. Liu conducted the experiments and analyzed the data. L.-L. Zhu and Y. Wang wrote the manuscript and the SI.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ob01622c.CCDC 2468172 (3b) contains the supplementary crystallographic data for this paper.12
Acknowledgements
We acknowledge the National Natural Science Foundation of China (22001272), the Science and Technology Research Project of Henan Province (242102110035), and the University Student Scientific Research Innovation Foundation of Zhoukou Normal University (ZKNUD2025103) for financial support.References
- (a) C. W. Nogueira, G. Zeni and J. B. T. Rocha, Chem. Rev., 2004, 104, 6255–6285 CrossRef CAS PubMed; (b) W. Hou and H. Xu, J. Med. Chem., 2022, 65, 4436–4456 CrossRef CAS; (c) J. Wang, M. Chen, Z. Zhang, L. Ma and T. Chen, Coord. Chem. Rev., 2023, 493, 215278 CrossRef CAS; (d) C. Morán-Serradilla, D. Plano, C. Sanmartín and A. K. Sharma, J. Med. Chem., 2024, 67, 7759–7787 CrossRef PubMed.
- (a) K. Sun, X. Wang, C. Li, H. Wang and L. Li, Org. Chem. Front., 2020, 7, 3100–3119 RSC; (b) L. Lu, D. Huang, Z. Wang and X. Wu, Adv. Synth. Catal., 2023, 365, 2310–2331 CrossRef CAS; (c) P. Qu, Y.-Q. Jiang, Y.-H. Wang and G.-Q. Liu, Green Chem., 2023, 25, 7485–7507 RSC.
- (a) E. Tang, W. Wang, Y. Zhao, M. Zhang and X. Dai, Org. Lett., 2016, 18, 176–179 CrossRef CAS PubMed; (b) Y. Liu, C. Li, S. Mu, Y. Li, R. Feng and K. Sun, Asian J. Org. Chem., 2018, 7, 720–723 CrossRef CAS; (c) L. Sun, Y. Yuan, M. Yao, H. Wang, D. Wang, M. Gao, Y. H. Chen and A. Lei, Org. Lett., 2019, 21, 1297–1300 CrossRef CAS PubMed; (d) X. Gao, L. Tang, L. Huang, Z.-S. Huang, Y. Ma and G. Wu, Org. Lett., 2019, 21, 745–748 CrossRef CAS PubMed; (e) B. Huang, Y. Li, C. Yang and W. Xia, Green Chem., 2020, 22, 2804–2809 RSC; (f) G.-Q. Liu, C.-F. Zhou, Y.-Q. Zhang, W. Yi, P.-F. Wang, J. Liu and Y. Ling, Green Chem., 2021, 23, 9968–9973 RSC; (g) F. Cui, Y. Hua, Y. Lin, J. Fei, L. Gao, X. Zhao and H. Xia, J. Am. Chem. Soc., 2022, 144, 2301–2310 CrossRef CAS PubMed; (h) X. Li, J. Huang, L. Xu, P. Liu and Y. Wei, J. Org. Chem., 2022, 87, 10684–10697 CrossRef CAS PubMed; (i) M. Kuang, H. Li, Z. Zeng, H. Gao, Z. Zhou, X. Hong, W. Yi and S. Wang, Org. Lett., 2023, 25, 8095–8099 CrossRef CAS PubMed; (j) R. Wang, N. Zhang, Y. Zhang, B. Wang, Y. Xia, K. Sun, W. Jin, X. Li and C. Liu, Green Chem., 2023, 25, 3925–3930 RSC; (k) Y.-Q. Zhang, Y.-Q. Jiang, Y.-H. Wang, C. Qi, Y. Ling, Y. Zhang and G.-Q. Liu, J. Org. Chem., 2023, 88, 7431–7447 CrossRef CAS PubMed; (l) J. Ji, X. Chen, Z. Hu, C. Guan, J. Liu, Y. Deng and S. Liu, Org. Chem. Front., 2024, 11, 3066–3071 RSC.
- (a) K. Sun, X. Wang, Y. Lv, G. Li, H. Jiao, C. Dai, Y. Li, C. Zhang and L. Liu, Chem. Commun., 2016, 52, 8471–8474 RSC; (b) K. Sun, X. Wang, C. Zhang, S. Zhang, Y. Cheng, H. Jiao and W. Du, Chem. – Asian J., 2017, 12, 713–717 CrossRef CAS PubMed; (c) S.-Y. Ren, Q. Zhou, H.-Y. Zhou, L.-W. Wang, O. M. Mulina, S. A. Paveliev, H.-T. Tang, A. O. Terentev, Y.-M. Pan and X.-J. Meng, J. Org. Chem., 2023, 88, 5760–5771 CrossRef CAS PubMed.
- C. G. Neochoritis, T. Zhao and A. Dömling, Chem. Rev., 2019, 119, 1970–2042 CrossRef CAS PubMed.
- A. Fanourakis, Y. Ali, L. Chen, P. Q. Kelly, A. J. Bracken, C. B. Kelly and M. D. Levin, Nature, 2025, 641, 646–652 CrossRef CAS PubMed.
- For selected examples of N-substituted tetrazoles, see: (a) G. Aridoss and K. K. Laali, Eur. J. Org. Chem., 2011, 6343–6355 CrossRef CAS; (b) N. Zhou, J. Zhao, C. Sun, Y. Lai, Z. Ruan and P. Feng, J. Org. Chem., 2021, 86, 16059–16067 CrossRef CAS PubMed; (c) N. Zhou, J. Yu, L. Hou, X. Wu, Z. Ruan and P. Feng, Adv. Synth. Catal., 2022, 364, 35–40 CrossRef CAS; (d) T. Kaszás, B. Szakács, É. Juhász-Tóth, I. Cservenyák, T. Kovács, L. Juhász, L. Somsák and M. Tóth, Eur. J. Org. Chem., 2022, e202201103 CrossRef; (e) S. P. Desai and M. S. Taylor, Org. Lett., 2022, 24, 7617–7621 CrossRef PubMed.
- For our recent work on N2-selective functionalization of triazoles, see: (a) L.-L. Zhu, X. Xu, J. Shi, B. Chen and Z. Chen, J. Org. Chem., 2016, 81, 3568–3575 CrossRef CAS PubMed; (b) L.-L. Zhu, L. Tian, H. Zhang, L. Xiao, W. Luo, B. Cai, H. Wang, C. Wang, G. Liu, C. Pei and Y. Wang, Adv. Synth. Catal., 2019, 361, 1117–1123 CrossRef CAS; (c) L.-L. Zhu, L. Tian, B. Cai, G. Liu, H. Zhang and Y. Wang, Chem. Commun., 2020, 56, 2979–2982 RSC; (d) L.-L. Zhu, L. Tian, K. Sun, Y. Li, G. Liu, B. Cai, H. Zhang and Y. Wang, J. Org. Chem., 2022, 87, 12963–12974 CrossRef CAS; (e) H. Zhang, L. Tian, Q. Li, H. Zheng, Y. Dai, J. Li, G. Liu, L.-L. Zhu and Y. Wang, Org. Biomol. Chem., 2025, 23, 7270–7274 RSC; (f) H. Zhang, Z. Zhang, L. Tian, Y. Han, P. Zhao, H. Yang, J. Li, G. Liu, L.-L. Zhu and Y. Wang, Org. Lett., 2025, 27, 1428–1433 CrossRef CAS PubMed and for a review on this topic, see: (g) Y. Zheng, L. Tian, V. Ramadoss, H. Zhang, L.-L. Zhu and Y. Wang, Synthesis, 2022, 2548–2560 CAS.
- (a) Carbohydrates: The Essential Molecules of Life, ed. R. V. Stick and S. Williams, Elsevier, 2nd edn, 2009 Search PubMed; (b) C. Xu and C. C. J. Loh, J. Am. Chem. Soc., 2019, 141, 5381–5391 CrossRef CAS PubMed.
- A. López-Francés, J. Rodrigues, S. Humbel, J. Rodriguez, M. Amatore and T. Constantieux, Eur. J. Org. Chem., 2025, e202500485 CrossRef.
- A. Toshimitsu, T. Aoai, H. Owada, S. Uemura and M. Okano, J. Org. Chem., 1981, 46, 4727–4733 CrossRef CAS.
- CCDC 2468172: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nvbgb.
| This journal is © The Royal Society of Chemistry 2026 |