Regioselective functionalization of sulfenamides: S-arylation with cyclic diaryl λ3-bromanes and λ3-chloranes
Jiahong
Chen
a,
Yuanyuan
Huang
a,
Nan
Wang
a,
Mengke
Wang
 *a,
Weichun
Huang
*a,
Weichun
Huang
 a,
Xinxing
Wu
a,
Xinxing
Wu
 a,
Xiaoping
Xu
a,
Xiaoping
Xu
 *b and
You
Zi
*b and
You
Zi
 *a
*a
aSchool of Chemistry and Chemical Engineering, Nantong University, Nantong 226019, P. R. China. E-mail: mengkewang@ntu.edu.cn; ziyou@ntu.edu.cn
bKey Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou 215123, P. R. China. E-mail: xuxp@suda.edu.cn
First published on 25th November 2025
Abstract
We report an S-selective and meta-regioselective arylation of sulfenamides with cyclic diaryl λ3-bromanes and λ3-chloranes. This metal-free strategy proceeds under mild conditions, tolerates diverse functional groups, and delivers S-aryl sulfilimines in good to excellent yields with high regioselectivity. The method is scalable, enabling scale-up synthesis and further transformations, highlighting the potential for efficient late-stage functionalization.
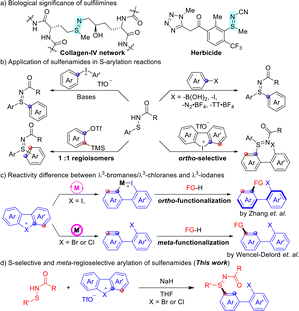
Sulfur-containing compounds are widely distributed in natural products and pharmaceuticals, with approximately 10% of U.S. food and drug administration (FDA)-approved drugs featuring sulfur-based functional groups.1 The remarkable structural diversity of these molecules largely stems from the variable oxidation states of sulfur atoms.2 Among them, sulfoxides represent one of the most prominent sulfur(IV) frameworks, finding extensive applications in pharmaceuticals, agrochemicals, and materials science.3 As the aza-analogues of sulfoxides by replacement of the oxygen atom with the nitrogen atom, sulfilimines have attracted growing attention in medicinal chemistry due to their unique properties compared to sulfoxides (Scheme 1a).4 The cross-linking of collagen-IV via sulfilimine bonds marks the first identification of this group in biomolecules.5 Incorporating sulfilimine moieties into biomacromolecules can impart novel chemical and biological properties.6 In synthetic chemistry, sulfilimines serve as ligands and nitrogen-radical precursors for heterocycle synthesis.7 Moreover, they can be readily transformed into higher-oxidation-state N–S frameworks, such as sulfoximines and sulfondiimines. Owing to their broad utility and potential in drug discovery, sulfilimines have emerged as valuable synthetic building blocks, prompting considerable research efforts toward the development of efficient synthetic methodologies.
A traditional approach to sulfilimine synthesis involves the oxidative imidation of thioethers with electrophilic nitrogen reagents to form the characteristic S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond.8 However, the oxidative conditions, reliance on potentially hazardous iminating reagents, and frequent use of transition-metal catalysts are drawbacks of this approach. [2,3]-sigmatropic rearrangement provides an alternative route, enabling the synthesis of ortho-sulfiliminyl phenols from oxyacetamides and electrophilic sulfenylation reagents, yet this method suffers from a narrow substrate scope.9 A consecutive addition of two Grignard reagents to sulfinylamines is feasible to afford the desired sulfilimines.10
N double bond.8 However, the oxidative conditions, reliance on potentially hazardous iminating reagents, and frequent use of transition-metal catalysts are drawbacks of this approach. [2,3]-sigmatropic rearrangement provides an alternative route, enabling the synthesis of ortho-sulfiliminyl phenols from oxyacetamides and electrophilic sulfenylation reagents, yet this method suffers from a narrow substrate scope.9 A consecutive addition of two Grignard reagents to sulfinylamines is feasible to afford the desired sulfilimines.10
Recently, elegant methods have been established for the synthesis of sulfilimines from sulfenamides through highly chemoselective sulfur functionalization pathways (Scheme 1b). Transition-metal-catalyzed approaches have enabled efficient coupling of sulfenamides with diverse partners such as boronic acids, sulfonylhydrazones, aryl iodides, diazonium salts, thianthrenium salts, and diazo compounds.11 In line with the principles of green chemistry, transition-metal-free strategies have also emerged. For example, alkyl halides serve as effective reagents for the S-alkylation of sulfenamides under basic conditions,12 while diaryliodonium salts enable S-arylation under metal-free conditions.13
Notably, in all these transformations, the reaction site is determined by the position directly bound to the functional group (e.g., boronic acid or halide). As highly reactive intermediates with two potential reactive sites, arynes have also been exploited for sulfilimine synthesis via S-arylation of sulfenamides.14 Although these reactions exhibit excellent efficiency, they are typically limited to symmetric arynes. The use of unsymmetric arynes often leads to mixtures of regioisomers in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Given these limitations, the development of a regioselective S-arylation of sulfenamides would provide a valuable complementary pathway for the synthesis of S-aryl sulfilimines.
1 ratio. Given these limitations, the development of a regioselective S-arylation of sulfenamides would provide a valuable complementary pathway for the synthesis of S-aryl sulfilimines.
Hypervalent compounds are indispensable tools in organic synthesis, with diaryl iodonium salts being among the most powerful and widely used examples.15 Typically, cyclic diaryl λ3-iodanes undergo transition-metal-catalyzed cross-coupling reactions smoothly, involving rapid oxidative addition into the C–I(III) bond to achieve ortho-functionalizations (Scheme 1c).16 Owing to the steric congestion introduced by the substituents, cyclic diaryl λ3-iodanes have often been employed in the synthesis of axially chiral biaryls. In this scenario, Zhang's group reported the construction of biaryl sulfilimines bearing both axial and S-central chirality from cyclic diaryl λ3-iodanes and sulfenamides (Scheme 1b).16b In sharp contrast, the chemistry of cyclic diaryl λ3-bromanes and λ3-chloranes has only recently attracted growing attention following Wencel–Delord's development of a general and efficient synthetic strategy.17 Due to the higher ionization potential and electronegativity of Br and Cl, hypervalent λ3-bromanes and λ3-chloranes display enhanced nucleofugality and reduced positive charge, enabling amplified reactivity under metal-free conditions.18 These features impart distinct reactivity profiles compared to their iodane counterparts, notably allowing their use as novel aryne precursors for selective C–C, C–O, and C–N bond formation at the meta-position.17a,18 Building on these precedents, we envisioned that cyclic diaryl λ3-bromanes and λ3-chloranes could serve as a promising platform for the regioselective meta-arylation pathway for the synthesis of structurally diverse S-aryl sulfilimines (Scheme 1d).
To initiate our study, we selected diaryl λ3-bromane 1a and sulfenamide 2a as model substrates for the regioselective meta-arylation. In the presence of Cs2CO3, no desired product was observed (Table 1, entry 1). A screening of bases, including NaOH, tBuOK, tBuONa, and NaH, revealed that NaH was uniquely effective, affording the S-aryl sulfilimine 3aa in 86% yield (Table 1, entries 2–5). Solvent optimization further identified THF as the most suitable medium, outperforming DCM, 1,4-dioxane, and toluene (Table 1, entries 6–8). Notably, both sulfenamide and base loadings significantly affected the reaction outcome, with a reduced loading delivering an improved yield of 94% (Table 1, entries 9 and 10). Time monitoring indicated that a reaction duration of 2 hours was sufficient to achieve the optimal yield (Table 1, entry 11).
| Entry | Base (equiv.) | Solvent | Yield of 3aab (%) |
|---|---|---|---|
| a Reaction conditions: cyclic diaryl λ3-bromane 1a (0.1 mmol), sulfenamide 2a (0.2 mmol), base, solvent (1.5 mL), −78 °C to rt, 3 h. b Isolated yield. c 1.5 equiv. of 2a was used. d 1.0 equiv. of 2a was used. e 2 hours. | |||
| 1 | Cs2CO3 (5) | THF | Trace |
| 2 | NaOH (5) | THF | 44 |
| 3 | t BuOK (5) | THF | 73 |
| 4 | t BuONa (5) | THF | 50 |
| 5 | NaH (5) | THF | 86 |
| 6 | NaH (5) | DCM | 47 |
| 7 | NaH (5) | 1,4-Dioxane | 34 |
| 8 | NaH (5) | Toluene | 25 |
| 9c | NaH (3) | THF | 93 |
| 10d | NaH (2) | THF | 94 |
| 11de | NaH (2) | THF | 99 |
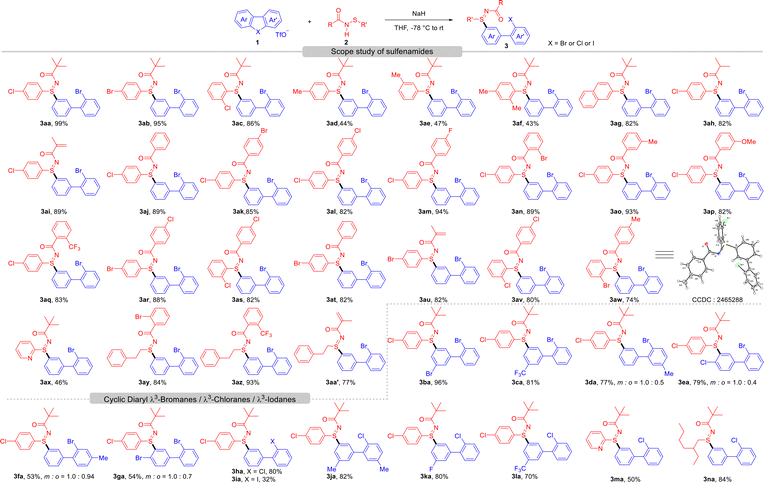
With the optimal conditions established, we next examined the generality of this strategy for S-selective and meta-regioselective arylation of sulfenamides with diaryl λ3-bromanes (Scheme 2). For aryl-substituted sulfenamides, halogen substituents were well tolerated, affording the desired products in good to excellent yields (3aa–3ac), with only a slight steric effect observed (86%, 3ac). In contrast, electron-donating groups reduced the yields due to decreased reactivity (43–47%, 3ad–3af). Extended conjugated systems also performed well, delivering the product in a good yield (82%, 3ag). We then evaluated the influence of acyl protecting groups. Alkanoyl- and methacryloyl-protected sulfenamides gave the corresponding sulfilimines in 82% and 89% yield, respectively (3ah–3ai). Benzoyl-protected substrates were highly reactive, affording 3aj in 89% yield. Benzoyl derivatives bearing Br, Cl, or F at different positions also reacted smoothly, producing 3ak–3am in 82–94% yield. Even the sterically hindered 2-bromo derivative was well tolerated (89%, 3an). Electron-rich benzoyl groups, such as methyl and methoxy, yielded 3ao (93%) and 3ap (82%), respectively, while CF3-substituted benzoyl sulfenamide afforded 3aq in 83% yield. A range of cross-substituted sulfenamides produced the corresponding sulfilimines in 74–88% yield (3ar–3aw). Pyridyl-substituted sulfenamide proved to be a competent candidate for this transformation, producing 3ax in 46% yield, and alkylated sulfenamides remained highly reactive, providing 3ay–3aa′ in 77–93% yield. The structure of 3aw has been further confirmed by single-crystal X-ray diffraction.
Then, we evaluated the scope of diaryl λ3-bromanes under the optimized conditions. A range of substrates bearing diverse substituents, including halogens, CF3, and methyl groups at various positions, proved compatible. Unsymmetric diaryl λ3-bromanes underwent smooth S-arylation, affording the desired products (3ba–3ga) in good yields. Notably, high regioselectivity was observed with 2-substituted diaryl λ3-bromanes, yielding S-aryl sulfilimines 3ba and 3ca exclusively. In contrast, regioisomers were obtained for 2-methyl substituted diaryl λ3-bromane (3da). Furthermore, 3-substituted analogues gave mixtures of regioisomers (3ea–3ga) due to competing arylation at different sites, likely a consequence of steric hindrance from adjacent substituents and the electrical properties of the aryl rings, which also led to diminished yields. In addition, we further explored the meta-arylation of sulfenamides with diaryl λ3-chloranes. Symmetric λ3-chloranes offered sulfilimines 3ha and 3ja in good yields (80% and 82%, respectively), whereas unsymmetric variants exhibited outstanding regioselectivity, providing 3ka and 3la efficiently. In addition, pyridyl- and alkyl-substituted sulfenamides were also successfully transformed to the desired sulfilimines in good yields (3ma–3na). Notably, a dramatic decrease of yield was observed for cyclic diaryl λ3-iodane under such conditions (3ia), underscoring the distinct reactivity of diaryl λ3-bromanes and λ3-chloranes.
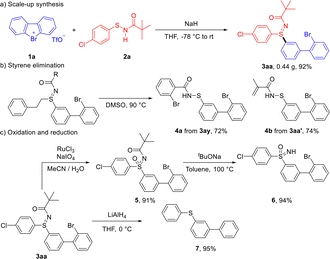
To further demonstrate the practicality and efficiency of this strategy, a scale-up reaction was carried out under the optimized conditions. As shown in Scheme 3a, the reaction proceeded smoothly to afford product 3aa in 92% yield, which is comparable to the yield obtained in the scope study, thereby highlighting the excellent scalability of this protocol and its potential for practical applications. We next explored divergent transformations of the sulfilimine products (Scheme 3b). Sulfilimines 3ay and 3aa′ underwent efficient dealkylation under mild conditions to furnish the corresponding sulfenamides 4a and 4b in good yields, providing a straightforward route to structurally diverse sulfenamides and enabling late-stage functionalization of sulfenamide-based molecules. In addition, sulfilimine 3aa was oxidized to sulfoximine 5 in excellent yield, which was readily deprotected to afford sulfoximine 6 in almost quantitative yield (Scheme 3c). Furthermore, treatment of 3aa with LiAlH4 delivered thioether 7 in good yield, further demonstrating the synthetic versatility of this methodology.
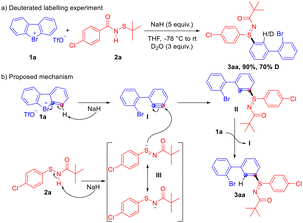
To gain further insight into the mechanism, a deuterated labelling experiment has been conducted (Scheme 4a). Product 3aa was obtained in 90% yield with 70% deuterium incorporation at the ortho-position, indicating the formation of an aryne intermediate. Based on the results and previous reports,17,18 a plausible reaction mechanism is outlined in Scheme 4b. In the presence of NaH, deprotonation of bromane 1a initiates the process, followed by C–Br bond cleavage to generate the aryne intermediate I. Concurrently, NaH deprotonates sulfenamide 2a to form the nucleophilic intermediate III. Given that N-functionalization of sulfenamides is often disfavored compared to the higher reactivity at the sulfur atom,11–14 nucleophilic attack occurs preferentially at the sulfur center of the deprotonated sulfenamide, which has an anionic character as represented in the resonance structure III, generating the anion intermediate II. An autocatalytic process may then occur,17a implying the attack of II on 1a to realize the protonation of II and the generation of aryne intermediate I, providing the desired S-selective and meta-regioselective arylation product 3aa.
In conclusion, we have developed an S-selective and meta-regioselective arylation of sulfenamides with cyclic diaryl λ3-bromanes and λ3-chloranes, enabling efficient synthesis of S-aryl sulfilimines. This protocol features a broad substrate scope, delivering products in good to excellent yields with outstanding selectivity, even for sterically hindered substrates. The method offers rapid access to structurally diverse sulfilimines and demonstrates excellent scalability, with scale-up synthesis providing comparable efficiency to that obtained in the scope study. Moreover, the obtained sulfilimines can be readily further transformed, underscoring the broad synthetic utility and application potential of this strategy in various fields.
We are grateful to the financial support from the National Natural Science Foundation of China (Grant No. 22201144).
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental procedures, characterization data, X-ray crystallographic data, and NMR spectra. See DOI: https://doi.org/10.1039/d5cc05327g.CCDC 2465288 contains the supplementary crystallographic data for this paper.19
Notes and references
- (a) K. A. Scott and J. T. Njardarson, Top. Curr. Chem., 2018, 376, 5 Search PubMed; (b) E. A. Ilardi, E. Vitaku and J. T. Njardarson, J. Med. Chem., 2013, 57, 2832–2842 Search PubMed.
- (a) A. Kausar, S. Zulfiqar and M. I. Sarwar, Polym. Rev., 2014, 54, 185 CrossRef CAS; (b) J. J. Petkowski, W. Bains and S. Seager, J. Nat. Prod., 2018, 81, 423–446 CrossRef CAS PubMed.
- (a) V. V. Chaban, Phys. Chem. Chem. Phys., 2016, 18, 10507–10515 RSC; (b) C. Jacob, Nat. Prod. Rep., 2006, 23, 851–863 RSC.
- (a) T. L. Gilchrist and C. J. Moody, Chem. Rev., 1977, 77, 409–435 CrossRef CAS; (b) N. Furukawa and S. Oae, Ind. Eng. Chem. Prod. Res. Dev., 1981, 20, 260–270 CrossRef CAS; (c) H. Takada, M. Oda, A. Oyamada, K. Ohe and S. Uemura, Chirality, 2000, 12, 299–312 CrossRef CAS PubMed.
- R. Vanacore, A.-J. L. Ham, M. Voehler, C. R. Sanders, T. P. Conrads, T. D. Veenstra, K. B. Sharpless, P. E. Dawson and B. G. Hudson, Science, 2009, 325, 1230–1234 CrossRef CAS PubMed.
- S. Lin, X. Yang, S. Jia, A. Weeks, M. Hornsby, P. S. Lee, R. V. Nichiporuk, A. T. Iavarone, J. A. Wells, F. D. Toste and C. J. Chang, Science, 2017, 355, 597–602 CrossRef CAS PubMed.
- (a) V. V. Thakur, N. S. C. Ramesh Kumar and A. Sudalai, Tetrahedron Lett., 2004, 45, 2915–2918 CrossRef CAS; (b) Q. Cheng, Z. Bai, S. Tewari and T. Ritter, Nat. Chem., 2022, 14, 898–904 CrossRef CAS.
- (a) F. Collet, R. H. Dodd and P. Dauban, Org. Lett., 2008, 10, 5473–5476 CrossRef CAS PubMed; (b) J. Wang, M. Frings and C. Bolm, Angew. Chem., Int. Ed., 2013, 52, 8661–8665 CrossRef CAS; (c) M. Yoshitake, H. Hayashi and T. Uchida, Org. Lett., 2020, 22, 4021–4025 CrossRef CAS PubMed; (d) Z. Liu, H. Wu, H. Zhang, F. Wang, X. Liu, S. Dong, X. Hong and X. Feng, J. Am. Chem. Soc., 2024, 146, 18050–18060 CrossRef CAS.
- (a) F. Xiong, L. Lu, T.-Y. Sun, Q. Wu, D. Yan, Y. Chen, X. Zhang, W. Wei, Y. Lu, W.-Y. Sun, J. J. Li and J. Zhao, Nat. Commun., 2017, 8, 15912 CrossRef PubMed; (b) Z. Xiao, M. Pu, Y. Li, W. Yang, F. Wang, X. Feng and X. Liu, Angew. Chem., Int. Ed., 2025, 64, e202414712 CrossRef CAS.
- Z.-X. Zhang, T. Q. Davies and M. C. Willis, J. Am. Chem. Soc., 2019, 141, 13022–13027 CrossRef CAS PubMed.
- (a) N. S. Greenwood, A. T. Champlin and J. A. Ellman, J. Am. Chem. Soc., 2022, 144, 17808–17814 CrossRef CAS; (b) N. S. Greenwood, N. P. Cerny, A. P. Deziel and J. A. Ellman, Angew. Chem., Int. Ed., 2024, 63, e202315701 CrossRef CAS PubMed; (c) N. S. Greenwood and J. A. Ellman, Org. Lett., 2023, 25, 2830–2834 CrossRef CAS; (d) N. S. Greenwood and J. A. Ellman, Org. Lett., 2023, 25, 4759–4764 CrossRef CAS PubMed; (e) Q. Liang, L. A. Wells, K. Han, S. Chen, M. C. Kozlowski and T. Jia, J. Am. Chem. Soc., 2023, 145, 6310–6318 CrossRef CAS PubMed; (f) X. Wu, M. Chen, F.-S. He and J. Wu, Green Chem., 2023, 25, 9092–9096 RSC; (g) Y. Han, Y. Yuan, S. Qi, Z.-K. Zhang, X. Kong, J. Yang and J. Zhang, Org. Lett., 2024, 26, 3906–3910 CrossRef CAS PubMed; (h) Y. Yuan, Y. Han, Z. K. Zhang, S. Sun, K. Wu, J. Yang and J. Zhang, Angew. Chem., Int. Ed., 2024, 63, e202409541 CrossRef CAS; (i) X. Zhuang, H. Li, Z. Feng and H. Wang, Org. Lett., 2025, 27, 4886–4892 CrossRef CAS.
- (a) Y. Chen, D.-m Fang, H.-S. Huang, X.-K. Nie, S.-Q. Zhang, X. Cui, Z. Tang and G.-X. Li, Org. Lett., 2023, 25, 2134–2138 CrossRef CAS; (b) G. Huang, X. Lu, K. Yang and X. Xu, Org. Lett., 2023, 25, 3173–3178 CrossRef.
- (a) G. Huang, X. Lu and F. Liang, Org. Lett., 2023, 25, 3179–3183 CrossRef CAS; (b) X. Wu, Y. Li, M. Chen, F.-S. He and J. Wu, J. Org. Chem., 2023, 88, 9352–9359 CrossRef CAS PubMed; (c) Q. Zhou, J. Li, T. Wang and X. Yang, Org. Lett., 2023, 25, 4335–4339 CrossRef CAS PubMed.
- (a) Y. Guo, Z. Zhuang, X. Feng, Q. Ma, N. Li, C. Jin, H. Yoshida and J. Tan, Org. Lett., 2023, 25, 7192–7197 CrossRef CAS; (b) X. Wu, M. Chen, F.-S. He and J. Wu, Org. Lett., 2023, 25, 5157–5161 CrossRef CAS.
- (a) Y.-X. Xu, Y.-Q. Liang, W.-M. Liu, H.-K. Fang, H.-K. Li, S.-J. Ji and Z.-J. Cai, Org. Lett., 2024, 26, 9005–9010 CrossRef CAS PubMed; (b) W.-M. Liu, Y.-J. Hao, Y. Zhang, X.-G. Li, S.-J. Ji and Z.-J. Cai, Org. Lett., 2024, 27, 363–368 CrossRef PubMed; (c) B. Kang, L. Wang, X. Sun, H. Liu, Z. Wen, Y. Ren, C. Qi and H. Jiang, Org. Chem. Front., 2023, 10, 5231–5241 RSC; (d) K. Zhao, S. Yang, Q. Gong, L. Duan and Z. Gu, Angew. Chem., Int. Ed., 2021, 60, 5788–5793 CrossRef CAS PubMed; (e) L. Duan, K. Zhao, Z. Wang, F.-L. Zhang and Z. Gu, ACS Catal., 2019, 9, 9852–9858 CrossRef CAS; (f) F. Heinen, E. Engelage, A. Dreger, R. Weiss and S. M. Huber, Angew. Chem., Int. Ed., 2018, 57, 3830–3833 CrossRef CAS.
- (a) W. Fang, Y. D. Meng, S. Y. Ding, J. Y. Wang, Z. H. Pei, M. L. Shen, C. Z. Yao, Q. Li, Z. Gu, J. Yu and H. J. Jiang, Angew. Chem., Int. Ed., 2024, 64, e202419596 CrossRef; (b) X. Liu, Z. Ye, J. Liu, Y. Wu and F. Zhang, Org. Lett., 2025, 27, 6695–6701 CrossRef CAS; (c) L. Duan, Z. Wang, K. Zhao and Z. Gu, Chem. Commun., 2021, 57, 3881–3884 RSC; (d) K. Zhu, Y. Wang, Q. Fang, Z. Song and F. Zhang, Org. Lett., 2020, 22, 1709–1713 CrossRef CAS PubMed; (e) K. Zhu, Z. Song, Y. Wang and F. Zhang, Org. Lett., 2020, 22, 9356–9359 CrossRef CAS; (f) H. Xie, S. Yang, C. Zhang, M. Ding, M. Liu, J. Guo and F. Zhang, J. Org. Chem., 2017, 82, 5250–5262 CrossRef CAS; (g) M. Ding, W. Hua, M. Liu and F. Zhang, Org. Lett., 2020, 22, 7419–7423 CrossRef CAS.
- (a) M. Lanzi, Q. Dherbassy and J. Wencel-Delord, Angew. Chem., Int. Ed., 2021, 60, 14852–14857 CrossRef CAS; (b) M. Lanzi, R. A. Ali Abdine, M. De Abreu and J. Wencel-Delord, Org. Lett., 2021, 23, 9047–9052 CrossRef CAS.
- (a) M. Lanzi, T. Rogge, T. S. Truong, K. N. Houk and J. Wencel-Delord, J. Am. Chem. Soc., 2022, 145, 345–358 CrossRef PubMed; (b) Y. Wang, Y.-N. Tian, S. Ren, R. Zhu, B. Huang, Y. Wen and S. Li, Org. Chem. Front., 2023, 10, 793–798 CAS; (c) D. Carter Martos, M. de Abreu, P. Hauk, P. Fackler and J. Wencel-Delord, Chem. Sci., 2024, 15, 6770–6776 CAS; (d) M. De Abreu, T. Rogge, M. Lanzi, T. J. Saiegh, K. N. Houk and J. Wencel-Delord, Angew. Chem., Int. Ed., 2024, 63, e202319960 CrossRef CAS; (e) K. Patra, M. P. Dey and M. Baidya, Chem. Sci., 2024, 15, 16605–16611 RSC; (f) B. Kang, W. Li, H. Jiang and C. Qi, Chem. Commun., 2025, 61, 3395–3398 RSC; (g) J.-Y. Shou and F.-L. Qing, Org. Lett., 2025, 27, 2815–2820 CrossRef CAS PubMed; (h) J.-Y. Fan, M.-C. Wang, J.-F. Zhou, T.-Q. Gan, W.-X. Zhang, L. Wei and B.-J. Li, Org. Chem. Front., 2025, 12, 1212–1216 RSC.
- CCDC 2465288: Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2nrbf6.
| This journal is © The Royal Society of Chemistry 2026 |