Electro-synergy for the degradation of refractory organic pollutants: coupling heterogeneous electro-Fenton and electro-oxidation
Yuhui
Cai
,
Hao
Liu
,
Jingru
Shu
,
Yuntai
Lin
and
Rui
Yang
 *
*
College of Materials and Chemical Engineering, Chengdu University of Technology, Chengdu, Sichuan 610059, China. E-mail: yangrui@cdut.edu.cn
First published on 28th November 2025
Abstract
This study reports the synthesis of an iron–nickel foam-derived cathode material (INF-600 °C) via a simple chemical impregnation and high-temperature calcination process. The as-prepared material was employed as a cathode to construct a synergistic system integrating heterogeneous electro-Fenton (EF) and electro-oxidation (EO) processes. This system efficiently generates hydroxyl radicals, the primary reactive oxygen species, enabling rapid degradation of tetracycline (TC) over a broad pH range (3–11).
The rapid industrialization has intensified global water pollution challenges.1,2 Refractory organic pollutants are increasingly detected in natural waters, municipal wastewater, and reclaimed water,3 posing significant risks to human health and ecological systems.4 In particular, emerging contaminants are often inadequately removed by conventional treatment technologies,5,6 which are frequently inefficient or ineffective.7–9 Therefore, there is an urgent need to develop advanced water treatment methods to address these growing environmental concerns.
Among numerous treatment technologies, electrochemical advanced oxidation processes (EAOPs) have gained widespread attention due to their high efficiency and environmental compatibility. EO, a representative EAOP, relies on the anodic generation of ˙OH, through water oxidation.10 Boron-doped diamond (BDD) electrodes have been shown to exhibit the highest efficiency for ˙OH production among known anode materials (eqn (S1) and (S2)).11,12 Notably, Nidheesh et al. demonstrated a synergistic effect between EO and EF processes, significantly enhancing the degradation of organic pollutants.13 Yang et al. further developed an integrated electro-oxidation–persulfate–electro-Fenton (EO–PS–EF) triple-coupling system using BDD as the anode,14 achieving efficient pollutant removal through the simultaneous generation of ˙OH and sulfate radicals.
EF technology is a key component of EAOPs, leveraging the Fenton reaction to generate ˙OH (eqn (S3)–(S5)) for effective contaminant degradation.15 Solid catalysts are central to this process. Most current heterogeneous EF catalysts consist of porous carbon matrices loaded with metal active sites;15 however, achieving uniform dispersion of these sites remains challenging due to steric hindrance.16 Additionally, their synthesis often involves complex procedures and harsh conditions, limiting scalability and practical application. In contrast, metallic foams offer advantages including mature fabrication techniques and superior structural stability under prolonged electrochemical operation compared to carbon-based supports.17 Nickel foam (NF) has been demonstrated to promote in situ H2O2 generation during EF processes. For instance, Zhu et al. utilized NF as a cathode in an oxygen mass transfer-enhanced reactor, achieving efficient H2O2 production.18 Sun et al. further revealed that NiO can act as an effective oxygen carrier, capable of adsorbing atmospheric oxygen.19 Meanwhile, iron oxides are widely used in EF systems owing to their catalytic efficiency, low cost, and abundance.20–22 Given the complementary properties of iron and nickel in iron–nickel foam (INF), constructing Ni–Fe composite metal oxides via surface self-growth presents promising potential for developing high-performance EF cathodes.
Building on these insights, this work develops an EO and EF coupling system (EO–EF) based on INF-600 °C (Fig. S1). The synthesized cathode materials were systematically characterized to elucidate their physicochemical properties. Process parameters were optimized using TC as a model pollutant. Furthermore, the practical applicability of the EO–EF system was evaluated through cycling stability tests, assessment of ionic interference, and experiments under varied water quality conditions. The abbreviations used in this study are summarized in Table S1 of the SI.
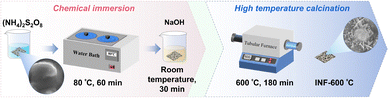
INF-600 °C was fabricated via chemical impregnation followed by calcination at 600 °C (Fig. 1) and subjected to comprehensive characterization. The results confirm that the material primarily consists of iron and nickel mixed oxides. Surface treatment induced significant roughening of the INF substrate (Fig. S2). At 600 °C, a well-developed, interlocked lamellar structure with a porous morphology formed on the surface. Consistent spatial distributions of O, Fe, and Ni (Fig. S2), lattice fringes observed in HRTEM (Fig. S3), and XRD patterns (Fig. S4) collectively verify the successful formation of crystalline metal oxides. XPS analysis revealed a high proportion of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups (32.41%) and an optimal Fe2+/Fe3+ ratio (57.11
O functional groups (32.41%) and an optimal Fe2+/Fe3+ ratio (57.11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42.89), both of which contribute to the enhanced catalytic activity of INF-600 °C (Fig. S5). Compared to INF, INF-600 °C exhibited significantly enhanced hydrophilicity, with a water contact angle approaching 0° (Fig. S6). Electrochemical characterization further demonstrated that INF-600 °C possesses superior electrocatalytic redox activity (Fig. S7)16 and reduced electrical resistance (Fig. S8 and Table S2). These favorable physicochemical properties synergistically improve the material's suitability for application in EAOPs.
42.89), both of which contribute to the enhanced catalytic activity of INF-600 °C (Fig. S5). Compared to INF, INF-600 °C exhibited significantly enhanced hydrophilicity, with a water contact angle approaching 0° (Fig. S6). Electrochemical characterization further demonstrated that INF-600 °C possesses superior electrocatalytic redox activity (Fig. S7)16 and reduced electrical resistance (Fig. S8 and Table S2). These favorable physicochemical properties synergistically improve the material's suitability for application in EAOPs.
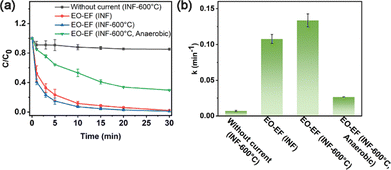
The EO–EF system depicted in Fig. S1 employed INF-600 °C as the cathode and a BDD electrode as the anode for the degradation of TC in aqueous solution. The degradation performance of various system configurations was subsequently evaluated and compared. As shown in Fig. 2, the EO–EF system achieves excellent TC degradation performance. Complete removal of TC was accomplished within 30 min using INF-600 °C as the cathode. The rate constant of 0.1432 min−1 was 5.38 times higher than that under anoxic conditions (k = 0.0266 min−1). Under anoxic conditions, anodic oxidation alone achieved 70.48% TC degradation. Furthermore, the adsorption capacity of the porous foam cathode was evaluated without current and found to be minimal (14.75%), indicating a negligible contribution to overall pollutant removal. Additionally, comparative analysis with other reported degradation systems (Table S3) confirms the superior efficiency of this coupled EO–EF system. These results collectively demonstrate that the integration of INF-600 °C into the EO–EF system effectively promotes TC degradation in water.
Moreover, the influence of six relevant process parameters, including electrode material calcination temperature, electrolyte concentration, and current density on system performance was systematically investigated (Fig. S9). Among these, calcination temperature, electrolyte concentration, and initial current density were found to have the most significant effects. As illustrated in Fig. S9a, INF-600 °C demonstrated the highest reaction rate constant (k = 0.1432 min−1), consistent with prior characterization and electrochemical tests indicating optimal physicochemical properties at a calcination temperature of 600 °C.
Electrolyte concentration and current density are critical factors influencing electrochemical processes. An appropriate Na2SO4 concentration facilitates mass transfer and enhances reaction kinetics.23 As depicted in Fig. S9b, increasing the Na2SO4 concentration from 5 g L−1 to 20 g L−1 resulted in an initial increase in TC degradation efficiency, peaking at 15 g L−1. Beyond this point, further increases led to decreased efficiency, likely due to ion accumulation and electrode passivation caused by excessive ionic strength. The applied current serves as the primary energy input for the system.24,25 Therefore, the effect of current densities ranging from 75 mA cm−2 to 150 mA cm−2 was examined. As shown in Fig. S9c, degradation performance varied significantly across this range, reaching maximum efficiency at 125 mA cm−2 (100% degradation within 30 min). At lower current densities, limited charge transfer restricts H2O2 generation, while excessively high currents promote parasitic side reactions, thereby reducing overall efficiency.26
In alkaline conditions, iron species involved in the Fenton reaction tend to form hydrogen oxygen complexes such as FeOH+, Fe(OH)2+, Fe(OH)3, and Fe(OH)4−, commonly referred to as iron sludge,14,27 which reduces the availability of catalytically active Fe2+ ions. This diminishes ˙OH production and consequently impairs pollutant degradation. Thus, acidic conditions are typically required to prevent sludge formation, necessitating substantial acid addition.28 However, acidic environments pose challenges including equipment corrosion and increased maintenance costs,27 which hinder the practical application of conventional Fenton processes. Therefore, the operational pH range is a crucial parameter for the EO–EF system. Notably, as shown in Fig. S9d, effective TC degradation was achieved over a broad pH range (3–11). A slight decline in efficiency at pH = 3 may be attributed to leaching of metal ions from the cathode under strong acidic conditions, leading to reduced catalytic activity.16 In neutral conditions, the decrease in degradation efficiency is not due to leaching but rather to suppressed H2O2 generation,29 which limits the formation of reactive oxygen species.
The air flow rate influences both H2O2 generation via two-electron oxygen reduction and mass transfer near the electrode surface. As shown in Fig. S9e, the degradation efficiency increased with air flow rate up to 300 mL min−1 (within the tested range of 200–400 mL min−1), beyond which it declined, likely due to inefficient gas utilization or bubble shielding effects. Given that pollutant concentrations vary widely in real wastewater streams, the effect of initial TC concentration on degradation efficiency was also investigated.30 As expected, Fig. S9f shows that the degradation rate decreases with increasing initial TC concentration.
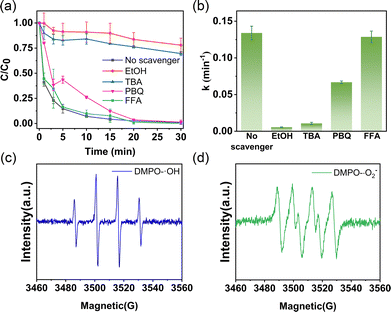
Scavenging experiments and electron paramagnetic resonance (EPR) analysis identified ˙OH as the primary reactive oxygen species (ROSs) in the system, with ˙O2− playing a secondary role. In the quenching experiments, quencher concentrations were optimized to ensure complete scavenging of the target ROS (Fig. S10). The addition of ethanol (EtOH) and tert-butanol (TBA) significantly inhibited TC degradation, reducing the removal efficiency to 22.12% and 30.41%, respectively (Fig. 3a and b). EtOH reacts with both ˙SO4− and ˙OH, whereas TBA selectively scavenges ˙OH, indicating that ˙OH is the dominant radical responsible for TC degradation.31 When p-benzoquinone (PBQ) was introduced as a ˙O2− scavenger, the degradation rate decreased (k = 0.0668 min−1), suggesting that ˙O2− also contributed to the early-stage degradation of TC. Nevertheless, complete degradation was still achieved within 30 min due to the continued presence of ˙OH as the primary oxidizing species. In contrast, the addition of furfuryl alcohol (FFA), a non-radical scavenger, had negligible impact on both the degradation rate (k = 0.1286 min−1) and overall efficiency (100%), indicating minimal contribution from non-radical pathways. These results collectively demonstrate that the radical-based oxidation pathway dominates in the EO–EF system, while non-radical mechanisms play a minor role.
The presence of ˙OH and ˙O2− was further confirmed by EPR spectroscopy. As shown in Fig. 3c, the DMPO–˙OH adduct exhibited a characteristic four-line signal with intensity ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, consistent with typical spin-trapping patterns for ˙OH. In Fig. 3d, the DMPO–˙O2− adduct displayed six distinct peaks – four major peaks with approximately equal intensities (1
1, consistent with typical spin-trapping patterns for ˙OH. In Fig. 3d, the DMPO–˙O2− adduct displayed six distinct peaks – four major peaks with approximately equal intensities (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and two weaker satellite peaks – matching the signature spectrum of ˙O2−. These findings provide direct evidence for the generation of both ˙OH and ˙O2− during the electrochemical process.
1) and two weaker satellite peaks – matching the signature spectrum of ˙O2−. These findings provide direct evidence for the generation of both ˙OH and ˙O2− during the electrochemical process.
The long-term stability and reusability of the INF-600 °C electrode were evaluated over ten consecutive TC degradation cycles. As illustrated in Fig. S11a, the electrode maintained complete degradation efficiency throughout all cycles, with no significant decline in the rate constant, demonstrating excellent catalytic durability. To assess the potential risks of heavy metal leaching, the concentrations of Fe and Ni ions were monitored (Fig. S11b). During the cycling tests, Fe leaching remained low at 2.6–4.6 µg L−1, and Ni leaching ranged from 6.5–12.1 µg L−1. These values are substantially lower than those reported for most conventional metal-based electrodes (Table S3) and well below the European Union Drinking Water Directive limits (Fe: 200 µg L−1; Ni: 20 µg L−1). This confirms the environmental safety of the INF-600 °C-based EO–EF system, with negligible risk of secondary metal pollution.
To evaluate practical applicability, the effects of common anions and cations, as well as different real water matrices, on TC degradation were investigated. As shown in Fig. 4a, cations exerted only a weak inhibitory effect, reducing TC removal by less than 9%, likely due to increased ionic strength slightly hindering mass transfer.32 In contrast, oxygen-containing anions (e.g., CO32−, HCO3−, NO3−) caused more pronounced inhibition (removal reduction of 5.41–15.77%, Fig. 4b), attributed to their competitive reaction with ˙OH radicals.
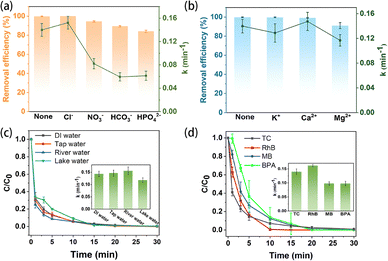 | ||
| Fig. 4 Effect of (a) cations and (b) anions on TC degradation. (c) Degradation of TC in different practical water quality. (d) The EO–HEEF system degrades other pollutants. | ||
Notably, the presence of Cl− enhanced TC degradation (k = 0.1523 min−1), which can be explained by two synergistic mechanisms: first, Cl− acts as a supporting electrolyte, improving charge transfer efficiency;32 second, it participates in chain reactions generating selective reactive chloride species (e.g., ˙Cl, Cl2−), providing an additional oxidative pathway.33 The performance of the system in various real water samples is shown in Fig. 4c. Efficient TC degradation (>90%) was achieved within 30 min in tap water, river water, and lake water, indicating robust performance across diverse real-water matrices. Notably, the rate constants in tap water and river water slightly increased, which may be attributed to the presence of Cl− ions that can generate reactive chlorine species such as ˙Cl, thereby promoting oxidative degradation.34
The energy consumption and operating costs of the EO–EF system were calculated (Table S4). Under optimal conditions, the system consumed 29.79 kWh m−3, with a corresponding operating cost of approximately ¥23.80 m−3, accounting for both electrical energy and chemical reagents. Despite its high degradation efficiency, the relatively high energy demand remains a key constraint for large-scale practical application. Therefore, future research should focus on process optimization to reduce both energy consumption and operational expenses.
Notably, the EO–EF system achieved complete degradation of rhodamine B (RhB), methylene blue (MB), and bisphenol A (BPA) without parameter optimization, demonstrating high removal efficiency across diverse pollutant classes (Fig. 4d). The corresponding pseudo-first-order rate constants reached 0.1616, 0.0979, and 0.0978 min−1, respectively, indicating promising practical application potential for effective elimination of diverse organic contaminants.
In summary, INF-600 °C was synthesized via a chemical impregnation method followed by high-temperature calcination in this work. An EO–EF coupled system was constructed using INF-600 °C as the cathode and a BDD electrode as the anode, exhibiting superior performance in degrading persistent aqueous pollutants. Importantly, the system significantly extended the operational pH range to 3–11, effectively overcoming the narrow pH limitation inherent to conventional Fenton processes. Moreover, it exhibited robust resistance to interference from common anions and maintained high efficiency in complex real water matrices, highlighting its environmental adaptability. The system also showed high degradation efficiency for a wide range of contaminants, including dyes, antibiotics, and phenolic compounds. This work advances the application of metal-based catalysts in electro-Fenton technology and demonstrates the great promise of EO–EF synergy for the remediation of refractory organic wastewater.
The authors gratefully acknowledge the National Natural Science Foundation of China (No. 22206015) and the Natural Science Foundation of Sichuan Province, China (No. 2023NSFSC1122) for their financial support.
Conflicts of interest
There are no conflicts to declare.Data availability
Data supporting this publication can be obtained from the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cc06093a.Notes and references
- Q. Ding, C. Li, H. Wang, C. Xu and H. Kuang, Chem. Commun., 2021, 57, 7215–7231 RSC.
- J. Yang, Y. Zhang, L. Zhang, H. Wang, J. Nie, Z. Qin, J. Li and W. Xiao, Chem. Commun., 2017, 53, 7477–7480 CAS.
- T. Aus Der Beek, F. A. Weber, A. Bergmann, S. Hickmann, I. Ebert, A. Hein and A. Küster, Environ. Toxicol. Chem., 2016, 35, 823–835 CrossRef CAS.
- J. Aguila-Rosas, F. J. Cano, A. Nagaya, C. T. Quirino-Barreda, M. de Jesús Martínez Ortiz, A. G. Vargas, I. A. Ibarra and E. Lima, Chem. Commun., 2025, 61, 11706–11731 RSC.
- Q. Shi, W. Zhu, W. Wang, F. Xie and Y. Feng, Chem. Eng. J., 2025, 517, 164407 CrossRef CAS.
- Q. Shi, Y. Feng, W. Wang, Z. Xu, Z. Li and W. Zhu, Process Saf. Environ. Prot., 2025, 196, 106947 CrossRef CAS.
- B. S. Rathi, P. S. Kumar and P.-L. Show, J. Hazard. Mater., 2021, 409, 124413 CrossRef CAS.
- Y. Gao, W. Zhu, C. Wang, X. Zhao, M. Shu, J. Zhang and H. Bai, Electrochim. Acta, 2020, 330, 135206 CrossRef CAS.
- Y. Gao, W. Zhu, J. Liu, P. Lin, J. Zhang, T. Huang and K. Liu, Appl. Catal., B, 2021, 295, 120273 CrossRef CAS.
- M. K. Wilsey, T. Taseska, Z. Meng, W. Yu and A. M. Müller, Chem. Commun., 2023, 59, 11895–11922 RSC.
- C. A. Martínez-Huitle and M. Panizza, Curr. Opin. Electrochem., 2018, 11, 62–71 CrossRef.
- Y. He, H. Lin, X. Wang, W. Huang, R. Chen and H. Li, Chem. Commun., 2016, 52, 8026–8029 RSC.
- P. V. Nidheesh, S. O. Ganiyu, M.-H. Carlos A, M. Emmanuel, O.-V. Hugo, T. Clément, Z. Minghua and M. A. Oturan, Crit. Rev. Environ. Sci. Technol., 2023, 53, 887–913 CrossRef CAS.
- W. Yang, Z. Deng, L. Liu, K. Zhou, P. E. Sharel, L. Meng, L. Ma and Q. Wei, Water Res., 2023, 243, 120312 CrossRef CAS PubMed.
- X. Wang, C. Xu, Y. Zhu, C. Zhou, Y. Yang, J. Miao, W. Zhou and Z. Shao, Surf. Interface, 2024, 44, 103820 CrossRef CAS.
- Z. Xu, X. Ma, F. He, M. Lu, J. Zhang, S. Wang, P. Dong and C. Zhao, J. Hazard. Mater., 2024, 465, 133193 CrossRef CAS.
- Y. Li, B. Yao, Y. Chen, Y. Zhou and X. Duan, Chem. Eng. J., 2023, 463, 142287 CrossRef CAS.
- Y. Zhu, Q. Shan, D. Fengxia, M. Fang, L. Guojun and Y. Zheng, Environ. Technol., 2020, 41, 730–740 CrossRef CAS.
- M. Sun, Y. Zhang, S.-Y. Kong, L.-F. Zhai and S. Wang, Water Res., 2019, 158, 313–321 CrossRef CAS PubMed.
- N. A. Zubir, C. Yacou, J. Motuzas, X. Zhang, X. S. Zhao and J. C. Diniz da Costa, Chem. Commun., 2015, 51, 9291–9293 RSC.
- G. Zhao, X. Peng, H. Li, J. Wang, L. Zhou, T. Zhao, Z. Huang and H. Jiang, Chem. Commun., 2015, 51, 7489–7492 RSC.
- L. Gong and L. Zhang, Chem. Commun., 2023, 59, 2081–2089 RSC.
- Y. Yao, J. Yang, C. Zhu, L. Lu, Q. Fang, C. Xu, Z. He, S. Song and Y. Shen, J. Hazard. Mater., 2024, 462, 132739 CrossRef CAS PubMed.
- H. Chen, G. Yoshida, F. J. Andriamanohiarisoamanana and I. Ihara, J. Water Process Eng., 2022, 47, 102672 CrossRef.
- B. Ou, J. Wang, Y. Wu, S. Zhao and Z. Wang, Chemosphere, 2020, 245, 125689 CrossRef CAS.
- A. H. Ltaïef, S. Sabatino, F. Proietto, S. Ammar, A. Gadri, A. Galia and O. Scialdone, Chemosphere, 2018, 202, 111–118 CrossRef.
- K. Wang, X. Liu, Y. Jia, L. Pan, M. Shi, W. Pan, N. Li and B. Tang, Chem. Commun., 2024, 60, 4773–4776 RSC.
- J. Lin, S. Chen, H. Xiao, J. Zhang, J. Lan, B. Yan and H. Zeng, Chem. Commun., 2020, 56, 6571–6574 RSC.
- J. Zhang, S. Qiu, H. Feng, T. Hu, Y. Wu, T. Luo, W. Tang and D. Wang, Chem. Eng. J., 2022, 428, 131403 CrossRef CAS.
- M. J. M. Bueno, M. J. Gomez, S. Herrera, M. D. Hernando, A. Agüera and A. R. Fernández-Alba, Environ. Pollut., 2012, 164, 267–273 CrossRef CAS PubMed.
- J. Yan, W. Gao, M. Dong, L. Han, L. Qian, C. P. Nathanail and M. Chen, Chem. Eng. J., 2016, 295, 309–316 CrossRef CAS.
- J. Wang and S. Wang, Chem. Eng. J., 2021, 411, 128392 CrossRef CAS.
- X. Luo, R. Zhu, L. Zhao and X. Gong, Sep. Purif. Technol., 2024, 346, 127451 CrossRef CAS.
- Z. Shen, Y. Zhang, C. Zhou, J. Bai, S. Chen, J. Li, J. Wang, X. Guan, M. Rahim and B. Zhou, Water Res., 2020, 170, 115357 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |