Open Access Article
Open Access ArticlePlatinum–copper nanowire networks with enhanced CO tolerance toward methanol oxidation electrocatalysis†
Shiyue
Xing
a,
Zhongliang
Liu
a,
Yingfang
Jiang
a,
Pinghui
Tang
a,
Jian
Zhang
a,
Jiatang
Chen
 b,
Huihui
Li
*a and
Chunzhong
Li
b,
Huihui
Li
*a and
Chunzhong
Li
 *a
*a
aKey Laboratory for Ultrafine Materials of Ministry of Education, School of Chemical Engineering, East China University of Science and Technology, Shanghai 200237, China. E-mail: huihuili@ecust.edu.cn; czli@ecust.edu.cn
bCornell High Energy Synchrotron Source, Wilson Laboratory, Cornell University, Ithaca, New York 14853, USA
First published on 18th April 2025
Abstract
Developing platinum-based electrocatalysts with high CO tolerance for the methanol oxidation reaction (MOR) is crucial for the practical application of direct methanol fuel cells (DMFCs). Herein, we employed a straightforward one-step method to synthesize PtxCuy network nanowires (NWNs), which exhibit the advantages of structural stability and bimetallic ensembles. The synergistic effect of compressive strain and the ligand effect, induced by Cu incorporation, can effectively lower the d-band center of Pt, thereby weakening the adsorption strength of CO on the catalyst surface. The optimized Pt42Cu58 NWNs deliver a peak mass activity of 1.33 A mgPt−1 and a specific activity of 4.43 mA cm−2 for the MOR, which are 3.03 and 4.03 times higher than those of commercial Pt/C, respectively. The CO stripping and in situ Fourier transform infrared spectroscopy results indicate their high anti-CO poisoning ability and methanol activation capacity. Moreover, the Pt42Cu58 NWNs also exhibit an excellent stability with high current densities observed after 3600 s of operation due to the enhanced CO tolerance and the stable three-dimensional (3D) network structure. This work provides a feasible strategy to suppress CO poisoning during the MOR and obtain highly efficient anode catalysts with enhanced durability in the DMFC field.
1 Introduction
Direct methanol fuel cells (DMFCs) represent a promising electrochemical power source, particularly in view of the usage of liquid methanol, which offers advantages in terms of convenience and security for transportation and storage through the existing gasoline infrastructure, as compared to hydrogen.1 Nevertheless, the sluggish kinetics of the methanol oxidation reaction (MOR), which entails six electron transfer steps at the anode, hinders the progress of DMFCs.2,3 During the stepwise dehydrogenation of methanol, carbonyl intermediates, particularly CO, tend to strongly adsorb onto the catalyst surface, blocking active sites for follow-up reactions.4,5 Platinum (Pt) nanoparticle electrocatalysts are presently recognized as the most effective anode catalysts.1,6,7 However, their catalytic efficiency is significantly hindered by the poisoning effect of the CO intermediate and Ostwald ripening during the MOR process.5,8–10The introduction of late transition metals (LTMs, such as Fe,11 Co,12 Ni13 and Cu14) into active Pt sites has emerged as a significant strategy to enhance the accelerated removal of CO. Incorporating LTMs enables the modification of the electronic structure of Pt and induces compressive strain on its surface, which results from the relatively smaller lattice constants of LTMs compared to those of Pt. Both the strain and ligand effects generated in the alloy can finely tune the d-band center, thereby reducing the adsorption strength of CO* intermediates on Pt active sites.15–20 Moreover, LTMs are abundant in nature and cost-effective, which can significantly reduce the expense of platinum-based catalysts, making them a more viable option for the MOR. Ultrathin one-dimensional nanowires (1D NWs), characterized by a typical diameter of less than 5 nm, exhibit distinctive properties such as high aspect ratios and enhanced atomic utilization efficiency.21 The NWs can readily self-assemble into a three-dimensional (3D) network structure, characterized by a large surface area with abundant exposed active sites, enhanced conductivity, and a stable structure that resists Ostwald ripening and degradation during the reaction.22 Therefore, adjusting the d-band center and enhancing structural stability are crucial for designing high-performance catalysts with superior anti-CO poisoning capabilities.
By comparing with Fe,23 Ni,24 Co,25etc., the Cu incorporation can optimize the d-band center to achieve an optimal balance between reduced CO binding energy and enhanced OH adsorption.26,27 The integration of Cu doping strategies with the distinctive structural advantages of NWs offers a promising approach for the design of MOR catalysts. Significant advancements have been achieved in PtCu NW systems. For instance, Zhang et al. synthesized screw thread-like Pt–Cu NWs featuring high-index facets, which provided an abundance of catalytically active sites and consequently enhanced the intrinsic catalytic activity.28 Other researches also demonstrated that the construction of atomic steps29 and the controlled introduction of surface defects26,29,30 can effectively increase active site density on NW surfaces, thereby boosting MOR performance. However, the critical role of composition-dependent strain–ligand synergy in optimizing CO tolerance and enhancing MOR activity remains underestimated. A more thorough investigation of this regulatory mechanism could reveal new avenues for the design of high-performance MOR catalysts.
Herein, we employed a simple one-step method to introduce Cu into Pt active sites, forming PtxCuy NW networks (NWNs) for the MOR. The characterization and in situ experimental results demonstrate that the synergistic effects of compressive strain and ligand effects, induced by Cu incorporation, can reduce CO adsorption, enhance CO oxidation, and liberate Pt active sites. This promotes MOR activity and improves resistance to CO poisoning. The optimized Pt42Cu58 NWNs demonstrate a peak mass activity (MA) of 1.33 A mgPt−1 and specific activity (SA) of 4.43 mA cm−2 for the MOR, 4.03 times and 5.03 times that of commercial Pt/C, respectively. Moreover, the Pt42Cu58 NWNs exhibit excellent stability with high current densities observed after 3600 s of continuous operation attributed to their superior resistance to CO poisoning and the stable 3D NWN structure.
2 Results and discussion
2.1 Synthesis and characterization
The PtxCuy alloy NWNs were synthesized via a simple one-step method, as illustrated in Fig. 1a, using H2PtCl6 and CuCl2·2H2O as metal precursors, sodium borohydride as a strong reducing agent, and the in situ generated hydrogen as the dynamic template for the formation of 1D structures31 (details in the Experimental section in the ESI†). In this synthesis, the addition of sodium borohydride at low temperatures is crucial in controlling the kinetics of nucleation and growth of NWs. Replacement with another strong reducing agent, such as N2H4·H2O, would result in the formation of nano-dendrites rather than NWs (Fig. S1†), due to the lack of the dynamic template of hydrogen. And the increase in temperature would lead to the formation of low-quality NWNs, due to the accelerated nanoparticle assembly (Fig. S2†).The morphology of Pt42Cu58 NWNs obtained from the scanning electron microscopy (SEM) in Fig. S3† and the low-magnification transmission electron microscope (TEM) in Fig. 1b reveal a 3D porous nano-network structure, which is primarily composed of interconnected wavy 1D NWs with a diameter of approximately 4.1 nm. The selected-area electron diffraction (SAED) pattern (inset of Fig. 1c) officially defines the polycrystalline nature of Pt42Cu58 NWNs. The detailed structural characterization of these NWs was further conducted using high-resolution transmission electron microscopy (HRTEM). HRTEM images reveal that the lattice spacing of the Pt42Cu58 NWNs is 0.215 nm, which is smaller than that of the surface cubic platinum (fcc) at 0.226 nm. This observation indicates the formation of an alloy structure with lattice contraction, as shown in Fig. 1d. The (111) crystal planes on the surface of NWNs are not oriented in parallel, which may lead to the development of wavy structures. The homogeneous distribution of Pt and Cu elements throughout the whole Pt42Cu58 NWN surface was observed in the STEM-EDX elemental mapping images (Fig. 1e). The EDS line-scanning analysis, conducted along the direction indicated by the yellow arrows in Fig. 1e (as shown in Fig. 1f), demonstrated a uniform distribution of Pt and copper Cu. In addition, the atomic ratio of Pt to Cu, as determined by STEM-EDS, was 41.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58.6, which is in agreement with the results obtained from inductively coupled plasma-optical emission spectrometry (ICP-OES) measurements (42.0
58.6, which is in agreement with the results obtained from inductively coupled plasma-optical emission spectrometry (ICP-OES) measurements (42.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58.0). Overall, the aforementioned results provide compelling evidence for the successful synthesis of ultrathin cross-linked Pt42Cu58 NWNs. The catalysts featuring analogous structures but varying Pt/Cu ratios as control samples were also successfully synthesized by adjusting the molar ratio of the two metal precursors. The morphology of the NWNs and the corresponding atomic ratios are presented in Fig. S4–S6 and Table S1.†
58.0). Overall, the aforementioned results provide compelling evidence for the successful synthesis of ultrathin cross-linked Pt42Cu58 NWNs. The catalysts featuring analogous structures but varying Pt/Cu ratios as control samples were also successfully synthesized by adjusting the molar ratio of the two metal precursors. The morphology of the NWNs and the corresponding atomic ratios are presented in Fig. S4–S6 and Table S1.†
2.2 Strain and ligand effects
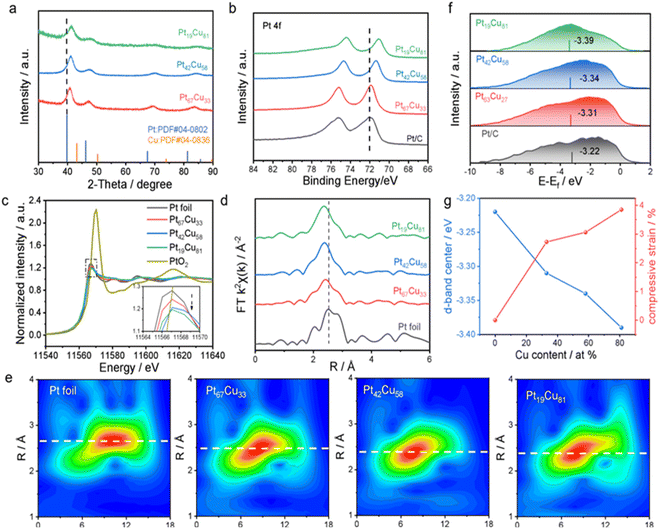
To investigate the phase and electronic structure of the prepared PtxCuy NWNs, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and X-ray adsorption fine structure (XAFS) were carried out. The XRD diffraction peaks of PtxCuy NWNs with different compositions (Fig. 2a) show a typical fcc polycrystalline structure and are located between the standard diffraction spectra of Pt metal (JCPDS card no. 04-0802) and Cu (JCPDS card no. 04-0836), indicating the formation of a PtCu binary alloy. The diffraction peak positions shift to higher angles with increasing Cu content relative to pure Pt, further confirming the presence of compressive strain due to lattice mismatch between Pt and the smaller atomic radius Cu. As shown in Table S2,† the compressive strains induced in the Pt (111) lattice of Pt67Cu33, Pt42Cu58, and Pt19Cu81 are 2.73%, 3.06%, and 3.85%, respectively.32–35XPS analysis revealed the electron transfer from Cu to Pt due to the difference in electronegativity (Fig. 2b and S10†). As shown in Fig. 2b, the binding energy of the Pt 4f peak shifts negatively with lower electronegativity Cu doping and that of Pt42Cu58 NWNs demonstrates a 0.69 eV negative shift compared to commercial Pt/C (Fig. S10†), which can lower the platinum d-band center for the increased number of filled orbitals.18,36 Concurrently, the Cu 2p spectra (Fig. S11†) reveal a gradual positive shift in binding energy with higher Cu content, confirming a reduction in electron density of Cu atoms. This complementary trend in binding energy for Pt 4f and Cu 2p directly validates the electron donation from Cu to Pt. Besides, we can also find that most of the Pt and Cu on the surface of Pt42Cu58 were in the metallic state (Fig. S10†), demonstrating the formation of the alloy.
Fig. 2c presents the normalized Pt L3-edge X-ray absorption near-edge structure (XANES) spectra of PtxCuy NWNs compared with Pt foil and PtO2. They exhibit a high degree of similarity to that of Pt foil, with a notable divergence from that of PtO2, demonstrating the Pt is mainly in a metallic state.37,38 As previously reported, the intensity of the white line is proportional to the transition of electrons from the Pt 2p3/2 core to the 5d state, and it can be used as an indicator of the electron occupancy of the Pt 5d band.20 A lower white line peak intensity is observed in PtxCuy NWNs compared to that in Pt foil. By integrating the Pt L3-edge white line areas,39,40 we quantified the 5d band holes (vacancies) as 0.785, 0.771, and 0.745 for Pt67Cu33, Pt42Cu58, and Pt19Cu81, respectively, systematically lower than those of pure Pt (1.000) and demonstrating a Cu-dependent reduction trend (Fig. S7, S8 and Table S3†). This progressive decline in 5d vacancies signifies enhanced electron density within the Pt 5d orbitals upon Cu incorporation, corroborating our XPS findings. This electron enrichment lowers the Pt d-band center, thereby weakening adsorbate binding strength.36 The Fourier transform extended XAFS (EXAFS) (Fig. 2d) and its fitting results (Fig. S9 and Table S3†) clearly confirmed the coordination environment of the Pt L3 edge for the catalysts. As shown in Fig. 2d, the k2-weighted EXAFS for PtxCuy NWNs at Pt L3-edge without phase correction shows a lower scattering peak (Pt67Cu33 at 2.479 Å, Pt42Cu58 at 2.446 Å, and Pt19Cu81 at 2.402 Å) in contrast to Pt foil (2.632 Å), which could be assigned to the Pt–Pt/Cu paths, confirming that the PtxCuy NWNs predominantly exhibit shortened Pt–M (M = Pt/Cu) metal bonds. This observation indicates the formation of the PtCu alloy structure with lattice contraction. Meanwhile, the fitting results in Table S3† can also prove the existence of Pt–Pt and Pt–Cu bonds in the PtCu NWWs. For instance, the main metal bonds are the Pt–Cu bond with a coordination number of 4.1 and the Pt–Pt bond with a coordination number of 5.0 in Pt42Cu58 NWNs (Table S4†). There are no Pt–O bonds in the PtxCuy NWNs compared to PtO2, which is also visually confirmed by the wavelet transform (WT) (Fig. 2e and S12†). Furthermore, it is noteworthy that the bond length of the Pt–M bond (about 2.65–2.72 Å) in R space has been shortened with more Cu content involved in comparison to the Pt–Pt bond (2.77 Å) in Pt foil from the EXAFS and WT analysis, demonstrating the compressive strain involved, which is in agreement with the HRTEM and the XRD results.41 The compressive strain can broaden the d-band and then downshift the d-band center, resulting from the increasing orbital overlap in Pt atoms after smaller metal atoms are involved.42,43
Prior research has demonstrated that a negative shift in the d-band center can weaken the binding energy between the catalyst surface and adsorbates.44,45 The findings illustrated above indicate that PtxCuy NWNs, which exhibit a rich-electron Pt surface and existing compressive strain, can synergistically decrease the d-band center, causing it to deviate from the Fermi level. Therefore, the XPS valence band spectrum of the catalysts was measured and analyzed (Fig. 2f). The results show that the d-band center positions of the compressive strained Pt67Cu33 NWNs, Pt42C58 NWNs, and Pt19Cu81 NWNs have decreased by 0.09 eV, 0.12 eV, and 0.17 eV, respectively, compared to that of Pt/C, suggesting that Cu incorporation leads to a downward shift in the d-band center, which may enhance the MOR activity by weakening the binding force between the Pt-based catalysts and the carbonyl intermediate species adsorbed on the surface. Furthermore, a significant positive correlation was observed among the compressive strain, the d-band center, and the Cu content (Fig. 2g). This indicates that an increase in Cu content corresponds to a greater compressive strain and a lower d-band center. Thus, the compressive strain and d-band electrons can be effectively tuned by merely modifying the Cu content, allowing for the regulation of intermediate adsorption strength and the optimization of catalyst performance for the MOR.
2.3 Electrocatalytic performances of the PtxCuy NWNs
The MOR activity and stability of the obtained PtxCuy NWNs and commercial Pt/C were evaluated under acid conditions. The details of the tests were in the Experimental section in the ESI.† The electrochemically active surface area (ECSA) was measured by CV in acid electrolyte at 50 mV s−1 (Fig. 3a). The calculated ECSA values for Pt67Cu33, Pt42Cu58, Pt19Cu81, and commercial Pt/C are 29.12, 30.09, 33.10, and 37.75 m2 gPt−1, respectively. Besides, the ECSA values of the three PtxCuy NWNs are comparable to those of Pt/C, indicating that the 3D nano-networks with ultrathin NWs could effectively expose active sites and improve atomic utilization.The MOR curves of catalysts were recorded in an acid electrolyte containing methanol (see Fig. 3b and c). As illustrated in Fig. 3d, the Pt42Cu58 NWNs demonstrate the highest mass activity (MA, 1.33 A mgPt−1), which is 1.36, 1.39, and 4.03 times higher than those of Pt67Cu33 NWNs (0.98 A mgPt−1), Pt19Cu81 NWNs (0.96 A mgPt−1), and commercial Pt/C (0.33 A mgPt−1), respectively. Furthermore, the specific activity (SA, 4.43 mA cm−2) of Pt42Cu58 NWNs obtained by normalizing the ECSA is also higher than those of Pt67Cu33 NWNs (3.39 mA cm−2), Pt19Cu81 NWNs (2.63 mA cm−2), and commercial Pt/C (0.88 mA cm−2), respectively. A significant volcano-type dependence on MOR activity was observed, influenced by the Cu component, compressive strain, and the position of the d-band center (Fig. S13†). The MOR activity of Pt42Cu58 NWNs is compared with that of previously reported Pt-based catalysts, as illustrated in Fig. 3d and summarized in Table S5.† The enhanced MOR activity may be attributed to the optimized electron structure of Pt, which has been tuned by strain and ligand effects after Cu doping and the presence of enriched active sites for the ultrathin 3D structure.
The catalytic stability was evaluated through chronoamperometry (CA) tests. As illustrated in Fig. 3d, the current densities for Pt42Cu58 NWNs remained superior to those of the other catalysts throughout the 3600 s CA tests. The structural change of Pt42Cu58 NWNs was negligible, except for a slight widening of the lattice stripes from 0.215 nm to 0.218 nm, which may result from the unavoidable leaching of Cu atoms during the reaction process (Fig. S14†). In contrast, the activity of Pt/C was poor, accompanied by severely damaged morphology due to CO poisoning and severe aggregation of the nanoparticles (Fig. S15†). The electrochemical tests above demonstrate that the excellent MOR catalytic activity and stability of Pt42Cu58 NWNs can be attributed to the robust CO tolerance after Cu doping and its advanced 3D structure.
To achieve a deeper understanding of the catalysts' activity, a series of kinetic studies were undertaken. Linear sweep voltammetry (LSV) measurements were conducted in acidic methanol media. As shown in Fig. S16a and b,† Pt42Cu58 NWNs have the lowest onset potential and Tafel slope (80.45 mV dec−1) compared to the other catalysts, indicating their enhanced MOR kinetics. As shown in Fig. S16c and Table S6,† Pt42Cu58 NWNs also possess the highest values of j0 and α, both of which are calculated from the Tafel equation, demonstrating accelerated electron transfer.46 The electrochemical impedance spectroscopy (EIS) analysis was further performed, as shown in Fig. S16d.† The impedance arc diameter of the Pt42Cu58 NWNs is significantly smaller compared to that of the other catalysts, indicating a lower charge transfer resistance and enhanced kinetic activity. These findings are in agreement with the previously mentioned results. Additionally, LSV curves at various scan rates were obtained for all samples (Fig. S17†). The plots of current density versus the square root of the scan rate for all electrocatalysts demonstrate a linear relationship, suggesting a diffusion-limited process.47 The Pt42Cu56 NWNs show the highest slope of 0.52, demonstrating accelerated mass/electron transfer.46 Finally, we also obtained LSV profiles at different temperatures to investigate the thermodynamics of the MOR for all samples. Fig. S18† illustrates the linear relationship between the current density ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j and 1/T for all the samples. The corresponding apparent activation energy values (Ea) calculated from the slope value of the Arrhenius plot can reflect the adsorption energy of methanol and intermediate species on the catalyst surface.48 The Pt42Cu58 NWNs show the smallest value of Ea (39.93 kJ mol−1) compared to the other catalysts, indicating that methanol oxidation is more facile for Pt42Cu58 NWNs.49 In brief, Pt42Cu58 NWNs that achieve an optimal balance between the strain and ligand effects can substantially reduce the energy barrier and enhance the kinetics of the MOR.
j and 1/T for all the samples. The corresponding apparent activation energy values (Ea) calculated from the slope value of the Arrhenius plot can reflect the adsorption energy of methanol and intermediate species on the catalyst surface.48 The Pt42Cu58 NWNs show the smallest value of Ea (39.93 kJ mol−1) compared to the other catalysts, indicating that methanol oxidation is more facile for Pt42Cu58 NWNs.49 In brief, Pt42Cu58 NWNs that achieve an optimal balance between the strain and ligand effects can substantially reduce the energy barrier and enhance the kinetics of the MOR.
2.4 Anti-CO poisoning ability of the PtxCuy NWNs
To further explore the anti-CO poisoning ability of Pt42Cu58 NWNs in the enhancement of MOR performance, we employed CO stripping and in situ attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy. CO is a key intermediate in the MOR process.50 As shown in Fig. 4a, the CO stripping peak potentials for all PtxCuy alloys shift negatively in comparison to commercial Pt/C (0.881 V vs. RHE), indicating improved CO oxidation capabilities and increased resistance to CO poisoning. Notably, the Pt42Cu58 NWNs (0.734 V vs. RHE) display a lower peak potential than the Pt19Cu81 NWNs (0.749 V vs. RHE) and Pt19Cu81 NWNs (0.741 V vs. RHE), suggesting that a moderate downshift in the d-band center is crucial for facilitating the removal of CO and enhancing the capacities for CO tolerance. | ||
| Fig. 4 (a) CO stripping tests of PtxCuy NWNs and commercial Pt/C. (b) Electrochemical in situ ATR-FTIR spectrum of the MOR on Pt42Cu58 NWNs and (c) Pt/C. | ||
ATR-FTIR spectroscopy was further used to detect the adsorbed species at the molecular level on the optimized Pt42Cu58 NWNs and commercial Pt/C for comparison (Fig. 4b and c). We can find featured bonds like the stretching vibration C–*OH (about 1022 cm−1) and the antisymmetric stretching vibration *COO (from 1500 to 1630 cm−1), which belong to methanol and the intermediate *COOH, respectively.5,51,52 With the potential improved, those bonds appeared first at 0.36 V in Pt42Cu58 NWNs, 100 mV lower than that of commercial Pt/C (0.46 V), implying the high methanol activation capacity of Pt42Cu58 NWNs. The stretching bonds O–H (about 3700 cm−1) detected in the two samples may be assigned to OH* from the water dissociation,53 which can oxidize the CO* to form *COOH and then dehydrogenate to CO2.14 The combined results of CO stripping and in situ ATR demonstrate that the Pt42Cu56 NWNs exhibit a high anti-CO poisoning ability and methanol activation capacity. This may be attributed to the weak adsorption of CO on Pt sites, which facilitates the removal of CO and then the liberation of active sites, thereby promoting methanol activation.
3 Conclusions
In conclusion, we designed a kind of compressive strained Pt42Cu58 NWN with enhanced catalytic activity and robust anti-CO poisoning capacity for the MOR. The experiments demonstrated that the synergy of compressive strain and the ligand effect endowed the catalysts with a downward shifted d-band center, which can weaken the CO adsorption strength on the Pt surface. The optimized Pt42Cu58 NWNs achieve a high MOR mass activity of 1.33 A mgPt−1 and specific activity of 4.43 mA cm−2, which are 4.03 and 5.03 times higher than those of Pt/C, respectively. The CO striping and in situ ATR-FTIR results further indicate their enhanced CO tolerance ability and methanol activation capacity. Moreover, the Pt42Cu58 NWNs exhibit excellent stability with high current densities observed after 3600 s of continuous operation attributed to their superior resistance to CO poisoning and the stable 3D NWN structure. This work provides a feasible strategy to suppress CO poisoning during methanol oxidation electrocatalysis and obtain highly efficient anode catalysts with enhanced durability in the DMFC field.Data availability
All data generated or analyzed during this study are included in this article and the ESI.†Author contributions
All of the authors contributed to the manuscript preparation. Shiyue Xing, Huihui Li and Chunzhong Li conceived the outline of the manuscript. Shiyue Xing, Huihui Li and Chunzhong Li wrote the original draft of the manuscript. Zhongliang Liu, Yingfang Jiang, Pinghui Tang, Jian Zhang, and Jiatang Chen discussed and helped revise the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (U22B20143, U24A20546, and 22478121), the Shanghai Municipal Science and Technology Major Project, and the Science and Technology Commission of Shanghai Municipality (22dz1205900).References
- J. Wang, B. Zhang, W. Guo, L. Wang, J. Chen, H. Pan and W. Sun, Toward Electrocatalytic Methanol Oxidation Reaction: Longstanding Debates and Emerging Catalysts, Adv. Mater., 2023, 35, 2211099 CrossRef CAS PubMed
.
- H. Li, Y. Pan, D. Zhang, Y. Han, Z. Wang, Y. Qin, S. Lin, X. Wu, H. Zhao, J. Lai, B. Huang and L. Wang, Surface oxygen-mediated ultrathin PtRuM (Ni, Fe, and Co) nanowires boosting methanol oxidation reaction, J. Mater. Chem. A, 2020, 8, 2323–2330 RSC
.
- F. Lyu, M. Cao, A. Mahsud and Q. Zhang, Interfacial engineering of noble metals for electrocatalytic methanol and ethanol oxidation, J. Mater. Chem. A, 2020, 8, 15445–15457 RSC
.
- A. R. Poerwoprajitno, L. Gloag, J. Watt, S. Cheong, X. Tan, H. Lei, H. A. Tahini, A. Henson, B. Subhash, N. M. Bedford, B. K. Miller, P. B. O'Mara, T. M. Benedetti, D. L. Huber, W. Zhang, S. C. Smith, J. J. Gooding, W. Schuhmann and R. D. Tilley, A single-Pt-atom-on-Ru-nanoparticle electrocatalyst for CO-resilient methanol oxidation, Nat. Catal., 2022, 5, 231–237 CrossRef CAS
.
- A. Xu, T. Liu, D. Liu, W. Li, H. Huang, S. Wang, L. Xu, X. Liu, S. Jiang, Y. Chen, M. Sun, Q. Luo, T. Ding and T. Yao, Edge-Rich Pt-O-Ce Sites in CeO2 Supported Patchy Atomic-Layer Pt Enable a Non-CO Pathway for Efficient Methanol Oxidation, Angew. Chem., Int. Ed., 2024, 63, e202410545 CrossRef CAS PubMed
.
- M. Zhou, C. Li and J. Fang, Noble-Metal Based Random Alloy and Intermetallic Nanocrystals: Syntheses and Applications, Chem. Rev., 2021, 121, 736–795 CrossRef CAS PubMed
.
- L. Huang, X. Zhang, Q. Wang, Y. Han, Y. Fang and S. Dong, Shape-Control of Pt–Ru Nanocrystals: Tuning Surface Structure for Enhanced Electrocatalytic Methanol Oxidation, J. Am. Chem. Soc., 2018, 140, 1142–1147 CrossRef CAS PubMed
.
- L. Hui, D. Yan, X. Zhang, H. Wu, J. Li and Y. Li, Halogen Tailoring of Platinum Electrocatalyst with High CO Tolerance for Methanol Oxidation Reaction, Angew. Chem., Int. Ed., 2024, 63, e202410413 CrossRef CAS PubMed
.
- R. Chen, Z. Wang, S. Chen, L. Wang, W. Wu, Y. Zhu and N. Cheng, Optimizing Intermediate Adsorption on Pt Sites via Triple-Phase Interface Electronic Exchange for Methanol Oxidation, Inorg. Chem., 2024, 63, 4364–4372 CrossRef CAS PubMed
.
- G. Bai, C. Liu, Z. Gao, B. Lu, X. Tong, X. Guo and N. Yang, Atomic Carbon Layers Supported Pt Nanoparticles for Minimized CO Poisoning and Maximized Methanol Oxidation, Small, 2019, 15, 1902951 CrossRef PubMed
.
- Q. Yang, S. Zhang, F. Wu, L. Zhu, G. Li, M. Chen, A. Pei and Y. Feng, Efficient and stable PtFe alloy catalyst for electrocatalytic methanol oxidation with high resistance to CO, J. Energy Chem., 2024, 90, 327–336 CrossRef CAS
.
- B. Y. Xia, H. B. Wu, N. Li, Y. Yan, X. W. D. Lou and X. Wang, One-Pot Synthesis of Pt-Co Alloy Nanowire Assemblies with Tunable Composition and Enhanced Electrocatalytic Properties, Angew. Chem., Int. Ed., 2015, 54, 3797–3801 CrossRef CAS PubMed
.
- T. Xia, K. Zhao, Y. Zhu, X. Bai, H. Gao, Z. Wang, Y. Gong, M. Feng, S. Li, Q. Zheng, S. Wang, R. Wang and H. Guo, Mixed-Dimensional Pt–Ni Alloy Polyhedral Nanochains as Bifunctional Electrocatalysts for Direct Methanol Fuel Cells, Adv. Mater., 2023, 35, 2206508 CrossRef CAS PubMed
.
- Y. Sun, S. Zhang, S. Sun, L. Wu, J. Tian, Y. Wu, Y. Chen and X. Liu, Cu Tailoring Pt Enables Branched-Structured Electrocatalysts with High Concave Surface Curvature Toward Efficient Methanol Oxidation, Small, 2023, 20, 2307970 CrossRef PubMed
.
- S. Chen, N. Liu, J. Zhong, R. Yang, B. Yan, L. Gan, P. Yu, X. Gui, H. Yang, D. Yu, Z. Zeng and G. Yang, Engineering Support and Distribution of Palladium and Tin on MXene with Modulation of the d-Band Center for CO-resilient Methanol Oxidation, Angew. Chem., Int. Ed., 2022, 61, e202209693 CrossRef CAS PubMed
.
- T. Singha, S. Tomar, S. Das and B. Satpati, D-Band Engineering in Pd-Based Nanowire Networks for Further Enhancement in Ethanol Electrooxidation Reaction, Small Methods, 2024, 8, 2400368 CrossRef CAS PubMed
.
- V. R. Stamenkovic, B. S. Mun, M. Arenz, K. J. Mayrhofer, C. A. Lucas, G. Wang, P. N. Ross and N. M. Markovic, Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces, Nat. Mater., 2007, 6, 241–247 CrossRef CAS PubMed
.
- J. Chen, F. Zhang, M. Kuang, L. Wang, H. Wang, W. Li and J. Yang, Unveiling synergy of strain and ligand effects in metallic aerogel for electrocatalytic polyethylene terephthalate upcycling, Proc. Natl. Acad. Sci. U.S.A., 2024, 121, e2318853121 CrossRef CAS PubMed
.
- Y. He, Y. Chen, R. Wu, Z. Xiao, M. Li, C. Shi and L. Wang, Ligand effect in surface atomic sites of group VI B transition metals on ultrathin Pt nanowires for enhanced oxygen reduction, Nano Res., 2024, 17, 5298–5304 CrossRef CAS
.
- L. Tao, K. Wang, F. Lv, H. Mi, F. Lin, H. Luo, H. Guo, Q. Zhang, L. Gu, M. Luo and S. Guo, Precise synthetic control of exclusive ligand effect boosts oxygen reduction catalysis, Nat. Commun., 2023, 14, 6893 CrossRef CAS PubMed
.
- H. Xu, H. Shang, C. Wang and Y. Du, Ultrafine Pt-Based Nanowires for Advanced Catalysis, Adv. Funct. Mater., 2020, 30, 2000793 CrossRef CAS
.
- Q. Shao, K. Lu and X. Huang, Platinum Group Nanowires for Efficient Electrocatalysis, Small Methods, 2019, 3, 1800545 CrossRef
.
- Y. Nie, X. Qi, R. Wu, R. Yang, H. Wang, M. Deng, S. Zhang, S. Lu, Z. Gu and X. Liu, Structurally ordered PtFe intermetallic nanocatalysts toward efficient electrocatalysis of methanol oxidation, Appl. Surf. Sci., 2021, 569, 151004 CrossRef CAS
.
- X. Chen, J. Zhao, J. Lian and X. Wang, Achieving superior methanol oxidation electrocatalytic performance by surface reconstruction of PtNi nanoalloys during acid etching process, Green Chem., 2023, 25, 3198–3207 RSC
.
- S. Papadimitriou, S. Armyanov, E. Valova, A. Hubin, O. Steenhaut, E. Pavlidou, G. Kokkinidis and S. Sotiropoulos, Methanol Oxidation at Pt–Cu, Pt–Ni, and Pt–Co Electrode Coatings Prepared by a Galvanic Replacement Process, J. Phys. Chem. C, 2010, 114, 5217–5223 CrossRef CAS
.
- K. Wang, D. Huang, Y. Guan, F. Liu, J. He and Y. Ding, Fine-Tuning the Electronic Structure of Dealloyed PtCu Nanowires for Efficient Methanol Oxidation Reaction, ACS Catal., 2021, 11, 14428–14438 CrossRef CAS
.
- H. Q. Pham and T. T. Huynh, Platinum–Copper Bimetallic Nanodendritic Electrocatalyst on a TiO2-Based Support for Methanol Oxidation in Alkaline Fuel Cells, ACS Appl. Nano Mater., 2021, 4, 4983–4993 CrossRef CAS
.
- N. Zhang, L. Bu, S. Guo, J. Guo and X. Huang, Screw Thread-Like Platinum–Copper Nanowires Bounded with High-Index Facets for Efficient Electrocatalysis, Nano Lett., 2016, 16, 5037–5043 CrossRef CAS PubMed
.
- D. Fang, L. Wan, Q. Jiang, H. Zhang, X. Tang, X. Qin, Z. Shao and Z. Wei, Wavy PtCu alloy nanowire networks with abundant surface defects enhanced oxygen reduction reaction, Nano Res., 2019, 12, 2766–2773 CrossRef CAS
.
- N. Guo, H. Xue, A. Bao, Z. Wang, J. Sun, T. Song, X. Ge, W. Zhang, K. Huang, F. He and Q. Wang, Achieving Superior Electrocatalytic Performance by Surface Copper Vacancy Defects during Electrochemical Etching Process, Angew. Chem., Int. Ed., 2020, 59, 13778–13784 CrossRef CAS PubMed
.
- L. Liu, L.-X. Chen, A.-J. Wang, J. Yuan, L. Shen and J.-J. Feng, Hydrogen bubbles template-directed synthesis of self-supported AuPt nanowire networks for improved ethanol oxidation and oxygen reduction reactions, Int. J. Hydrogen Energy, 2016, 41, 8871–8880 CrossRef CAS
.
- Y. Zheng, A. S. Petersen, H. Wan, R. Hübner, J. Zhang, J. Wang, H. Qi, Y. Ye, C. Liang, J. Yang, Z. Cui, Y. Meng, Z. Zheng, J. Rossmeisl and W. Liu, Scalable and Controllable Synthesis of Pt-Ni Bunched-Nanocages Aerogels as Efficient Electrocatalysts for Oxygen Reduction Reaction, Adv. Energy Mater., 2023, 13, 2204257 CrossRef CAS
.
- S. Wang, T. Sheng and Q. Yuan, Low-Pt Octahedral PtCuCo Nanoalloys: “One Stone, Four Birds” for Oxygen Reduction and Methanol Oxidation Reactions, Inorg. Chem., 2023, 62, 11581–11588 CrossRef CAS PubMed
.
- H. Guo, L. Li, Y. Chen, W. Zhang, C. Shang, X. Cao, M. Li, Q. Zhang, H. Tan, Y. Nie, L. Gu and S. Guo, Precise Strain Tuning Boosts Electrocatalytic Hydrogen Generation, Adv. Mater., 2023, 35, 2302285 CrossRef CAS PubMed
.
- K. Peng, N. Bhuvanendran, S. Ravichandran, W. Zhang, Q. Ma, Q. Xu, L. Xing, L. Khotseng and H. Su, Bimetallic Pt3Mn nanowire network structures with enhanced electrocatalytic performance for methanol oxidation, Int. J. Hydrogen Energy, 2020, 45, 30455–30462 CrossRef CAS
.
- J. T. L. Gamler, H. M. Ashberry, S. E. Skrabalak and K. M. Koczkur, Random Alloyed versus Intermetallic Nanoparticles: A Comparison of Electrocatalytic Performance, Adv. Mater., 2018, 30, 1801563 CrossRef PubMed
.
- Y. Lv, L. Lin, R. Xue, P. Zhang, F. Ma, T. Gan, J. Zhang, D. Gao, X. Zheng, L. Wang, Y. Qin, H. Zhao, Y. Dong, Y. Wang and Y. Zhu, Electronegativity Induced d-Band Center Offset for Pt-Rh Dual Sites in High-Entropy Alloy Boosts Liquid Fuels Electrooxidation, Adv. Energy Mater., 2024, 14, 2304515 CrossRef CAS
.
- T. Shen, D. Xiao, Z. Deng, S. Wang, L. An, M. Song, Q. Zhang, T. Zhao, M. Gong and D. Wang, Stabilizing Diluted Active Sites of Ultrasmall High-Entropy Intermetallics for Efficient Formic Acid Electrooxidation, Angew. Chem., Int. Ed., 2024, 63, e202403260 CrossRef CAS PubMed
.
- J. Chen, Y. M. Yiu, Z. Wang, D. Covelli, R. Sammynaiken, Y. Z. Finfrock and T.-K. Sham, Elucidating the Many-Body Effect and Anomalous Pt and Ni Core Level Shifts in X-ray Photoelectron Spectroscopy of Pt–Ni Alloys, J. Phys. Chem. C, 2020, 124, 2313–2318 CrossRef CAS
.
- D. Wang, X. Cui, Q. Xiao, Y. Hu, Z. Wang, Y. M. Yiu and T. K. Sham, Electronic behaviour of Au-Pt alloys and the 4f binding energy shift anomaly in Au bimetallics- X-ray spectroscopy studies, AIP Adv., 2018, 8, 065210 CrossRef
.
- Y. Nie, Y. Sun, B. Song, Q. Meyer, S. Liu, H. Guo, L. Tao, F. Lin, M. Luo, Q. Zhang, L. Gu, L.-M. Yang, C. Zhao and S. Guo, Low-Electronegativity Mn-Contraction of PtMn Nanodendrites Boosts Oxygen Reduction Durability, Angew. Chem., Int. Ed., 2023, 136, e202317987 CrossRef
.
- D. Wang, Z. Chen, Y.-C. Huang, W. Li, J. Wang, Z. Lu, K. Gu, T. Wang, Y. Wu, C. Chen, Y. Zhang, X. Huang, L. Tao, C.-L. Dong, J. Chen, C. V. Singh and S. Wang, Tailoring lattice strain in ultra-fine high-entropy alloys for active and stable methanol oxidation, Sci. China Mater., 2021, 64, 2454–2466 CrossRef CAS
.
- K. Xu, L. Liang, T. Li, M. Bao, Z. Yu, J. Wang, S. M. Thalluri, F. Lin, Q. Liu, Z. Cui, S. Song and L. Liu, Pt1.8Pd0.2CuGa Intermetallic Nanocatalysts with Enhanced Methanol Oxidation Performance for Efficient Hybrid Seawater Electrolysis, Adv. Mater., 2024, 36, e2403792 CrossRef PubMed
.
- X. Yang, Y. Wang, X. Tong and N. Yang, Strain Engineering in Electrocatalysts: Fundamentals, Progress, and Perspectives, Adv. Energy Mater., 2022, 12, 2102261 CrossRef CAS
.
- Q. Yao, Z. Yu, L. Li and X. Huang, Strain and Surface Engineering of Multicomponent Metallic Nanomaterials with Unconventional Phases, Chem. Rev., 2023, 123, 9676–9717 CrossRef CAS PubMed
.
- T. T. Huynh, Q. Huynh, Q. V. Nguyen and H. Q. Pham, Lattice Strain and Composition Effects on the Methanol Oxidation Performance of Platinum–Ruthenium–Nickel Ternary Nanocatalysts, Inorg. Chem., 2023, 62, 20477–20487 CrossRef CAS PubMed
.
- J. Hu, C. Fang, X. Jiang, D. Zhang and Z. Cui, Ultrathin and Porous 2D PtPdCu Nanoalloys as High-Performance Multifunctional Electrocatalysts for Various Alcohol Oxidation Reactions, Inorg. Chem., 2022, 61, 9352–9363 CrossRef CAS PubMed
.
- T. T. Huynh, Q. Huynh, A. Q. K. Nguyen and H. Q. Pham, Strong Component-Interaction in N-doped 2D Ti3C2Tx-Supported Pt Electrocatalyst for Acidic Ethanol Oxidation Reaction, Adv. Sustainable Syst., 2025, 9, 2400995 CrossRef CAS
.
- K. Zhang, H. Wang, J. Qiu, J. Wu, H. Wang, J. Shao, Y. Deng and L. Yan, Multi-dimensional Pt/Ni(OH)2/nitrogen-doped graphene nanocomposites with low platinum content for methanol oxidation reaction with highly catalytic performance, Chem. Eng. J., 2021, 421, 127786 CrossRef CAS
.
- T. He, W. Wang, F. Shi, X. Yang, X. Li, J. Wu, Y. Yin and M. Jin, Mastering the surface strain of platinum catalysts for efficient electrocatalysis, Nature, 2021, 598, 76–81 CrossRef CAS PubMed
.
- L. He, M. Li, L. Qiu, S. Geng, Y. Liu, F. Tian, M. Luo, H. Liu, Y. Yu, W. Yang and S. Guo, Single-atom Mo-tailored high-entropy-alloy ultrathin nanosheets with intrinsic tensile strain enhance electrocatalysis, Nat. Commun., 2024, 15, 2290 CrossRef CAS PubMed
.
- F. Yang, J. Ye, L. Gao, J. Yu, Z. Yang, Y. Lu, C. Ma, Y. J. Zeng and H. Huang, Ultrathin PtNiGaSnMoRe Senary Nanowires with Partial Amorphous Structure Enable Remarkable Methanol Oxidation Electrocatalysis, Adv. Energy Mater., 2023, 13, 2301408 CrossRef CAS
.
- J. Zhu, L. Xia, R. Yu, R. Lu, J. Li, R. He, Y. Wu, W. Zhang, X. Hong, W. Chen, Y. Zhao, L. Zhou, L. Mai and Z. Wang, Ultrahigh Stable Methanol Oxidation Enabled by a High Hydroxyl Concentration on Pt Clusters/MXene Interfaces, J. Am. Chem. Soc., 2022, 144, 15529–15538 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc00656b |
| This journal is © The Royal Society of Chemistry 2025 |