Open Access Article
Open Access ArticleThiophene-backbone arcuate graphene nanoribbons: shotgun synthesis and length dependent properties†
Ruiying
Zhang
a,
Xinyu
Chen
 a,
Lingyun
Zhu
a,
Yanxia
Huang
a,
Zi'ang
Zhai
a,
Qiang
Wang
a,
Lingding
Wang
a,
Taosong
Wang
a,
Wei-Zhen
Wang
a,
Ke-Yin
Ye
a,
Lingyun
Zhu
a,
Yanxia
Huang
a,
Zi'ang
Zhai
a,
Qiang
Wang
a,
Lingding
Wang
a,
Taosong
Wang
a,
Wei-Zhen
Wang
a,
Ke-Yin
Ye
 *a and
Yuanming
Li
*a and
Yuanming
Li
 *ab
*ab
aKey Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian 350108, China. E-mail: yuanming.li@fzu.edu.cn; kyye@fzu.edu.cn
bState Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071, China
First published on 19th March 2025
Abstract
Efficient synthetic methods are urgently needed to produce graphene nanoribbons (GNRs) with diverse structures and functions. Precise control over the topological edges of GNRs is also crucial for achieving diverse molecular topologies and desirable electro-optical properties. This study demonstrates a highly efficient “shotgun” synthesis of thiophene-backbone arcuate GNRs, offering a significant advantage over tedious iterative synthesis. This method utilizes a one-pot, three component Suzuki–Miyaura coupling for the precursor, followed by a Scholl reaction for cyclization. The resulting arcuate GNRs have sulfur atoms embedded in the carbon backbone with a combined armchair, cove, and fjord edge structure. This multi-edge architecture is further modified by high-yield oxidation of the electron-rich sulfur atoms to electron-deficient sulfones, enabling precise regulation of the GNRs' electronic properties. These arcuate GNRs with diverse edge structures, heteroatom doping and precise lengths open exciting avenues for their application in optoelectronic devices.
Introduction
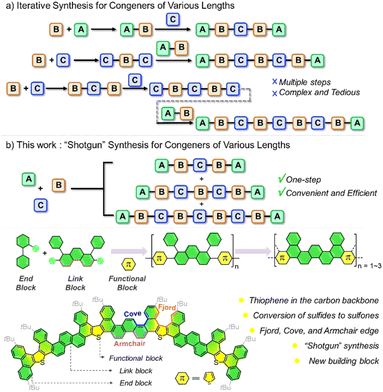
Atomically precise graphene nanoribbons (GNRs) have attracted significant attention recently due to their intriguing electronic properties and applications in next-generation optoelectronic devices.1–8 Their optical and electronic properties strongly depend on their structural parameters, such as lengths, widths, shapes, and edges.9–13 Besides control over the above-mentioned structural parameters, properties can be fine-tuned by incorporation of heteroatoms into the conjugated nanoribbon backbones.14–17 Organic chemists have focused on developing new synthetic strategies and easily accessible structure-rich building blocks for the production of GNRs and heteroatom-doped GNRs over decades.18–21The solution-phase synthesis that is the formation of a linearly extended precursor followed by a ring-closing step is one of the most promising strategies for the preparation of well-defined GNRs on a large scale.22–30 For the study of length-dependent photophysical properties via comparison with their linear counterparts, GNRs with different lengths are usually prepared by iterative synthesis (Fig. 1a).12,17,31,32 These methods often involve complex and expensive starting materials, restricting structural and functional diversity. The “shotgun” approach to macrocycles33–37 involves using a one-pot multicomponent cyclization of small monomers, to access the macrocycle. This approach shows great advantages to quickly construct macrocycles with diverse sizes and functionalities. In addition, phenanthrene is an important polycyclic aromatic hydrocarbon (PAH), which has an armchair side. We envision that 1,8-diaryl phenanthrene derivatives would be promising building blocks for the “shotgun” synthesis of GNRs, which are readily available.38
Herein, we report a series of arcuate GNRs with thiophene backbones with high efficiency. The precursor of GNRs was constructed by Suzuki–Miyaura coupling with 1,8-diaryl phenanthrene as a key building block and then dehydrogenation to obtain GNRs of different lengths. In this case, three GNRs (AR-11, AR-17, and AR-23) with multiple edge structures were achieved (the numbering indicates the number of longitudinally fused rings along their backbone) (Fig. 1a). Oxidizing electron-rich sulfur atoms to electron-deficient sulfones yielded ARO-11 and ARO-17 with high efficiency, respectively. Detailed theoretical and experimental studies demonstrate that length and the oxidation state greatly affect not only molecular conformations but also electronic structures and photophysical properties.
Results and discussion
Synthesis
To achieve the synthesis of GNRs, a key building block, that is, 1,8-diaryl phenanthrene-2,7-diboronic acid pinacol ester 3 was synthesized in three steps on a gram scale (Scheme S2†),38 which features armchair and cove type edges (Scheme 1a). The introduction of sulfur into GNRs is achieved by the addition of 2,5-dibromothiophene 2 as the functional block. In addition, 2-substituted biphenyl 1 was introduced as the end block. Tert-butyl substituents were installed respectively on 1 and 3 to ensure the good solubility of the intermediates and the corresponding GNRs. With the key link block 3, functional block 2, and end block 1 in hand, a series of precursors of sulfur-doped AR-n were obtained by one-pot synthesis and isolated through column chromatography in 53% (4a), 19% (4b), and 12% (4c) yield, respectively. By varying the end groups and adjusting the molar ratio of the three components, congeners with diverse lengths can be obtained (see Scheme S3 and Table S2† for further details).39 This “shotgun” approach demonstrates significant advantages over conventional iterative synthesis in terms of time efficiency and material economy. A direct comparison reveals that the iterative strategy requires six reactions spanning 216 hours to produce 4a, 4b, and 4c, while our protocol achieves identical product formation in just 36 hours (an 84% reduction in total reaction time). Critically, this method also exhibits marked improvement in synthetic economy: equimolar quantities of target products can be obtained using significantly less starting material compared to the traditional approach (Scheme S4†). The drastic reduction in both operational steps (from six to one) and experimental duration highlights the exceptional practicality and scalability of this synthesis strategy for multi-component molecular construction.The following ring-closing reactions of 4a and 4b by intramolecular oxidative cyclodehydrogenation in the presence of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and CF3SO3H gave the desired GNRs AR-11 and AR-17 in 95% and 93% yields, which were well-characterized by NMR analysis and HRMS. Due to the poor solubility of AR-23, well-resolved 1H and 13C NMR spectra could not be recorded. However, characterization was achieved by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), Fourier transform infrared spectroscopy (FT-IR) and the Raman spectrum (Scheme 1c and Fig. S6–S8†). The Scholl reaction is conducted using super stoichiometric oxidants, limiting its scalability. To address these challenges, we also developed an electrochemical continuous flow Scholl reaction. This method eliminates the need for oxidants and enables the production of AR-11 in larger quantities. The arc lengths and radians of AR-11, AR-17 and AR-23 are 2.10 nm and 37.2° for AR-11, 3.29 nm and 51.4° for AR-17, and 4.49 nm and 70.6° for AR-23 based on density functional theory (DFT) optimized structures (Scheme 1a and Fig. S9–S13†). The source code of the program for the measurement of lengths and the radians is listed in Table S5.† In addition, AR-11 and AR-17 were oxidized to give orange solid ARO-11 and ARO-17 with excessive meta-chloroperoxybenzoic acid (mCPBA) in 90% yields (Scheme 1b).
X-ray crystal structures
The arcuate and curved shape structures of AR-11 and ARO-11 were confirmed by single-crystal X-ray diffraction analysis (Fig. 2). Slow diffusion of acetone into a CDCl3/CS2 solution of AR-11 at room temperature resulted in the formation of AR-11 single crystals. The single crystals of ARO-11 were obtained by the slow diffusion of CH3OH into the CHCl3 solution. Single-crystal structure analysis indicated that the π-skeletons of AR-11 and ARO-11 are highly twisted along their longitudinal axes (Fig. 2a and b). With the presence of bulky tert-butyl groups on the fjord edges of AR-11 and ARO-11, the benzenoid rings adopt an interlacing “up–down” conformation with dihedral angles ranging from 36° to 47° (also see Fig. S2†). The end-to-end arc lengths for the π-backbone of AR-11 and ARO-11 are 2.05 nm and 2.06 nm, respectively, which correspond to the DFT optimized structures (Scheme 1a). In the packing structures of AR-11 and ARO-11 (Fig. 2c and d), every two identical molecules (shown in green and blue) surround each other in the form of face to edge, forming a dimer. This dimer presents a slip-stacked packing mode along the plane of the a-axis and c-axis. In the stacking structures of AR-11, the dimer has intermolecular π–π interactions with an interlayer distance as short as 3.3 Å (Fig. 2c and S2b†). In the crystal structure of ARO-11 the distance of π–π interactions between the adjacent molecules is 3.0 Å. Besides, there are face-to-edge intermolecular C–H…O hydrogen bonding interactions (2.5 Å) and the arrangement is closer for ARO-11 (Fig. 2d and S2b†).Calculations and photophysical properties
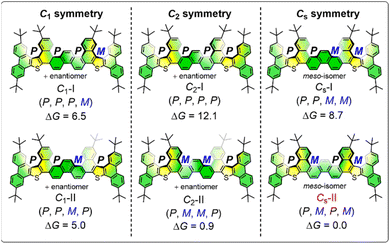
Considering the 4-fold helicity of AR-11, we can depict ten stereoisomers including four pairs of enantiomers and two meso isomers (six diastereomers in total, Fig. 3), which are categorized as follows: C1 symmetry (C1-I and C1-II), C2 symmetry (C2-I and C2-II), and Cs symmetry (Cs-I and Cs-II) (Fig. 3). Density Functional Theory (DFT) calculations were performed to explore their relative stability. Calculation details are given in the ESI (Fig. S14 and S15)†40–46 Based on the DFT calculations, the (P,M,P,M) conformation is the most stable, which is consistent with the X-ray crystal structure result, followed by the alternated (P,M,M,P) (+0.9 kcal mol−1). The conversion barrier of AR-11 from the (P,M,M,P) to the (P,M,P,M) is 20.1 kcal mol−1 (Fig. S15†). Furthermore, AR-17 exhibits 6-fold helicity and affords 36 types of isomers with varying Gibbs free energies (Fig. S16†). Semi-empirical calculations were conducted over them. Based on the calculations, the (P,M,P,M,P,M,P) conformation is the most stable, followed by the alternated conformation (P,M,P,M,P,P,M) (+0.8 kcal mol−1).To gain insight into the length-dependent photophysical properties of AR-n and ARO-n, UV/vis absorption and fluorescence emission were measured in diluted CH2Cl2 solutions at room temperature (Fig. 4a and b). The results are summarized in Table 1. AR-17 (λabs = 434 nm, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε = 5.24) exhibited a bathochromic shift compared to AR-11 (λabs = 415 nm, log
ε = 5.24) exhibited a bathochromic shift compared to AR-11 (λabs = 415 nm, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε = 5.07), which was attributed to the extended π-backbone. ARO-11 exhibited a pronounced bathochromic shift in its absorption maximum (λabs = 466 nm, log
ε = 5.07), which was attributed to the extended π-backbone. ARO-11 exhibited a pronounced bathochromic shift in its absorption maximum (λabs = 466 nm, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε = 4.79) compared to AR-11 as well, attributable to the integration of the electron-withdrawing thiophene-S,S-dioxide unit into the extended π-system. Notably, ARO-11 displayed broader absorption features than AR-11. Similar trends were observed for ARO-17 and AR-17.
ε = 4.79) compared to AR-11 as well, attributable to the integration of the electron-withdrawing thiophene-S,S-dioxide unit into the extended π-system. Notably, ARO-11 displayed broader absorption features than AR-11. Similar trends were observed for ARO-17 and AR-17.
| Compound | λ abs [nm] | ε max [105 M−1 cm−1] | λ em [nm] | Φ , [%] | τ , [ns] | λ em [nm] | Φ , [%] | τ , [ns] | E HOMO [eV] | E HOMO [eV] | E LUMO [eV] | E g [eV] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Measured in CH2Cl2 solution (1.0 × 10−5 M). b Measured in the solid state. c Fluorescence quantum yield. d Fluorescence lifetime. e From CVs measured in DCM (1.0 × 10−4 M). The HOMO energy levels are adjusted according to the redox half potential of Fc/Fc+ and estimated according to the formula EHOMO (eV) = –(4.80 + Eox1onset − E(Fc/Fc+)) (also see Scheme S5). f Calculations were performed at the B3LYP/6-31G(d) level. | ||||||||||||
| AR-11 | 415/392 | 1.19/1.01 | 459 | 15 | 2.07 | 464 | 4.7 | 0.67 | −5.34 | −5.06 | −1.70 | 3.36 |
| AR-17 | 434/410 | 1.74/1.56 | 479 | 37 | 4.02 | 506 | 0.2 | 0.54 | −5.66 | −5.03 | −1.79 | 3.24 |
| ARO-11 | 466/441 | 0.65/0.56 | 511 | 65 | 1.77 | 529 | 5.9 | 0.74 | −5.60 | −5.43 | −2.40 | 3.03 |
| ARO-17 | 486/463 | 1.04/0.92 | 530 | 71 | 1.77 | 549 | 1.5 | 1.24 | −5.41 | −5.40 | −2.51 | 2.89 |
Compared to AR-11 (λem = 459 nm), the bathochromic shift in the photoluminescence spectra was pronounced for AR-17 (λem = 479 nm) due to its extended conjugated backbone, or for ARO-11 (λem = 511 nm) due to incorporation of the electron-withdrawing thiophene-S,S-dioxide unit (Fig. 4b). The absolute fluorescence quantum yields (ΦF) were measured by using an integrating sphere detector.47 In CH2Cl2 solution, ΦF values increased with π-backbone extension in AR-11 (15%) and AR-17 (37%). Conversely, ARO-11 (65%) and ARO-17 (71%) displayed high, π-backbone-independent ΦF values. In the solid state, however, ΦF values decreased to 5.9% for ARO-11 and 1.5% for ARO-17, likely due to aggregation-caused quenching via strong intermolecular π-overlap. Similar trends were observed for AR-11 (4.7%) and AR-17 (0.2%).48–50 Additionally, the emission wavelengths of the compounds in the solid state were measured. Owing to the high molecular rigidity of the compounds, the emission wavelengths in both the solid and solution states showed minimal red-shift (Fig. S5†). To further elucidate the photophysical properties, the energy diagrams and frontier molecular orbitals of AR-n and ARO-n were calculated by the time-dependent density functional theory (TD-DFT) method at the PBE0-D3(BJ)/6-31G(d,p) level of theory (Fig. 4d, S19 and S20†).51 The lowest energy absorption bands of AR-n and ARO-n are attributed to the S0 → S1 excitation (ΔE, 3.12 eV for AR-11, 2.74 eV for ARO-11, 2.97 eV for AR-17, and 2.61 eV for ARO-17), which mainly arises from the HOMO → LUMO transition (95% for AR-11, 92% for ARO-11, 90% for AR-17, and 87% for ARO-17) and has an oscillator strength (f) of 1.77 for AR-11, 1.19 for ARO-11, 3.07 for AR-17, and 2.11 for ARO-17 (Tables S6–S9†). The electrochemical properties of these sulfur-doped GNRs were studied in CH2Cl2 solution by cyclic voltammetry (CV) and differential pulse voltammetry (DPV). All compounds exhibited an obvious reduction process, with Epred1 values of −1.51 eV (AR-11), −1.76 eV (ARO-11), −1.52 eV (AR-17), and −1.57 eV (ARO-17). Oxidation potentials (Epox1) were 0.65 eV (AR-11), 0.74 eV (ARO-11), 0.80 eV (AR-17), and 0.92 eV (ARO-17). Based on Epox1 values, HOMO energy levels were estimated to be −5.34 eV (AR-11), −5.60 eV (ARO-11), −5.66 eV (AR-17), and −5.41 eV (ARO-17), demonstrating good agreement with the computational study (Fig. 4c, S3† and Table 1).
The energy levels of the calculated frontier molecular orbitals of AR-n and ARO-n are shown in Fig. 4d, S19 and S20,† for example, the HOMO and LUMO of AR-17 and ARO-17 spread over the whole π-system. After converting the electron-rich thiophene ring into an electron-poor thiophene-S,S-dioxides, the sulfone dioxide unit considerably reduces the HOMO energy level; the LUMO energy level has a greater decrease. Hence, the energy gap of the molecule is greatly reduced, which leads to a large redshift of fluorescence emission. Notably, the band gaps of AR-n and ARO-n are well regulated from 3.90 eV of AR-5 to 3.11 eV of AR-23 and 3.43 eV of ARO-5 to 2.81 eV of ARO-23. The energies of frontier orbitals of AR-n and ARO-n are plotted in Fig. 4e (also see Fig. S21–S24†). The decrement of the LUMO energy level and increment of the HOMO energy level are observed from AR-5 to AR-23, which leads to a clear shrinkage of Eg in the same sequence. The decreased HOMO level for ARO-n compared with AR-n strongly suggests the enhanced affinity of ARO-n to electrons.
Notably, to illustrate the electronic structures and the molecular aromaticity of S-doped GNRs, nucleus-independent chemical shift (NICS) and anisotropy of the induced current density (ACID) calculations on AR-11 and ARO-11 are conducted at the B3LYP/6-31G(d) level of theory based on the optimized structures (Fig. 4f, S17 and S18†).52 The largely negative NICS(1)ZZ values (−22.9 to −27.6 of AR-11 and −24.1 to −28.6 of ARO-11 in yellow) indicate that these six-membered rings exhibit local aromaticity. These analyses are further supported by ACID calculations, which show clockwise (diamagnetic) ring currents mainly distributed at hexagonal rings A/B/C/D/E/F at the outer rims of the whole π-framework. In addition, rings J/L in AR-11 and ARO-11 also display very negative NICS(1)ZZ values (−20.3 of AR-11 and −20.6 of ARO-11), manifesting that they also contribute to the aromatic structures. However, the positive NICS(1)ZZ values of 13.3 suggest that these sulfur dioxide rings have no contribution to the overall π-conjugation in Fig. 4f. The induced ring currents are relatively weak in the fused sulfur dioxide rings H/N of ARO-11, resulting in a break for the global delocalization of the π-electrons (Fig. 4f). These results demonstrate that the doping of sulfur dioxide achieves the delocalization of π-electrons in GNRs.
Conclusions
Graphene nanoribbons (GNRs) hold significant promise for optoelectronic applications, but their development is hampered by the limitations of traditional iterative synthetic methods. These methods often involve complex and expensive starting materials, restricting structural and functional diversity. This hinders the rapid exploration of structure–property relationships, especially length-dependent properties of GNRs, and the discovery of high-performance materials. A more efficient approach is to employ a modular synthesis, using end blocks, link blocks, and functional blocks as key components. This strategy allows for the facile synthesis of GNRs with a wide range of structures and functionalities by simply varying the functional block. We have demonstrated a highly efficient synthetic approach for sulfur-doped GNRs featuring a unique cove-armchair-fjord edge combination. Notably, sulfur incorporation into the backbone yielded arc-shaped GNRs. The unambiguous crystallographic characterization of AR-11 and ARO-11 reveals that the combined cove and fjord edge structure causes it to deviate from planarity due to steric repulsion. By oxidizing the electron-rich sulfur atoms to electron-deficient sulfones, we effectively tune the electronic properties of GNRs. Expanding the GNR family further, we're currently exploring the versatility of 1,8-diaryl phenanthrene by crafting GNRs with diverse functional blocks.Data availability
The crystallographic data for AR-11 and ARO-11 reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC: 2303699 and 2303698, respectively. Other data supporting this study are available in the article and ESI.†Author contributions
Y. L. conceived and directed the project. K. Y. directed the electrochemical synthesis section. R. Z. conducted most of the experiments. X. C. performed the DFT calculations. L. Z. and Y. H. and Z. Z. contributed to the synthesis. Q. W. and L. W. and T. W. analyzed the data. W. W. conducted the electrochemical synthesis. All authors discussed the results and commented on the manuscript.Conflicts of interest
The authors declare no competing interests.Acknowledgements
Project supported by the Joint Funds of the National Natural Science Foundation of China (No. U24A20567) and the Natural Science Foundation of Fujian Province, China (No. 2024J01236). We thank Prof. Weiguo Huang (Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences) for the single crystal measurement and Prof. Kenichiro Itami (Nagoya University), Prof. Akiko Yagi (Nagoya University), Prof. Chenguang Wang (Jilin University) and Prof. Chaolumen (Inner Mongolia University) for constructive criticism of the manuscript.Notes and references
- J. Wu, W. Pisula and K. Müllen, Graphenes as Potential Material for Electronics, Chem. Rev., 2007, 107(3), 718–747 CrossRef CAS PubMed.
- X. Li, X. Wang, L. Zhang, S. Lee and H. Dai, Chemically Derived, Ultrasmooth Graphene Nanoribbon Semiconductors, Science, 2008, 319(5867), 1229–1232 CrossRef CAS PubMed.
- Y. Segawa, H. Ito and K. Itami, Structurally uniform and atomically precise carbon nanostructures, Nat. Rev. Mater., 2016, 1(1), 15002 CAS.
- K.-Y. Yoon and G. Dong, Liquid-phase bottom-up synthesis of graphene nanoribbons, Mater. Chem. Front., 2020, 4(1), 29–45 CAS.
- Y. Yano, N. Mitoma, H. Ito and K. Itami, A Quest for Structurally Uniform Graphene Nanoribbons: Synthesis, Properties, and Applications, J. Org. Chem., 2020, 85(1), 4–33 CrossRef CAS PubMed.
- R. S. K. Houtsma, J. de la Rie and M. Stöhr, Atomically precise graphene nanoribbons: interplay of structural and electronic properties, Chem. Soc. Rev., 2021, 50(11), 6541–6568 RSC.
- Y. Gu, Z. Qiu and K. Müllen, Nanographenes and Graphene Nanoribbons as Multitalents of Present and Future Materials Science, J. Am. Chem. Soc., 2022, 144(26), 11499–11524 CrossRef CAS PubMed.
- M.-W. Wang, W. Fan, X. Li, Y. Liu, Z. Li, W. Jiang, J. Wu and Z. Wang, Molecular Carbons: How Far Can We Go?, ACS Nano, 2023, 17(21), 20734–20752 CrossRef PubMed.
- O. V. Yazyev, A Guide to the Design of Electronic Properties of Graphene Nanoribbons, Acc. Chem. Res., 2013, 46(10), 2319–2328 CrossRef CAS PubMed.
- J. Liu, B.-W. Li, Y.-Z. Tan, A. Giannakopoulos, C. Sanchez-Sanchez, D. Beljonne, P. Ruffieux, R. Fasel, X. Feng and K. Müllen, Toward Cove-Edged Low Band Gap Graphene Nanoribbons, J. Am. Chem. Soc., 2015, 137(18), 6097–6103 CrossRef CAS PubMed.
- X. Yao, W. Zheng, S. Osella, Z. Qiu, S. Fu, D. Schollmeyer, B. Müller, D. Beljonne, M. Bonn, H. I. Wang, K. Müllen and A. Narita, Synthesis of Nonplanar Graphene Nanoribbon with Fjord Edges, J. Am. Chem. Soc., 2021, 143(15), 5654–5658 CrossRef CAS PubMed.
- Y. Wang, Y. Huang, T. Huang, J. Zhang, T. Luo, Y. Ni, B. Li, S. Xie and Z. Zeng, Perylene-Based Linear Nonalternant Nanoribbons with Bright Emission and Ambipolar Redox Behavior, Angew. Chem., Int. Ed., 2022, 61(21), e202200855 CrossRef CAS PubMed.
- K. Liu, W. Zheng, S. Osella, Z.-L. Qiu, S. Böckmann, W. Niu, L. Meingast, H. Komber, S. Obermann, R. Gillen, M. Bonn, M. R. Hansen, J. Maultzsch, H. I. Wang, J. Ma and X. Feng, Cove-Edged Chiral Graphene Nanoribbons with Chirality-Dependent Bandgap and Carrier Mobility, J. Am. Chem. Soc., 2024, 146(1), 1026–1034 CrossRef CAS PubMed.
- S. Kawai, S. Nakatsuka, T. Hatakeyama, R. Pawlak, T. Meier, J. Tracey, E. Meyer and A. S. Foster, Multiple heteroatom substitution to graphene nanoribbon, Sci. Adv., 2018, 4(4), eaar7181 CrossRef PubMed.
- X.-Y. Wang, X. Yao, A. Narita and K. Müllen, Heteroatom-Doped Nanographenes with Structural Precision, Acc. Chem. Res., 2019, 52(9), 2491–2505 Search PubMed.
- A. Borissov, Y. K. Maurya, L. Moshniaha, W.-S. Wong, M. Żyła-Karwowska and M. Stępień, Recent Advances in Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds, Chem. Rev., 2022, 122(1), 565–788 CrossRef CAS PubMed.
- Y. Sano, T. Shintani, M. Hayakawa, S. Oda, M. Kondo, T. Matsushita and T. Hatakeyama, One-Shot Construction of BN-Embedded Heptadecacene Framework Exhibiting Ultra-narrowband Green Thermally Activated Delayed Fluorescence, J. Am. Chem. Soc., 2023, 145(21), 11504–11511 CrossRef CAS PubMed.
- L. Chen, Y. Hernandez, X. Feng and K. Müllen, From Nanographene and Graphene Nanoribbons to Graphene Sheets: Chemical Synthesis, Angew. Chem., Int. Ed., 2012, 51(31), 7640–7654 CAS.
- J. Liu and X. Feng, Synthetic Tailoring of Graphene Nanostructures with Zigzag-Edged Topologies: Progress and Perspectives, Angew. Chem., Int. Ed., 2020, 59(52), 23386–23401 CAS.
- W. Chen, F. Yu, Q. Xu, G. Zhou and Q. Zhang, Recent Progress in High Linearly Fused Polycyclic Conjugated Hydrocarbons (PCHs, n > 6) with Well-Defined Structures, Adv. Sci., 2020, 7(12), 1903766 CAS.
- A. Jolly, D. Miao, M. Daigle and J.-F. Morin, Emerging Bottom-Up Strategies for the Synthesis of Graphene Nanoribbons and Related Structures, Angew. Chem., Int. Ed., 2020, 59(12), 4624–4633 CAS.
- M. Grzybowski, K. Skonieczny, H. Butenschön and D. T. Gryko, Comparison of Oxidative Aromatic Coupling and the Scholl Reaction, Angew. Chem., Int. Ed., 2013, 52(38), 9900–9930 CrossRef CAS PubMed.
- Y. Zhang, S. H. Pun and Q. Miao, The Scholl Reaction as a Powerful Tool for Synthesis of Curved Polycyclic Aromatics, Chem. Rev., 2022, 122(18), 14554–14593 CAS.
- Y. Zhong, B. Kumar, S. Oh, M. T. Trinh, Y. Wu, K. Elbert, P. Li, X. Zhu, S. Xiao, F. Ng, M. L. Steigerwald and C. Nuckolls, Helical Ribbons for Molecular Electronics, J. Am. Chem. Soc., 2014, 136(22), 8122–8130 CrossRef CAS PubMed.
- M. Daigle, A. Picard-Lafond, E. Soligo and J.-F. Morin, Regioselective Synthesis of Nanographenes by Photochemical Cyclodehydrochlorination, Angew. Chem., Int. Ed., 2016, 55(6), 2042–2047 CrossRef CAS PubMed.
- K. Kato, Y. Segawa, L. T. Scott and K. Itami, A Quintuple [6]Helicene with a Corannulene Core as a C5-Symmetric Propeller-Shaped π-System, Angew. Chem., Int. Ed., 2018, 57(5), 1337–1341 CrossRef CAS PubMed.
- W. Hagui, H. Doucet and J.-F. Soulé, Application of Palladium-Catalyzed C(sp2)–H Bond Arylation to the Synthesis of Polycyclic (Hetero)Aromatics, Chem, 2019, 5(8), 2006–2078 CAS.
- W. Yang, A. Lucotti, M. Tommasini and W. A. Chalifoux, Bottom-Up Synthesis of Soluble and Narrow Graphene Nanoribbons Using Alkyne Benzannulations, J. Am. Chem. Soc., 2016, 138(29), 9137–9144 CrossRef CAS PubMed.
- Y. L. Li, C.-T. Zee, J. B. Lin, V. M. Basile, M. Muni, M. D. Flores, J. Munárriz, R. B. Kaner, A. N. Alexandrova, K. N. Houk, S. H. Tolbert and Y. Rubin, Fjord-Edge Graphene Nanoribbons with Site-Specific Nitrogen Substitution, J. Am. Chem. Soc., 2020, 142(42), 18093–18102 CrossRef CAS PubMed.
- A. Narita, Z. Chen, Q. Chen and K. Müllen, Solution and on-surface synthesis of structurally defined graphene nanoribbons as a new family of semiconductors, Chem. Sci., 2019, 10(4), 964–975 RSC.
- X. Yang, S. M. Elbert, F. Rominger and M. Mastalerz, A Series of Soluble Thieno-Fused Coronene Nanoribbons of Precise Lengths, J. Am. Chem. Soc., 2022, 144(22), 9883–9892 CrossRef CAS PubMed.
- A. Boddeda, M. M. Hossain, M. Saeed Mirzaei, S. V. Lindeman, S. Mirzaei and R. Rathore, Angular ladder-type meta-phenylenes: synthesis and electronic structural analysis, Org. Chem. Front., 2020, 7(20), 3215–3222 RSC.
- R. Jasti, J. Bhattacharjee, J. B. Neaton and C. R. Bertozzi, Synthesis, Characterization, and Theory of [9]-, [12]-, and [18]Cycloparaphenylene: Carbon Nanohoop Structures, J. Am. Chem. Soc., 2008, 130(52), 17646–17647 CrossRef CAS PubMed.
- Y. Segawa, S. Miyamoto, H. Omachi, S. Matsuura, P. Šenel, T. Sasamori, N. Tokitoh and K. Itami, Concise Synthesis and Crystal Structure of [12]Cycloparaphenylene, Angew. Chem., Int. Ed., 2011, 50(14), 3244–3248 CrossRef CAS PubMed.
- L. Zhu, J. Xu, B. Lan, X. Chen, H. Kono, H. Xu, J. Yan, W. Li, A. Yagi, Y. Yuan, K. Itami and Y. Li, Ferrocene-based conjugated macrocycles: shotgun synthesis, size-dependent properties and tunable fluorescence intensity, Org. Chem. Front., 2024, 11(18), 5130–5137 RSC.
- J. Xu, B. Lan, L. Zhu, H. Xu, X. Chen, W. Li, Y. Yuan, J. Yan and Y. Li, Synthesis and Properties of Ferrocene Conjugated Macrocycles with Illusory Topology of the Penrose Stairs, Chem. Res. Chin. Univ., 2024, 40(5), 881–886 CrossRef CAS.
- B. Lan, J. Xu, L. Zhu, X. Chen, H. Kono, P. Wang, X. Zuo, J. Yan, A. Yagi, Y. Zheng, S. Chen, Y. Yuan, K. Itami and Y. Li, Side-Chain Type Ferrocene Macrocycles, Precis. Chem., 2024, 2(4), 143–150 CrossRef CAS PubMed.
- Y. Li, A. Yagi and K. Itami, Synthesis of sterically hindered 4,5-diarylphenanthrenes via acid-catalyzed bisannulation of benzenediacetaldehydes with alkynes, Chem. Sci., 2019, 10(21), 5470–5475 RSC.
- When 2-bromo-4,4'-di-tert-butyl-1,1'-biphenyl was employed as the end-capping compound, the yields of 4a and 4b decreased to 30% and 16%, respectively (Scheme S3†)..
- See the ESI† for a full citation of Gaussian 16..
- F. Neese, Software update: The ORCA program system—Version 5.0, WIREs Com. Mol. Sci., 2022, 12(5), e1606 CrossRef.
- F. Neese, Software update: the ORCA program system, version 4.0, WIREs Com. Mol. Sci., 2018, 8(1), e1327 CrossRef.
- N. Mardirossian and M. Head-Gordon, ωB97M-V: A combinatorially optimized, range-separated hybrid, meta-GGA density functional with VV10 nonlocal correlation, J. Chem. Phys., 2016, 144, 214110 CrossRef PubMed.
- J.-D. Chai and M. Head-Gordon, Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections, Phys. Chem. Chem. Phys., 2008, 10(44), 6615–6620 RSC.
- F. Weigend, Accurate Coulomb-fitting basis sets for H to Rn, Phys. Chem. Chem. Phys., 2006, 8(9), 1057–1065 RSC.
- F. Weigend and R. Ahlrichs, Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy, Phys. Chem. Chem. Phys., 2005, 7(18), 3297–3305 RSC.
- C. Würth, M. Grabolle, J. Pauli, M. Spieles and U. Resch-Genger, Relative and absolute determination of fluorescence quantum yields of transparent samples, Nat. Protoc., 2013, 8(8), 1535–1550 CrossRef PubMed.
- D. L. Dexter and J. H. Schulman, Theory of Concentration Quenching in Inorganic Phosphors, J. Chem. Phys., 1954, 22, 1063–1070 CrossRef CAS.
- M. Kasha, H. Rawls and M. El-Bayoumi, The exciton model in molecular spectroscopy, Pure Appl. Chem., 1965, 11, 371–392 CAS.
- R. F. Chen and J. R. Knutson, Mechanism of fluorescence concentration quenching of carboxyfluorescein in liposomes: energy transfer to nonfluorescent dimers, Anal. Biochem., 1988, 172(1), 61–77 CrossRef CAS PubMed.
- T. Lu and F. Chen, Multiwfn: A multifunctional wavefunction analyzer, J. Comput. Chem., 2012, 33(5), 580–592 CrossRef CAS PubMed.
- M. Randić, Aromaticity of Polycyclic Conjugated Hydrocarbons, Chem. Rev., 2003, 103(9), 3449–3606 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2303698 and 2303699. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc08353a |
| This journal is © The Royal Society of Chemistry 2025 |