Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Modulator approach for the design and synthesis of anisotropic multi-domain metal–organic frameworks†
Yiwen
He
 a,
Zhehao
Li
a,
Zhehao
Li
 a,
Zoe M.
Soilis
a,
Zoe M.
Soilis
 a,
Gefan
He
a,
Gefan
He
 a and
Nathaniel L.
Rosi
a and
Nathaniel L.
Rosi
 *ab
*ab
aDepartment of Chemistry, University of Pittsburgh, Pittsburgh, Pennsylvania 15260, USA. E-mail: nrosi@pitt.edu
bDepartment of Chemical and Petroleum Engineering, University of Pittsburgh, Pittsburgh, Pennsylvania 15261, USA
First published on 20th March 2025
Abstract
Multi-domain metal–organic frameworks (MD-MOFs) consist of chemically-distinct interconnected MOF domains. Most commonly they are isotropic, with core–shell and stratified MOFs representing classic examples in which a core MOF is concentrically encased in one or more MOF shells. Anisotropic multi-domain MOFs (AMD-MOFs) are much rarer and are projected to exhibit unique properties that depend on domain sequence, composition, and 3-D spacial distribution. However, straightforward approaches for their synthesis and construction are underdeveloped. We present and describe a modulator-based strategy for preparing a diverse collection of AMD-MOFs. Designed coordination modulators were used to inhibit secondary domain growth along certain facets of seed MOF crystals. Through multistep syntheses, this strategy allows for controlled construction of AMD-MOFs with different domain distributions that depend on modulator identity and domain synthesis sequence. The reported results represent important steps toward realizing a more general synthetic approach for fabricating arbitrarily complex AMD-MOFs, which is crucial for enabling broader exploration and study of their properties, functions, and applications.
Introduction
The connectivity and spatial disposition of chemical moieties and different functional domains underscore the properties of all matter. Precise synthetic control over the placement of these constituents allows for the design and rational fabrication of functional molecules and materials, from complex natural products1,2 and multiblock polymers3–6 to multicomponent and multi-domain nanoparticles7,8 and metal–organic frameworks (MOFs).9–14MOFs, in particular, exhibit multiple levels of chemical and structural complexity. At the molecular level, they consist of periodically interconnected metal and organic components (n), where n ≥ 2, and their properties and functions are largely defined by the identity and 3-D connectivity (i.e., MOF topology) of these components.15–17 Strategies for further increasing MOF complexity have emerged which rely on considering MOFs themselves as domain building blocks18,19 in larger scale ‘MOF-on-MOF’ architectures.20–35 While fabrication methods for such materials have advanced considerably, it remains challenging to rationally construct anisotropic multi-domain MOFs (AMD-MOFs) consisting of multiple different interconnected MOF domains.29,36–40
To contextualize this challenge, it is instructive to relate multi-domain MOF syntheses to the derivatization of organic molecular substrates. If the substrate has multiple functional groups with similar reactivity, an undiscriminating reactant may react with all of them. Analogously, if MOF shell growth is equally likely on all facets of a MOF seed crystal, then isotropic shell growth will occur, resulting in the formation of isotropic core–shell MOF products.20,21,25,41 Targeting reactions to specific functional groups of an organic substrate can result in selective derivatization; however, this requires careful choice of reactants and synthetic conditions. For comparison, there are rare cases of multi-domain MOF syntheses that result in facet-selective shell growth and the formation of AMD-MOFs, yet they necessitate meticulous selection of MOF seed and shell pairs.29,36–40 In organic synthesis, a general strategy to prevent reaction at some functional groups—and thus direct reactions toward others—involves use of protecting groups. A similar widely applicable approach for prescribing secondary MOF domain growth to specific facets of a MOF seed crystal is lacking.
Drawing inspiration from organic protecting group strategies, we envisioned that molecular coordination modulators could be designed to prevent shell growth on specific MOF facets while allowing it to occur on others.42 Indeed, introducing molecular modulators to MOF syntheses has proven to be an effective way of controlling MOF crystal growth and influencing crystal morphology.42–46 Modulators can affect the pH of the reaction medium or competitively coordinate to metal sites, limiting crystal growth along certain axes. A modulator-based approach could make AMD-MOF fabrication more straightforward and potentially more general than current established methods.
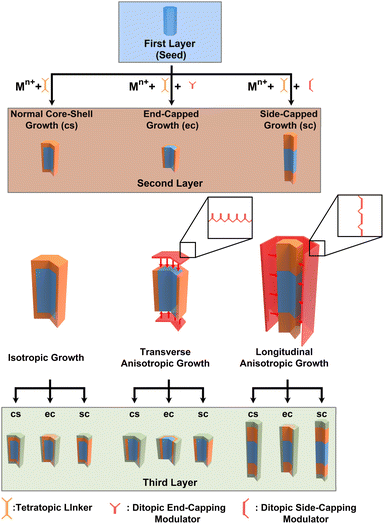
Our proposed strategy for controlling domain growth is illustrated in Fig. 1. In a typical synthesis, isotropic shell growth yields a core–shell (cs) MOF (left). To direct either transverse or longitudinal secondary domain growth, a molecular modulator (red) designed to competitively bind to either the seed crystal's ends (end cap, ec) or sides (side cap, sc), respectively, would be added to the synthesis (middle and right). After secondary domain growth, which could result in three potential binary domain MOFs, a third phase of modulated or unmodulated growth would yield a diverse collection of 9 different ternary domain MOFs, with 8 exhibiting anisotropic disposition of domains. Successive domain growth steps could be implemented to generate increasingly complex families of AMD-MOFs.
In this study, we design ditopic modulators to direct the formation and structure of AMD-MOFs. We first synthesize binary domain MOFs and characterize their morphology and composition using a variety of microscopic and spectroscopic methods. After demonstrating modulator-based control over secondary domain growth, we explore the scope of the methodology through design and synthesis of multiple different ternary AMD-MOFs. The results (i) illustrate the utility of a modulator-guided synthetic approach for fabricating diverse collections of AMD-MOFs; (ii) contribute to the fundamental understanding of MOF growth mechanisms; and (iii) provide practical routes for creating multi-MOF systems with tailorable structures and compositions.
Results and discussion
Anisotropic growth of binary domain MOFs
Gu et al. introduced a linker scissoring strategy to control the synthesis and growth of non-cubic MOF crystals constructed using tetratopic carboxylate linkers.47 They designed ditopic modulators derived from the tetratopic linkers to direct either transverse or longitudinal MOF crystal growth, resulting in formation of either nanoplatelets or nanorods. We reasoned a similar strategy could be adopted to positionally direct secondary MOF growth onto MOF seed crystals, thereby facilitating the controlled fabrication of AMD-MOFs.We chose PCN-608-OMe48 to investigate the applicability of this approach. PCN-608-OMe consists of Zr6O8 clusters interlinked by the D2h-symmetric tetratopic ligand, 4,4′-dimethoxybiphenyl-3,3′,5,5′-tetra(phenyl-4-carboxylate) (MeO-TPCB) (Fig. 2A). Within the PCN-608-OMe crystal structure, the long axis of MeO-TPCB aligns with the c crystallographic axis. Rod-like seed crystals of PCN-608-OMe were first synthesized. The average crystal length (1.26 ± 0.05 μm), width (0.39 ± 0.02 μm), and aspect ratio (3.22 ± 0.13) were measured from scanning electron microscopy (SEM) images (100 counts; Fig. 2B, F, G, S18 and Table S2†). Secondary domain growth solutions consisting of HfCl4, trifluoroacetic acid (TFA), H4-MeO-TPCB and dimethylformamide (DMF) were then prepared; Hf(IV) was used instead of Zr(IV) to distinguish domains using elemental mapping (vide infra). The seed crystals were immersed and heated (120 °C, 20 h) in the secondary growth solution yielding PCN-608-OMe(Zr)⊂PCN-608-OMe(Hf), binary ‘core⊂shell’ product hereafter denoted as cs-PCN. Powder X-ray diffraction (PXRD) analyses reveal that both the seed and core⊂shell MOFs are crystalline, phase pure, and isostructural to PCN-608 (Fig. S17 and S23†). cs-PCN crystals were longer (1.50 ± 0.13 μm) and wider (0.43 ± 0.03 μm) than the seed crystals (Fig. 2C, F, S25 and Table S3†), yet their aspect ratio (3.47 ± 0.32) (Fig. 2G and Table S3†) was comparable.
Having established synthetic conditions for preparing well-defined seed crystals and for secondary domain growth, we proceeded to explore syntheses that incorporated modulators to positionally direct growth of secondary domains onto specific seed crystal facets. Guided by the linker scissoring strategy summarized above,47 we prepared two different ditopic modulators based on the tetratopic MeO-TPCB linker. (1,1′,3′,1′′-Terphenyl)-4,4′′-dicarboxylic acid was designed to competitively coordinate along (001) and serve as an end-capping modulator (M-ec1) for directing transverse secondary domain growth. (1,1′,3′,1'':3′′,1′′′-Quaterphenyl)-4,4′′′-dicarboxylic acid was designed as a side-capping modulator (M-sc1) to coordinate along both (100) and (010) and thus direct longitudinal secondary domain growth. PCN-608-OMe seed crystals were immersed and heated in secondary domain growth solutions containing either M-ec1 or M-sc1 (120 °C, 20 h). These syntheses yielded anisotropic binary domain MOFs denoted as either end-capped PCN-608-OMe(Zr)⊂PCN-608-OMe(Hf) (ec-PCN) or side-capped PCN-608-OMe(Zr)⊂PCN-608- OMe(Hf) (sc-PCN). PXRD patterns revealed that ec-PCN and sc-PCN are isostructural to PCN-608 (Fig. S23†), and SEM was used to determine their size distributions and aspect ratios (Fig. 2D and E). The dimensions of the ec-PCN and sc-PCN were compared to the dimensions of the seed crystals to assess how the modulators influenced shell growth. The length of ec-PCN was determined to be 1.27 ± 0.11 μm (Fig. 2F, S26 and Table S3†), indicating minimal growth along the longitudinal axis (cf. 1.26 ± 0.05 μm for seed crystal). In contrast, the length of sc-PCN was 4.54 ± 0.43 μm (Fig. 2F, S27 and Table S3†), approximately 3× longer than the seed crystals. ec-PCN and sc-PCN exhibited widths of 1.38 ± 0.06 μm and 0.50 ± 0.02 μm, respectively (Fig. 2F, S26, S27 and Table S3†), indicating transverse secondary domain growth was significant for ec-PCN and limited for sc-PCN (cf. 0.39 ± 0.02 μm for seed crystals). The differences become even more pronounced when comparing the aspect ratios of ec-PCN and sc-PCN to that of the seed crystals. As previously mentioned, the aspect ratios of cs-PCN and the PCN-608-OMe(Zr) seeds are nearly identical. However, both are three times higher than that of ec-PCN (0.92 ± 0.06, Fig. 2G and Table S3†). A similarly large disparity is observed for sc-PCN, which exhibits an aspect ratio of 9.00 ± 0.72 (Fig. 2G and Table S3†), nearly three times greater than the original PCN-608-OMe(Zr) seed. Based on the dimensions and aspect ratios of ec-PCN and sc-PCN, we conclude that addition of M-ec1 or M-sc1 modulators to the syntheses significantly affects the location and direction of secondary domain growth.
Scanning transmission electron microscopy (STEM) was used to collect high-angle annular dark-field (HAADF) images and energy dispersive X-ray spectroscopy (EDS) line-scans to further elucidate and map MOF domain distribution (Fig. 3). In each case, the outer secondary domain is brighter than the inner seed region due to the presence of Hf. The HAADF image of cs-PCN indicated growth of PCN-608-OMe(Hf) in both the longitudinal and transverse directions. This observation was further corroborated by STEM-EDS line-scans, which revealed Zr signals in the inner region and Hf signals in the outer region of the crystals. During secondary domain growth, both metal and linker exchange is possible, and these processes have been documented in this context in previous publications by us and others.24,28 From the data presented, we can reasonably conclude that the secondary domain primarily contains Hf4+ and the seed domain is primarily Zr4+. As a control experiment, PCN-608-OMe seed crystals were immersed and heated (120 °C, 20 h) in the same secondary growth solution but without the ligand or modulator. HAADF image and STEM-EDS confirm the absence of Hf4+ in the resulting MOFs, indicating undetectable metal exchange during secondary growth (Fig. S19†). HAADF images and EDS line-scans of products from the modulated syntheses are starkly different than those for cs-PCN. We observe that ec-PCN almost exclusively exhibits transverse secondary domain growth while secondary domain growth for sc-PCN is almost solely along the longitudinal direction. Collectively, the data indicate that the designed ditopic modulators (i) inhibit secondary domain growth on certain facets; (ii) direct the placement and growth of domains to specific locations; and (iii) enable rational construction of anisotropic binary domain MOFs.
 | ||
| Fig. 3 HAADF images for binary domain PCN MOFs (top); STEM-EDS line-scans along longitudinal (middle) and transverse (bottom) directions. | ||
We applied this strategy to a second MOF system, Zr-BBI,49 to explore its versatility. Like PCN-608-OMe, Zr-BBI consists of Zr6O8 cluster nodes interconnected by tetratopic D2h symmetric linkers, BBI (4,4′,4′′,4′′′-(1,4-phenylenebis(1H-imidazole-2,4,5- triyl))tetrabenzoate). We used a brominated version of BBI (BBI-Br2, Fig. 4A), to prepare seed crystals so that we could identify its location using SEM-EDS Br mapping. Secondary domains were grown using non-brominated BBI. Zr-BBI-Br2 seeds were immersed and heated in a growth solution (100 °C, 20 h) to synthesize Zr-BBI-Br2⊂Zr-BBI (cs-BBI). For anisotropic secondary domain growth, the ditopic modulators 4,4′-(2-phenyl-1H-imidazole-4,5-diyl)dibenzoic acid (M-ec2) or 4-(2-(4-(5-(4-carboxyphenyl)-4-phenyl-1H-imidazole-2-yl)phenyl)-5-phenyl-1H-imidazole-4-yl)benzoic acid (M-sc2) were added to the growth solutions to prepare either ec-BBI and or sc-BBI, respectively. PXRD confirmed that the resulting binary domain Zr-BBI MOFs were crystalline and isostructural to Zr-BBI (Fig. S24†). We note that the Zr-BBI-Br2 seeds were polydisperse in size. Therefore, for this system, comparing the crystal dimensions would not provide meaningful information regarding differences in domain growth between cs-, ec-, and sc-BBI products. We instead relied exclusively on SEM-EDS line-scans, conducted for Br and Zr, to reveal domain distribution (Fig. 4B). cs-BBI exhibited secondary domain growth in both the longitudinal and transverse directions, while ec-BBI and sc-BBI displayed growth exclusively along the transverse and longitudinal directions, respectively. Although the applicability of this method to two different MOFs does not imply generality, it does indicate that it could potentially be broadly applied to many non-cubic MOF systems.
Anisotropic growth of ternary domain MOFs
An outstanding challenge in MOF chemistry involves the construction of arbitrarily complex multi-domain MOF systems.10 Having successfully created anisotropic binary domain MOFs, we were motivated to apply our strategy to achieve even greater degrees of MOF hierarchical complexity. Building from the established PCN-608-OMe system, we used cs-PCN, ec-PCN and sc-PCN as seeds for growth of Zr-based tertiary domains in either the absence or presence of the ditopic modulators. These syntheses resulted in 9 different ternary domain MOF products. Prior to preparing the ternary domain MOFs, we quantified the amount of M-ec1 within the ec-PCN MOF seeds. 1H NMR spectra of the dissolved seeds indicated a 0.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 modulator to ligand ratio, which we expected would negligibly affect tertiary domain growth.
1 modulator to ligand ratio, which we expected would negligibly affect tertiary domain growth.
HAADF imaging was again used to image the MOF domains (Fig. 5). In the absence of modulator, all three MOF products exhibit darker shell domains in the HAADF images, indicating the presence of Zr. Due to the larger size of the seed MOFs, shell growth in the longitudinal direction was more easily discernible. STEM-EDS line-scans, however (Fig. 5), clearly indicate both longitudinal and transverse tertiary domain growth, which further confirm that any small amount of ditopic modulator remaining in the binary MOF seeds does not affect tertiary domain growth. With the addition of M-ec1, transverse tertiary domain growth onto all three binary domain seeds was observed while minimal growth was observed in the longitudinal direction. Specifically, STEM-EDS line scans revealed distinct Zr signals at the outermost region along the transverse direction of the ternary domain MOFs, but Hf signal is strongest at the ends of the rod-like crystals. Similar conclusions can be drawn from HAADF images and EDS line-scans of the ternary domain MOFs formed with addition of M-sc1. In these cases, the resulting MOFs exhibited significant growth along the longitudinal directions but no observable growth in the transverse directions. In summary, we successfully synthesized nine different ternary domain MOFs with diverse anisotropic domain distributions by varying the type of ditopic modulator added to the synthesis.
Conclusion
In this study, we demonstrated that modulators designed to selectively coordinate to specific crystalline facets can positionally direct the growth of secondary and tertiary MOF domains, resulting in the formation of AMD-MOFs. We systematically applied this strategy to two different MOF platforms, PCN-608-OMe and Zr-BBI, resulting in a diverse collection of hierarchically complex MOFs. In the case of PCN-608-OMe, we successfully applied multistep modulated syntheses to prepare various ternary domain MOFs via controlled anisotropic domain growth. These results underscore a robust and straightforward synthetic approach for designing and forming AMD-MOFs, which is critical for expanding access to such materials and ultimately more broadly exploring their properties and applications. Further development of these methods will investigate the effects of MOF seed morphology and modulator concentration in influencing secondary MOF domain growth and the formation of AMD-MOFs with other MOF platforms.Data availability
The data supporting this article have been uploaded as part of the ESI.†Author contributions
Y. H.: conceptualisation, investigation, methodology, formal analysis, writing original draft, writing review and editing; Z. L.: investigation, formal analysis; Z. M. S.: investigation, formal analysis; G. H.: investigation, formal analysis; N. L. R.: conceptualisation, funding acquisition, project administration, supervision, writing original draft, writing review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was supported by Defense Threat Reduction Agency (Grant no. HDTRA1-21-1-0019) and funds generously provided by the Covestro Endowment. This work was performed, in part, at the Nanoscale Fabrication and Characterization Facility, a laboratory of the Gertrude E. and John M. Petersen Institute of NanoScience and Engineering, housed at the University of Pittsburgh (PXRD, SEM, and STEM Instrumentation).Notes and references
- J. Clardy and C. Walsh, Nature, 2004, 432, 829–837 CrossRef CAS PubMed.
- T. Rodrigues, D. Reker, P. Schneider and G. Schneider, Nat. Chem., 2016, 8, 531–541 CrossRef CAS PubMed.
- P. A. Rupar, L. Chabanne, M. T. Winnik and I. Manners, Science, 2012, 337, 559–562 CrossRef CAS PubMed.
- J.-F. Lutz, M. Ouchi, D. R. Liu and M. Sawamoto, Science, 2013, 341, 1238149 CrossRef PubMed.
- K. Kawamoto, M. Zhong, K. R. Gadelrab, L.-C. Cheng, C. A. Ross, A. Alexander-Katz and J. A. Johnson, J. Am. Chem. Soc., 2016, 138, 11501–11504 CrossRef CAS PubMed.
- R. Liang, Y. Xue, X. Fu, A. N. Le, Q. Song, Y. Qian, Q. Xie, R. Dong, Z. Sun, C. O. Osuji, J. A. Johnson, W. Li and M. Zhong, Nat. Mater., 2022, 33, 1434–1440 CrossRef PubMed.
- P.-C. Chen, X. Liu, J. L. Hedrick, Z. Xie, S. Wang, Q.-Y. Lin, M. C. Hersam, V. P. Dravid and C. A. Mirkin, Science, 2016, 352, 1565–1569 CrossRef CAS PubMed.
- B. C. Steimle, J. L. Fenton and R. E. Schaak, Science, 2020, 367, 418–424 CrossRef CAS PubMed.
- W. Xu, B. Tu, Q. Liu, Y. Shu, C. S. Diercks, O. M. Yaghi, Y. Zhang, H. Deng and Q. Li, Nat. Rev. Mater., 2020, 5, 764–779 CrossRef CAS.
- L. Feng, K.-Y. Wang, J. Willman and H.-C. Zhou, ACS Cent. Sci., 2020, 6, 359–367 CrossRef CAS PubMed.
- Y. Luo, M. Ahmad, A. Schug and M. Tsotsalas, Adv. Mater., 2019, 31, 1901744 CrossRef PubMed.
- M. Wu, Y. Wang, G. Zhou and X. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 54285–54305 CrossRef CAS PubMed.
- R. Haldar and C. Woll, Nano Res., 2021, 14, 355–368 CrossRef CAS.
- H. Ha and H. R. Moon, CrystEngComm, 2021, 23, 2337–2354 RSC.
- M. Eddaoudi, D. B. Moler, H. Li, B. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34(4), 319–330 CrossRef CAS PubMed.
- S. Kitagawa, R. Kitaura and S.-i. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- X. Yang, S. Yuan, L. Zou, H. Drake, Y. Zhang, J. Qin, A. Alsalme and H.-C. Zhou, Angew. Chem., Int. Ed., 2018, 57, 3927 CrossRef CAS PubMed.
- T. Y. Luo, C. Liu, X. Y. Gan, P. F. Muldoon, N. A. Diemler, J. E. Millstone and N. L. Rosi, J. Am. Chem. Soc., 2019, 141, 2161 CrossRef CAS PubMed.
- K. Koh, A. G. Wong-Foy and A. J. Matzger, Chem. Commun., 2009, 6162 RSC.
- S. Furukawa, K. Hirai, K. Nakagawa, Y. Takashima, R. Matsuda, T. Tsuruoka, M. Kondo, R. Haruki, D. Tanaka, H. Sakamoto, S. Shimomura, O. Sakata and S. Kitagawa, Angew. Chem., Int. Ed., 2009, 121, 1798 CrossRef.
- Y. Yoo and H. Jeong, Cryst. Growth Des., 2010, 10(3), 1283–1288 CrossRef CAS.
- T. Fukushima, S. Horike, H. Kobayashi, M. Tsujimoto, S. Isoda, M. Foo, Y. Kubota, M. Takata and S. Kitagawa, J. Am. Chem. Soc., 2012, 134(32), 13341–13347 CrossRef CAS PubMed.
- X. Song, T. K. Kim, H. Kim, D. Kim, S. Jeong, H. R. Moon and M. S. Lah, Chem. Mater., 2012, 24, 3065 CrossRef CAS.
- T. Li, J. E. Sullivan and N. L. Rosi, J. Am. Chem. Soc., 2013, 135, 9984–9987 CrossRef CAS PubMed.
- G. Zhang and H. C. Zeng, Nat. Commun., 2018, 9, 3778 CrossRef PubMed.
- X. Liu, F. Zhang, T. Goh, Y. Li, Y. Shao, L. Luo, W. Huang, Y. Long, L. Chou and C. Tsung, Angew. Chem., Int. Ed., 2018, 57, 2110 CrossRef CAS PubMed.
- Y. He, M. De Souza, T.-Y. Luo, S. K. Achar, J. K. Johnson and N. L. Rosi, Angew. Chem., Int. Ed., 2024, e202409150 CAS.
- H. Lee, J. Cho, W. Cho and M. Oh, ACS Nano, 2013, 7(1), 491–499 CrossRef PubMed.
- Z. Wang, J. Liu, B. Lukose, Z. Gu, P. G. Weidler, H. Gliemann, T. Heine and C. Woll, Nano Lett., 2014, 14(3), 1526–1529 CrossRef CAS PubMed.
- L. Yuan, S. Li, J.-L. Li, K.-Y. Wang, G. S. Day, P. Zhang, Y. Wang and H.-C. Zhou, ACS Cent. Sci., 2018, 4(12), 1719–1726 CrossRef PubMed.
- O. Kwon, J. Y. Kim, S. Park, J. H. Lee, J. Ha, H. Park, H. R. Moon and J. Kim, Nat. Commun., 2019, 10, 3620 CrossRef PubMed.
- F. Wang, S. He, H. Wang, S. Zhang, C. Wu, H. Huang, Y. Pang, C.-K. Tsung and T. Li, Chem. Sci., 2019, 10, 7755–7761 RSC.
- S. Jeong, J. Seong, S. W. Monn, J. Lim, S. B. Baek, S. K. Min and M. S. Lah, Nat. Commun., 2022, 13, 1027 CrossRef CAS PubMed.
- Y. Zou, C. Liu, C. Zhang, L. Yuan, J. Li, T. Bao, G. Wei, J. Zou and C. Yu, Nat. Commun., 2023, 14, 5780 CrossRef CAS PubMed.
- S. Choi, T. Kim, H. Ji, H. Lee and M. Oh, J. Am. Chem. Soc., 2016, 138(43), 14434–14440 CrossRef CAS PubMed.
- D. Kim, G. Lee, S. Oh and M. Oh, Chem. Commun., 2019, 55, 43–46 RSC.
- G. Lee, S. Lee, S. Oh, D. Kim and M. Oh, J. Am. Chem. Soc., 2020, 142(6), 3042–3049 CrossRef CAS PubMed.
- C. Liu, Q. Sun, L. Lin, J. Wang, C. Zhang, C. Xia, T. Bao, J. Wan, R. Huang, J. Zou and C. Yu, Nat. Commun., 2020, 11, 4971 CrossRef CAS PubMed.
- F. Wang, Y. Fan, Y. Ma and T. Li, Cryst. Growth Des., 2021, 21(8), 4571–4578 CrossRef CAS.
- T. Y. Luo, C. Liu, X. Y. Gan, P. F. Muldoon, N. A. Diemler, J. E. Millstone and N. L. Rosi, J. Am. Chem. Soc., 2019, 141, 2161 CrossRef CAS PubMed.
- A. Umemura, S. Diring, S. Furukawa, H. Uehara, T. Tsuruoka and S. Kitagawa, J. Am. Chem. Soc., 2011, 133(39), 15506–15513 CrossRef CAS PubMed.
- S. Amirjalayer, M. Tafipolsky and R. Schmid, J. Phys. Chem. Lett., 2014, 5, 3206–3210 CrossRef CAS PubMed.
- G. Zhan and H. C. Zeng, Adv. Funct. Mater., 2016, 26, 3268–3281 CrossRef CAS.
- X.-Y. Liu, W.-S. Lo, C. Wu, B. P. Williams, L. Luo, Y. Li, L.-Y. Chou, Y. Lee and C.-K. Tsung, Nano Lett., 2020, 20, 1774–1780 CrossRef CAS PubMed.
- Z. Wang, L. Ge, D. Feng, Z. Jiang, H. Wang, M. Li, R. Lin and Z. Zhu, Cryst. Growth Des., 2021, 21, 926–934 CrossRef CAS.
- M. Xu, P. Cai, S.-S. Meng, Y. Yang, D.-S. Zheng, Q.-H. Zhang, L. Gu, H.-C. Zhou and Z.-Y. Gu, Angew. Chem., Int. Ed., 2022, 61, e202207786 CrossRef CAS PubMed.
- J. Pang, S. Yuan, J. Qin, C. Liu, C. Lollar, M. Wu, D. Yuan, H.-C. Zhou and M. Hong, J. Am. Chem. Soc., 2017, 139, 16939–16945 CrossRef CAS PubMed.
- X. Wang, Y. Zhang, Z. Shi, T. Lu, Q. Wang and B. Li, ACS Appl. Mater. Interfaces, 2021, 13(45), 54217–54226 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc07985j |
| This journal is © The Royal Society of Chemistry 2025 |