 Open Access Article
Open Access ArticleSCN–IgG functionalized NaYF4:Yb3+/(Er3+,Tm3+) upconversion nanoparticles for targeted fluorescence imaging of liver cancer cells
Ha Thi Phuong a,
Tran Thu Huong
a,
Tran Thu Huong *b,
Le Thi Vinh*c,
Do Thi Thaod,
Le Anh Tu
*b,
Le Thi Vinh*c,
Do Thi Thaod,
Le Anh Tu b,
Tong Quang Congb,
Nguyen Duc Vanb and
Tran Quoc Tien
b,
Tong Quang Congb,
Nguyen Duc Vanb and
Tran Quoc Tien b
b
aDepartment of Chemistry, Hanoi Medical University, 1 Ton That Tung, Hanoi 100000, Vietnam
bInstitute of Materials Science, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Hanoi 100000, Vietnam. E-mail: huongtt.ims@gmail.com
cFaculty of Basic Science, Hanoi University of Mining and Geology, 18 Pho Vien, Hanoi 100000, Vietnam. E-mail: levinhmdc@gmail.com
dInstitute of Biology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Hanoi 100000, Vietnam
First published on 2nd July 2025
Abstract
Lanthanide-doped upconversion nanoparticles (UCNPs) offer significant potential for bioimaging due to their ability to convert near-infrared (NIR) excitation into visible emission. In this study, NaYF4:Yb3+/(Er3+,Tm3+) UCNPs were synthesized via a hydrothermal method and sequentially functionalized with a silica shell, amine, thiocyanate (SCN), and immunoglobulin G (IgG) to enhance their biocompatibility and targeting capabilities. Structural characterization confirmed the formation of highly crystalline β-NaYF4 cores with uniform morphology and successful surface modification. The functionalized nanoparticles exhibited strong upconversion luminescence under 980 nm excitation, with multicolor emission dominated by red light (∼660 nm). Fluorescence microscopy and flow cytometry demonstrated selective labeling of HepG2 liver cancer cells, achieving a labeling efficiency of 18.10% for SCN–IgG-conjugated nanoparticles—significantly higher than in unmodified controls. These findings demonstrate that SCN–IgG-functionalized UCNPs are effective and selective nanoprobes for targeted fluorescence imaging and hold promise for the early diagnosis of liver cancer.
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent and lethal forms of liver cancer, often diagnosed at advanced stages due to the lack of sensitive early detection methods. In recent years, nanotechnology-based diagnostic platforms have shown great promise in improving cancer detection, particularly through the development of fluorescent nanoprobes for targeted imaging.1–4 Various applications of upconversion nanomaterials systems have been explored, e.g., triplet–triplet annihilation (TTA) for energy conversion, NaYF4:Yb3+,Er3+ UCNPs for bioimaging and photodynamic therapy (PDT), as well as rare-earth-modified inorganic semiconductor photocatalysts for efficient solar energy conversion. Additionally, plasmon-enhanced UCNPs with tunable emission properties have also been reported.5–8 Among these, lanthanide-doped upconversion nanoparticles (UCNPs) have attracted significant interest due to their unique ability to convert near-infrared (NIR) light into visible emission via multiphoton excitation. This upconversion luminescence (UCL) provides several key advantages, including deep tissue penetration, low background autofluorescence, and minimal photodamage, making UCNPs highly suitable for biological imaging applications.9–14NaYF4 has been widely recognized as the most efficient host lattice for upconversion processes due to its low phonon energy and high luminescence efficiency. In particular, NaYF4:Yb3+/Er3+ systems have been extensively studied for their strong green (∼540 nm) and red (∼650 nm) emissions under 980 nm excitation.15,16 However, to broaden the emission spectrum and enhance optical output, co-doping strategies involving both Er3+ and Tm3+ ions have been adopted. Co-doping with ytterbium (Yb3+), erbium (Er3+), and thulium (Tm3+) enables multicolor emission—blue (∼475 nm), green (∼545 nm), and red (∼660 nm)—under a single NIR excitation source, thereby facilitating multiplexed imaging and improving detection sensitivity.
Despite their promising optical properties, the biomedical application of UCNPs remains limited by challenges related to surface modification and biofunctionalization. These include the need for colloidal stability, biocompatibility, and selective cell-targeting capability.17–19 To overcome these limitations, various surface engineering strategies have been explored. Among them, silica encapsulation has proven particularly effective in providing a chemically robust and optically transparent shell that preserves luminescence while offering functional groups for subsequent modification.20–25
In particular, 3-thiocyanatopropyltriethoxysilane (SCN–PTES) offers a direct route to introduce thiocyanate (–SCN) groups, which can form covalent thiourea linkages with primary amines in biomolecules such as antibodies. This approach enables stable and site-specific conjugation without disrupting the optical properties of the UCNPs.26–31
In this study, we report the synthesis of NaYF4:Yb3+/(Er3+,Tm3+) UCNPs, their surface modification with a silica shell and SCN groups, and subsequent conjugation with immunoglobulin G (IgG) for targeted bioimaging of HepG2 liver cancer cells. Structural and spectroscopic analyses confirmed successful functionalization without compromising crystal phase or luminescence intensity. Most importantly, fluorescence microscopy and flow cytometry demonstrated that the SCN–IgG-conjugated UCNPs enabled highly selective labeling of HepG2 cells, with significantly higher efficiency than non-functionalized controls. These findings support the potential of SCN-based biofunctionalization as a robust strategy for developing selective and efficient upconversion nanoprobes for cancer diagnostics.
2. Materials and methods
2.1. Materials
All chemicals were of analytical grade and used without further purification. Yttrium(III) nitrate hexahydrate Y(NO3)3·6H2O, (99.9%), ytterbium(III) nitrate pentahydrate Yb(NO3)3·5H2O, (99.9%), erbium(III) nitrate pentahydrate Er(NO3)3·5H2O (99.9%), thulium(III) nitrate pentahydrate Tm(NO3)3·5H2O (99.9%), tetraethyl orthosilicate (TEOS (99.9%)), sodium fluoride NaF (99%), ammonium hydroxide (NH4OH (25%)), 3-triethoxysilylpropyl amine APTES (98%), and 3-triethoxysilylpropyl thiocyanate SCNPTES (95%) were purchased from Sigma-Aldrich. Trisodiumcitrate dihydrate HOC(COONa)(CH2COONa)2·2H2O (99%), sodium hydroxide NaOH (99%) were provided from Merck. Ethanol C2H5OH (99.8%) and oleic acid CH3(CH2)7CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)7COOH (92%) were supplied by Fisher Scientific. HepG2 cells were a kind gift from Prof. Chi-Ying F. Huang, National Yang Ming Chiao Tung, Taipei, Taiwan.
CH(CH2)7COOH (92%) were supplied by Fisher Scientific. HepG2 cells were a kind gift from Prof. Chi-Ying F. Huang, National Yang Ming Chiao Tung, Taipei, Taiwan.
2.2. Synthesis of NaYF4:Yb3+/(Er3+, Tm3+) nanoparticles
The upconversion nanoparticles were synthesized using a hydrothermal method. Rare-earth precursors were mixed in a molar ratio of Y3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb3+
Yb3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+
Er3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tm3+ = 78
Tm3+ = 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixture was combined with NaF, NaOH, and oleic acid in an ethanol–water solution, then vigorously stirred and transferred to a Teflon-lined stainless-steel autoclave. The autoclave was sealed and heated at 200 °C for 24 hours. The resulting product was collected by centrifugation, washed thoroughly with ethanol and deionized water, and dried at 70 °C.
1. The mixture was combined with NaF, NaOH, and oleic acid in an ethanol–water solution, then vigorously stirred and transferred to a Teflon-lined stainless-steel autoclave. The autoclave was sealed and heated at 200 °C for 24 hours. The resulting product was collected by centrifugation, washed thoroughly with ethanol and deionized water, and dried at 70 °C.
2.3. Silica coating and surface functionalization
The obtained nanoparticles were dispersed in ethanol and coated with a silica shell via the Stöber method, involving hydrolysis of TEOS in the presence of NH4OH. Subsequently, the silica-coated nanoparticles were functionalized with amine groups (–NH2) by reacting with APTES for 24 hours. For thiocyanate functionalization, SCN–PTES was added to the amine-modified nanoparticles and stirred in phosphate-buffered saline (PBS, 0.5 M, pH 5) at a concentration of 5 g L−1 for 30 minutes. The SCN-modified nanoparticles were then incubated with varying concentrations of IgG in the presence of glycerol at room temperature for 6 hours. Finally, the biofunctionalized nanoparticles were centrifuged at 5900 rpm, washed three times with water, and stored at 4 °C in sealed containers.2.4. Liver cancer cell culture, fluorescence imaging of cells
In this study, the experiments were implemented on HepG2 liver cancer cells which were maintained in Dulbecco's Modified Eagle Medium (DMEM – Invitrogen) with fetal bovine serum (10%) (Sigma) and gentamicin (50 μg mL−1) at 37 °C and 5% CO2 in a humidified atmosphere. To study the uptake capacity of the functionalized NaYF4:Yb3+/(Er3+,Tm3+)@silica–NH–SCN–IgG, the liver cancer cells (104 cells per ml) at log phase were seeded in 24 well plates, then incubated for 24 hours. Polyethylene glycol 1500 (Sigma) and UCNP samples NaYF4:Yb3+/(Er3+,Tm3+), NaYF4:Yb3+/(Er3+,Tm3+)@silica–NH–SCN–IgG were added to the wells at a final concentration of 2% and incubated for 3 hours. After the assigned time, the cultured medium was discarded. The cells were then washed with phosphate buffer saline (PBS pH 7.4) three times before fixed with formaldehyde 10% in 30 min at room temperature. The fixed cells were then formaldehyde discarded, PBS washed three times before staining with Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 solution (2 μg mL−1) for 15 minutes at room temperature. After staining, the solution was removed, and the cells were washed with PBS three times again. The cell images were obtained using the fluorescence-inverted microscope ZEISS AXIOSCOPE A1 with 200× magnification.
342 solution (2 μg mL−1) for 15 minutes at room temperature. After staining, the solution was removed, and the cells were washed with PBS three times again. The cell images were obtained using the fluorescence-inverted microscope ZEISS AXIOSCOPE A1 with 200× magnification.
2.5. Cellular uptake analysis using flowcytometry
The HepG2 cells were cultured in the DMEM supplemented with L-glutamine, sodium pyruvat, NaHCO3, penicillin/streptomycin, 10% FBS (Fetal Bovine Serum). Cells at the density of 1 × 104 cells per mL were pre-cultured in 6-well plate at 37 °C, 5% CO2 in the incubator for 24 hours. Then, the medium was replaced with DMEM (w/o FBS) added 2% of either NaYF4:Yb3+/(Er3+,Tm3+) or NaYF4:Yb3+/(Er3+,Tm3+)@silica–NH–SCN–IgG and incubated for 3 hours at 37 °C, 5% CO2 incubator. Then, the cells were detached with 0.05% trypsin–EDTA, centrifuged at 1000 rpm for 5 minutes to obtain the cell pellets. The pellets were washed with cold PBS twice before resuspending in PBS 1× for analyzing with flowcytometry Novocyte system (ACEA Bioscience inc.) and NovoExpress software. The cells were requested for light protection.2.6. Characterization techniques
Structural and morphological characterizations were performed using X-ray diffraction (XRD; D8 Advance, Bruker, Germany) and field-emission scanning electron microscopy (FESEM; S-4800, Hitachi, Japan).Surface functionalization was confirmed by Fourier-transform infrared spectroscopy (FTIR; Spectrum Two, PerkinElmer, USA). FTIR spectra were recorded in the transmittance mode over the range of 4000–400 cm−1. For FTIR analysis, the powdered nanoparticle samples were thoroughly dried and then finely ground with potassium bromide (KBr) at a ratio of approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (sample
100 (sample![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KBr, w/w). The mixture was pressed into thin pellets using a hydraulic press.
KBr, w/w). The mixture was pressed into thin pellets using a hydraulic press.
The upconversion luminescence properties were analyzed using a photoluminescence measurement system (iHR320, Horiba) under 980 nm continuous-wave (CW) laser excitation (estimated optical power density in the range of several tens of W cm−2). Measurements were conducted on dry powder samples, with equal amounts of each sample gently pressed into standard sample holders featuring a 1 mm diameter well. All measurement parameters—including excitation power, acquisition time, and optical configuration—were kept constant across samples to ensure reliable intensity comparison. To minimize light scattering due to particle aggregation, the samples were thoroughly dried, finely ground, and uniformly dispersed prior to measurement.
In vitro bioimaging experiments were performed using HepG2 liver cancer cells incubated with either bare NaYF4:Yb3+/(Er3+,Tm3+) nanoparticles or functionalized NaYF4:Yb3+/(Er3+,Tm3+)@silica–NH–SCN–IgG nanoparticles. Fluorescence microscopy was then employed to evaluate their targeting performance.
3. Results and discussion
3.1. Morphological characterization
The morphology of the synthesized NaYF4:Yb3+/(Er3+,Tm3+) nanoparticles was examined using field-emission scanning electron microscopy (FESEM), as shown in Fig. 1. | ||
| Fig. 1 FESEM images of NaYF4:Yb3+/(Er3+,Tm3+) nanoparticles: (a) high-magnification image at 200 nm scale and (b) lower magnification image at 500 nm scale, respectively. | ||
The high-resolution image (Fig. 1a) reveals that the particles exhibit well-defined morphology with near-spherical. The measured particle diameters fall within the desired range of 45–55 nm. The lower-magnification view (Fig. 1b) further confirms that the sample is well-dispersed with minimal aggregation, indicating efficient synthesis during the hydrothermal process.
After surface modification with silica, amine, thiocyanate, and subsequent IgG conjugation, the morphology of the nanoparticles was re-examined by FESEM (Fig. 2). The NaYF4:Yb3+/(Er3+,Tm3+)@silica–NH–SCN–IgG particles retain their overall shape and uniformity, with a slight increase in diameter (∼50–60 nm) compared to the unmodified sample. The increase in particle size and slightly rougher surface texture are consistent with the formation of an amorphous silica shell and subsequent bioconjugation – thiocyanate groups with IgG molecules. This change in morphology supports the successful formation of the silica layer and further functionalization, which is essential for subsequent bioconjugation with targeting ligands or antibodies. These results confirm that the nanoparticles retain their structural integrity after surface modification and are morphologically suitable for biomedical applications, including cellular.
3.2. Structure characterization
The crystalline phase of the synthesized nanoparticles were evaluated by X-ray diffraction (XRD), and the results are shown in Fig. 3. The diffraction peaks of the uncoated sample NaYF4:Yb3+/(Er3+,Tm3+) (red line-(1)) match well with the standard hexagonal β-NaYF4 phase (JCPDS no. 28-1192), indicating the successful formation of the desired crystal structure.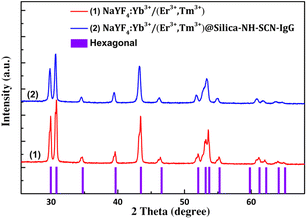 | ||
| Fig. 3 X-ray diffraction patterns of the (1) NaYF4:Yb3+/(Er3+, Tm3+) and (2) NaYF4:Yb3+/(Er3+, Tm3+)@silica–NH–SCN–IgG nanoparticles. | ||
Characteristic peaks at 2θ values of approximately ∼28.2, 30.0, 32.7, 34.8, 40.0, 43.5, 47.0, 52.7, 55.5, 58.3, 60.3, 62.9° correspond to the (1 0 0), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (2 0 0), (1 1 2), (2 0 1), (1 0 4), (2 0 2), (2 1 0) lattice planes of β-NaYF4, respectively. The narrow and intense diffraction peaks suggest high crystallinity of the as-prepared nanomaterials. After silica coating and amine, thiocyanate functionalization with IgG-conjugated (blue line – (2)), the diffraction pattern remains largely unchanged, retaining all the key peaks associated with the β-NaYF4 phase. This indicates that the silica, amine, thiocyanate groups promoted the covalent IgG conjugation shell is amorphous and does not interfere with the crystal structure of the core material.
The diffraction intensity slightly decreases without peak broadening or shifting, suggesting that the shell did not alter the crystal structure of the core material. No additional peaks are observed, confirming the phase purity of the coated nanoparticles.
To estimate the average crystallite size, the Scherrer equation was applied to the most intense diffraction peak located at approximately 2θ ≈ 30.0°:
This crystallite size is in good agreement with the particle size range of 45–60 nm observed in FESEM images, suggesting that the nanoparticles are largely monocrystalline. The consistency between XRD and FESEM results indicates that almost each nanoparticle corresponds to a single crystal domain with minimal aggregation or polycrystallinity.
Fourier-transform infrared (FTIR) spectroscopy was used to confirm the surface modification and functional group incorporation on the synthesized nanoparticles. Fig. 4 displays the FTIR spectra of NaYF4:Yb3+/(Er3+,Tm3+) nanoparticles before (sample 1) and after surface modification with silica, amine, thiocyanate (SCN), and immunoglobulin G (IgG) (sample 2).
 | ||
| Fig. 4 FTIR spectra of NaYF4:Yb3+/(Er3+,Tm3+) nanoparticles before (1) and after (2) surface functionalization with silica–NH–SCN–IgG. | ||
Both samples exhibit a broad absorption band in the range of 3350–3450 cm−1, which is attributed to the O–H stretching vibrations of adsorbed water or surface hydroxyl groups. The absorption band at approximately 2930 cm−1 corresponds to the asymmetric and symmetric stretching vibrations of C–H bonds, likely originating from residual organic surfactants such as oleic acid used during synthesis. In sample 2, a distinct absorption band at ∼1660 cm−1 corresponds to N–H bending vibrations from amine groups (introduced via APTES) and/or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching from IgG molecules, indicating successful protein conjugation. A weak but distinct peak at ∼2050 cm−1 is exclusively observed in sample 2 and corresponds to the C
O stretching from IgG molecules, indicating successful protein conjugation. A weak but distinct peak at ∼2050 cm−1 is exclusively observed in sample 2 and corresponds to the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching vibration, which is characteristic of thiocyanate (–SCN) groups. Despite its low intensity, this band provides compelling evidence of successful SCN incorporation via amine linkers onto the silica surface.32–34
N stretching vibration, which is characteristic of thiocyanate (–SCN) groups. Despite its low intensity, this band provides compelling evidence of successful SCN incorporation via amine linkers onto the silica surface.32–34
Notably, strong absorption bands observed between 1000 and 1150 cm−1 in sample 2 are characteristic of Si–O–Si stretching vibrations, confirming the presence of a silica shell. In the low wavenumber region below 1000 cm−1, both spectra exhibit absorption bands associated with lanthanide–fluoride (Ln–F) vibrations, particularly around 460–690 cm−1, validating the NaYF4:Yb3+/(Er3+,Tm3+) core structure.35,36
In summary, the FTIR results clearly confirm the stepwise surface modification process—including silica coating, amination, SCN functionalization, and IgG conjugation—which is crucial for enhancing colloidal stability, biocompatibility, and targeted bioimaging performance of the upconversion nanoparticles.
3.3. Luminescence properties
Fig. 5 presents the upconversion photoluminescence (UCPL) spectra of NaYF4:Yb3+/(Er3+,Tm3+) nanoparticles before and after surface modification.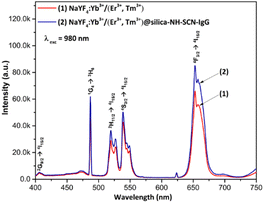 | ||
| Fig. 5 UCPL spectra of NaYF4:Yb3+/(Er3+,Tm3+) (1) and NaYF4:Yb3+/(Er3+,Tm3+)@silica–NH–SCN–IgG nanoparticles (2) under 980 nm excitation. | ||
Under 980 nm excitation, at ∼475 nm (blue) corresponds to the 1G4 → 3H6 transition of Tm3+, ∼410 nm band: corresponds to 2G9/2 → 4I15/2 transition, at ∼520–560 nm band (green): attributed to overlapping (2H11/2, 4S3/2) → 4I15/2 transitions of Er3+, respectively. These results confirm efficient energy transfer from Yb3+ sensitizers to both Er3+ and Tm3+ activators within the NaYF4 host matrix.
Notably, the surface-modified nanoparticles NaYF4:Yb3+/(Er3+,Tm3+)@silica–NH–SCN–IgG (curve 2) exhibits enhanced emission intensity compared to the uncoated core (curve 1). This enhancement may be attributed to the silica shell's ability to reduce surface-related quenching, protect the luminescent core, and potentially improve light scattering or local field effects. The preservation and even enhancement of dual-color emission (blue and red) after surface modification confirms that the core crystal quality is maintained and the functional coating is compatible with upconversion luminescence. Among the three emission bands, the red emission (∼660 nm) is dominant. This result supports the material's suitability for fluorescence imaging in biological labeling applications.
3.4. In vitro cellular labeling
To evaluate the bio-recognition performance of the synthesized nanomaterials, fluorescence labeling experiments were applied in HepG2 liver cancer cells. Fig. 6 presents the comparative fluorescence microscopy images of: (1) negative control, (2) HepG2 cells treated with NaYF4:Yb3+/(Er3+,Tm3+), and (3) HepG2 cells treated with NaYF4:Yb3+/(Er3+,Tm3+)@silica–NH–SCN–IgG. For each condition, red channel (upconversion luminescence), blue channel (Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 nuclear stain), bright-field (transition) and merged images are provided.
342 nuclear stain), bright-field (transition) and merged images are provided.
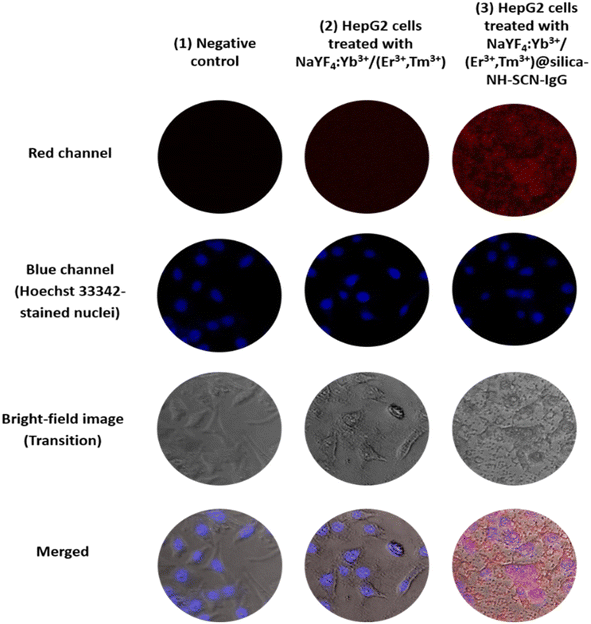 | ||
| Fig. 6 Fluorescence microscopy images of HepG2 cells treated with (1) negative control (no nanoparticles), (2) NaYF4:Yb3+/(Er3+,Tm3+) and (3) NaYF4:Yb3+/(Er3+,Tm3+)@silica–NH–SCN–IgG. | ||
In the negative control group, no red fluorescence was observed, as expected. Cells treated with unmodified UCNPs NaYF4:Yb3+/(Er3+,Tm3+) showed weak and diffuse red signals, likely resulting from nonspecific adsorption or limited uptake. In stark contrast, HepG2 cells treated with SCN–IgG-functionalized nanoparticles NaYF4:Yb3+/(Er3+,Tm3+)@silica–NH–SCN–IgG exhibited intense and localized red fluorescence co-localized with the nuclei, demonstrating effective and selective cellular targeting.
The enhanced targeting is attributed to the role of thiocyanate (–SCN) groups, which form stable thiourea bonds with the primary amine groups of IgG antibodies. This covalent conjugation preserves the bioactivity of IgG, allowing it to serve as a molecular recognition element that specifically binds to surface antigens on HepG2 cells. Notably, no significant red signal was detected in the normal control cells (HEK-293A, data not shown), supporting the selective targeting capability of the SCN–IgG system.
The merged fluorescence images further highlight this selectivity, with clear co-localization of red (UCNP) and blue (nucleus) signals, confirming successful surface binding without off-target fluorescence. These results demonstrate that the developed nanoprobes effectively combine bright upconversion luminescence with ligand-directed specificity for potential use in targeted liver cancer diagnostics.
3.5. Cellular uptake analysis using flow cytometry
To evaluate the cellular penetration ability of the synthesized upconversion nanoprobes, flow cytometry analysis was conducted on HepG2 liver cancer cells incubated with different nanoparticle formulations. The fluorescence signal (PE-A channel) was quantitatively analyzed to determine the percentage of fluorescent cell population as shown in Table 1 and illustrated in Fig. 7.| Samples | Fluorescent cell population (%) |
|---|---|
| Negative control (no nanoparticles) | 3.68 |
| HepG2 – NaYF4:Yb3+/(Er3+,Tm3+) | 3.13 |
| HepG2 – NaYF: Yb3+/(Er3+,Tm3+)@silica–NH–SCN–IgG | 18.10 |
 | ||
| Fig. 7 Flow cytometry analysis of HepG2 cells labelled with: (a) negative control (no treatment), (b) NaYF4:Yb3+/(Er3+,Tm3+) and (c) NaYF4:Yb3+/(Er3+,Tm3+)@silica–NH–SCN–IgG. | ||
Table 1 shows that both the negative control (untreated cells) and the group treated with non-functionalized NaYF4:Yb3+/(Er3+,Tm3+) nanoparticles exhibited minimal fluorescence, with positive populations of only 3.68 and 3.13%, respectively. This indicates negligible non-specific cellular uptake. In contrast, HepG2 cells treated with the IgG-conjugated nanoprobes (NaYF4:Yb3+/(Er3+,Tm3+)@SiO2–NH–SCN–IgG) displayed a markedly higher fluorescent cell population of 18.10%, clearly indicating enhanced cellular binding and uptake due to IgG-mediated recognition. These findings are further corroborated by the flow cytometry histograms in Fig. 7, which demonstrate a significant shift in fluorescence intensity in the IgG-conjugated group compared to the other two. The enhanced signal confirms the successful targeting ability of the functionalized nanoprobes.
Thus, the results of cellular uptake analysis provide compelling evidence that the surface modification—particularly IgG conjugation – significantly improves the targeting and cellular uptake of the upconversion nanoparticles. This reinforces their potential applicability in selective liver cancer cell imaging.
4. Conclusions
In this study, NaYF4:Yb3+/(Er3+,Tm3+) upconversion nanoparticles were successfully synthesized and sequentially functionalized with silica, thiocyanate, and IgG to enhance their biocompatibility and targeting capability. The resulting nanoprobes exhibited high crystallinity, uniform nanoscale morphology, and strong upconversion luminescence under 980 nm excitation, with dominant red emission.Surface modification with silica and SCN–IgG preserved or enhanced luminescence intensity while enabling selective cellular recognition. Fluorescence microscopy and flow cytometry confirmed specific labeling of HepG2 liver cancer cells, achieving a labeling efficiency of 18.10% in the IgG-conjugated group – significantly higher than in control samples.
These results demonstrate the potential of SCN–IgG-functionalized NaYF4:Yb3+/(Er3+,Tm3+) nanoparticles as highly selective and luminescent nanoprobes for targeted fluorescence imaging in liver cancer diagnostics.
Data availability
All data supporting the findings of this study, including figures and tables, are available within the article. Additional datasets are available from the corresponding author upon reasonable request.Author contributions
Ha Thi Phuong: methodology, investigation, formal analysis, data curation, conceptualization. Le Thi Vinh: writing – original draft, methodology, investigation, formal analysis. Do Thi Thao: methodology, investigation, formal analysis. Le Anh Tu: methodology, formal analysis. Tong Quang Cong: methodology, investigation. Nguyen Duc Van: writing – review & editing. Tran Quoc Tien: methodology, formal analysis, writing – review and editing. Tran Thu Huong: writing – review & editing, methodology, investigation, formal analysis, conceptualization and editing the final manuscript. All the authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare that there is no conflicts of interest regarding the publication of this paper.Acknowledgements
This research is funded by the Vietnam Academy of Science and Technology under Grant No. VAST03.03/23-24.References
- J. Zhou, Z. Liu and F. Li, Upconversion Nanophosphors for Small-Animal Imaging, Chem. Soc. Rev., 2012, 41, 1323–1349 RSC.
- Y. Liu, D. Tu, H. Zhu, E. Ma and X. Chen, Lanthanide-doped luminescent nano-bioprobes: from fundamentals to biodetection, Nanoscale, 2013, 5, 1369–1384 RSC.
- G. Chen, H. Qiu, P. N. Prasad and X. Chen, Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics, Chem. Rev., 2014, 114(10), 5161–5214 CrossRef CAS PubMed.
- M. Wang, G. Abbineni, A. Clevenger, C. Mao and S. Xu, Upconversion Nanoparticles: Synthesis, Surface Modification and Biological Applications, Nanomed. Nanotechnol. Biol. Med., 2011, 7(6), 710–729 CrossRef CAS PubMed.
- K. Chen, Q. Luan, T. Liu, B. Albinsson and L. Hou, Semiconductor nanocrystals-based triplet-triplet annihilation photon-upconversion: Mechanism, materials and applications, Responsive Mater., 2025, 3(1), e20240030 CrossRef CAS.
- A. A. Doronkina, V. I. Kochubey and A. V. Maksutova, et al., NaYF4:Yb,Er Upconversion Nanoparticles for Imaging: Effect on Red Blood Cells, Photonics, 2023, 10, 1386 CrossRef CAS.
- Y. Yaoguang, C. Gang, Z. Yansong and H. Zhonghui, Recent advances in rare-earth elements modification of inorganic semiconductor-based photocatalysts for efficient solar energy conversion, J. Rare Earths, 2015, 33(5), 453–462 CrossRef.
- F. Kang, J. He, T. Sun, Z. Y. Bao, F. Wang and D. Y. Lei, Plasmonic Dual-Enhancement and Precise Color Tuning of Gold Nanorod@SiO2 Coupled Core–Shell–Shell Upconversion Nanocrystals, Adv. Funct. Mater., 2017, 27(36), 1701842 CrossRef.
- F. Wang and X. Liu, Upconversion Multicolor Fine-Tuning: Visible to Near-Infrared Emission from Lanthanide-Doped NaYF4 Nanoparticles, J. Am. Chem. Soc., 2008, 130, 5642–5643 CrossRef CAS PubMed.
- F. Wang and X. Liu, Recent Advances in the Chemistry of Lanthanide-Doped Upconversion Nanocrystals, Chem. Soc. Rev., 2009, 38, 976–989 RSC.
- J. Zhou, Q. Liu, W. Feng, Y. Sun and F. Li, Upconversion Luminescent Materials: Advances and Applications, Chem. Rev., 2015, 115(1), 395–465 CrossRef CAS PubMed.
- B. Zhou, B. Shi, D. Jin and X. Liu, Controlling Upconversion Nanocrystals for Emerging Applications, Nat. Nanotechnol., 2015, 10, 924–936 CrossRef CAS PubMed.
- M. Haase and H. Schäfer, Upconverting Nanoparticles, Angew. Chem., Int. Ed., 2011, 50(26), 5808–5829 CrossRef CAS PubMed.
- L. Gaofeng, W. Haojie, S. Hao, W. Haitao, Z. Mengxi, J. Aihua, L. Jinghua and L. Guangda, Recent progress in the development of upconversion nanomaterials in bioimaging and disease treatment, J. Nanobiotechnol., 2020, 18, 154 CrossRef PubMed.
- T. T. Huong, H. T. Phuong, L. T. Vinh, H. T. Khuyen, D. T. Thao, L. D. Tuyen, T. K. Anh and L. Q. Minh, Upconversion NaYF4:Yb3+/Er3+@silica-TPGS bio-nano complexes: synthesis, characterization, and in vitro test for labelling cancer cells, J. Phys. Chem. B, 2021, 125, 9768–9775 CrossRef PubMed.
- H. T. Phuong, L. T. Vinh, T. Q. Cong, T. Q. Tien, N. D. Van, V. T. H. Ha, V. N. Phan, L. T. Hoi, P. D. Thang, D. T. Thao and T. T. Huong, Optimized NaYF4: Er3+/Yb3+ Upconversion Nanocomplexes via Oleic Acid for Biomedical Applications, Inorganics, 2025, 13, 1–22 CrossRef.
- S. Wen, J. Zhou, K. Zheng, A. Bednarkiewicz, X. Liu and D. Jin, Advances in highly doped upconversion nanoparticles, Nat. Commun., 2018, 9, 2415 CrossRef PubMed.
- F. Zhang, Y. Wan, T. Yu, F. Zhang, Y. Shi, S. Xie, Y. Li, L. Xu, B. Tu and D. Zhao, Uniform Nanostructured Arrays of Sodium Rare-Earth Fluorides for Highly Efficient Multicolor Upconversion Luminescence, Angew. Chem., Int. Ed., 2007, 46(42), 7976–7979 CrossRef CAS PubMed.
- D. Gao, G. Dangli, Z. Xiangyu, Z. Hairong, S. Peng, L. Long and L. Yawen, Codopant ion-induced tunable upconversion emission in β-NaYF4:Yb3+/Tm3+ nanorods, Dalton Trans., 2013, 42, 1834–1841 RSC.
- C. Hongjin, X. Juan, Z. Baozhou, L. Botong, X. Shuilin, R. Na, X. Xiaoji, H. Ling and H. Wei, Rare Earth Ion-Doped Upconversion Nanocrystals: Synthesis and Surface Modification, Nanomaterials, 2015, 5, 1–25 Search PubMed.
- A. J. Rufaihah and Z. Yong, Biocompatibility of silica coated NaYF4 upconversion fluorescent nanocrystals, Biomaterials, 2008, 29(30), 4122–4128 CrossRef PubMed.
- H. Dong, L.-D. Sun and C.-H. Yan, Energy Transfer in Lanthanide Upconversion Studies for Extended Optical Applications, Chem. Soc. Rev., 2015, 44, 1608–1634 RSC.
- M. K. Gnanasammandhan, N. M. Idris, A. Bansal, K. Huang and Y. Zhang, Near-infrared photoactivation using mesoporous silica–coated NaYF4:Yb,Er/Tm upconversion nanoparticles, Nat. Protoc., 2016, 11, 688–713 CrossRef CAS PubMed.
- X. Tang, Y. Han, W. Zhou, W. Shen and Y. Wang, A FRET Based Composite Sensing Material Based on UCNPs and Rhodamine Derivative for Highly Sensitive and Selective Detection of Fe3+, J. Fluoresc., 2023, 33(6), 2219–2228 CrossRef CAS PubMed.
- Z. Li, Y. Zhang and S. Jiang, Multicolor Core/Shell-Structured Upconversion Fluorescent Nanoparticles, Adv. Mater., 2008, 20(24), 4765–4769 CrossRef CAS.
- H. Chu, T. Cao, G. Dai, B. Liu, H. Duan, C. Kong, N. Tian, D. Hou and Z. Sun, Recent Advances in Functionalized Upconversion Nanoparticles for Light-Activated Tumor Therapy, RSC Adv., 2021, 11, 35472–35488 RSC.
- C. P. Alonso, et al., Synthesis, characterization and application in HeLa cells of NIR light responsive doxorubicin delivery system based on NaYF4:Yb,Tm@SiO2-PEG nanoparticles, ACS Appl. Mater. Interfaces, 2015, 7(27), 14992–14999 CrossRef PubMed.
- C. Yueying, W. Jiehua, Z. Xianlin, L. Yanling, A. P. James, L. Yiqing and H. P. Nicolle, Assessing the activity of antibodies conjugated to upconversion nanoparticles for immunolabeling, Anal. Chim. Acta, 2022, 1209, 339863 CrossRef PubMed.
- C. Wang, L. Cheng and Z. Liu, Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics, Theranostics, 2013, 3(5), 317–330 CrossRef PubMed.
- R. Wei, W. Shihui, A. T. Sherif, P. S. Qian, L. Gungun, A. J. Lining, J. F. Michael, G. Harshad, M. O. Antoine and J. Dayong, Anisotropic functionalization of upconversion nanoparticles, Chem. Sci., 2018, 9(18), 4352–4358 RSC.
- H. Chu, L. Wang, Y. Zhu, X. Yu and Y. Wang, Recent advances in functionalized upconversion nanoparticles for light-activated tumor therapy, RSC Adv., 2021, 11(56), 35472–35488 RSC.
- M. B. Audet, B. Byrne and S. G. Kazarian, High-Throughput Thermal Stability Analysis of a Monoclonal Antibody by Attenuated Total Reflection FT-IR Spectroscopic Imaging, Anal. Chem., 2014, 86, 9786–9793, DOI:10.1021/ac502529q.
- A. Sadat and I. J. Joye, Peak Fitting Applied to Fourier Transform Infrared and Raman Spectroscopic Analysis of Proteins, Appl. Sci., 2020, 10(5918), 1–16, DOI:10.3390/app10175918.
- S. Niekamp, N. Stuurman and R. D. Vale, A 6-nm ultra-photostable DNA FluoroCube for fluorescence imaging, Nat. Methods, 2020, 17, 437–441 CrossRef CAS PubMed.
- R. G. Geitenbeek, P. T. Prins, W. Albrecht, A. V. Blaaderen, B. M. Weckhuysen and A. Meijerink, NaYF4:Er3+,Yb3+/SiO2 Core/Shell Upconverting Nanocrystals for Luminescence Thermometry up to 900 K, J. Phys. Chem. C, 2017, 121, 3503–3510 CrossRef CAS PubMed.
- T. M. Stauffer, Applications of Molecular Spectroscopy to Current Research in the Chemical and Biological Sciences - Fourier Transform Infrared and Raman Characterization of Silica-Based Materials, Larissa Brentano Capeletti and João Henrique Zimnoch, Intech, 2016, ch. 1, pp. 1–20 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |


