 Open Access Article
Open Access ArticleFabrication of inorganic@organic V2O5@PEDOT nanocomposite cathode for advanced aqueous manganese-ion batteries†
Xianyu Liu *a,
Jianan Zhaob,
Zhigang Fan*c,
Yingchun Xiaoa,
Yande Zhaoa and
Qing Guoa
*a,
Jianan Zhaob,
Zhigang Fan*c,
Yingchun Xiaoa,
Yande Zhaoa and
Qing Guoa
aBailie School of Petroleum Engineering, Lanzhou City University, Lanzhou 730070, China. E-mail: xyliu15@mail.ustc.edu.cn
bDepartment of Materials Science and Engineering, College of Transportation Engineering, Dalian Maritime University, Dalian, 116026, PR China
cNational Petroleum and Natural Gas Pipeline Network Group Co., Ltd, Yunnan Branch, Kunming 650032, China. E-mail: fengdediaolin@163.com
First published on 2nd July 2025
Abstract
Aqueous manganese-ion batteries (AMIBs) show promise for energy storage because Mn anodes exhibit high capacity (976 mAh g−1) and low potential (−1.18 V vs. SHE). However, cathode development faces challenges due to solvated Mn2+ with a large radius, resulting in slow ion diffusion, structural instability and limited capacity. Herein, we synthesized inorganic@organic V2O5@PEDOT nanocomposite via a facile in situ polymerization method by combining the EDOT monomer with V2O5. The resulting PEDOT coating exhibited strong adhesion to the V2O5 substrate owing to the redox reaction at the organic–inorganic interface, creating a unique hybrid architecture with enhanced charge transfer properties. Furthermore, the PEDOT composite significantly enhanced electrochemical performance by simultaneously suppressing vanadium dissolution and improving electronic conductivity, resulting in exceptionally high specific capacity (340.3 mAh g−1 at 0.5 A g−1) and rate capability (211.8 mAh g−1 at 5 A g−1). Systematic mechanism characterization confirmed the structural stability and high reversibility of Mn2+ insertion/extraction. The practical applicability of the nanocomposite was further demonstrated in a full-cell configuration (Mn‖V2O5@PEDOT), demonstrating high capacity. This study presents a high-performance cathode material for advanced AMIBs and provides new insights into design principles.
1. Introduction
Aqueous battery systems have emerged as promising candidates for large-scale energy storage applications primarily because of their inherent safety features and environmental advantages.1–3 Among the various aqueous battery technologies, manganese (Mn) metal stands out because of its low redox potential (−1.18 V vs. standard hydrogen electrode), suggesting that aqueous manganese-ion batteries (AMIBs) can achieve higher operating voltages.4 Furthermore, Mn anodes offer additional advantages, including abundant natural reserves, low costs, nontoxicity, and high capacity (976 mAh g−1 based on the Mn/Mn2+ redox couple), positioning AMIBs as highly competitive candidates for next-generation aqueous rechargeable batteries.5,6 The electrochemical performance of AMIBs is largely governed by their cathode materials. Current research has focused on three major categories of cathode materials: vanadium-based compounds, manganese-based oxides and organic materials.7–9Among these cathode materials, vanadium-based materials have attracted particular attention owing to their cost-effectiveness, multivalent oxidation states, and high theoretical capacities.10,11 Nevertheless, the practical application of V2O5-based cathodes is limited by sluggish reaction kinetics and rapid capacity fading due to poor electrical conductivity, strong Mn2+ electrostatic interactions, and material dissolution issues.12–14 To address these technical challenges, research efforts have been devoted to developing effective modification strategies, which primarily focus on defect engineering,15 heteroatom doping,16,17 conductive network construction18,19 and electrolyte optimization.20,21 These modification approaches synergistically enhance electrochemical performance by simultaneously improving charge transfer kinetics, structural stability, and interfacial compatibility.22 For instance, Al3+ was introduced as a pillar in layered V2O5 to develop an AlVO cathode via facile one-step hydrothermal synthesis, demonstrating exceptional compatibility with AMIBs. This cathode material exhibited remarkable electrochemical properties and rapid reaction kinetics.5 However, metal-ion-intercalated V2O5 electrodes typically exhibit poor electronic conductivity, which significantly limits their electrochemical performance. Moreover, conducting poly(3,4-ethylenedioxythiophene) (PEDOT) has been widely employed as an electrode material in energy storage systems because of its relatively high conductivity.23,24 PEDOT has emerged as an ideal candidate for enhancing the electrical conductivity of composite materials because of its excellent compatibility with inorganic materials. The incorporation of PEDOT can significantly improve the overall performance of electrode materials by optimizing electron conduction pathways.25,26 PEDOT polymers can be incorporated into V2O5 to fabricate inorganic@organic nanocomposites, significantly enhancing the stable host structures.
In this study, we develop an inorganic@organic V2O5@PEDOT nanocomposite via an in situ polymerization approach utilizing the interfacial redox reaction between V2O5 and the EDOT monomer at room temperature. The in situ formed PEDOT chains form conductive networks and mixed V5+/V4+ valences, which significantly improve the electronic conductivity and ionic diffusion coefficients for AMIBs. Benefiting from these synergistic effects, the as-prepared inorganic@organic V2O5@PEDOT nanocomposite manifests exceptional electrochemical performance, including a high capacity of 340 mAh g−1 at 0.5 A g−1, a remarkable rate capability (maintaining 211.8 mAh g−1 at 5 A g−1) and outstanding cycling stability after 1000 cycles in an MnSO4 electrolyte. This approach demonstrates exceptional potential for practical applications, combining facile synthesis with outstanding electrochemical performance.
2. Experimental section
2.1 Material preparation
Initially, 7 g of a V2O5 powder was dispersed in 70 mL of deionized water under continuous stirring at room temperature to form a homogeneous suspension. Subsequently, 3 mL of the EDOT monomer was added to the system drop-wise, followed by continuous stirring for 144 h at room temperature. During this process, a distinct color transition from yellow to green was observed, indicating the occurrence of polymerization. The resultant V2O5@PEDOT products were obtained via vacuum-drying at 80 °C for 15 h.2.2 Material characterization
The crystalline phases and structural properties of the synthesized V2O5@PEDOT were characterized by X-ray diffraction (XRD) using a Rigaku D-Max-3A diffractometer with Cu Kα radiation (λ = 1.5418 Å). Morphological analysis was performed by scanning electron microscopy (SEM; Zeiss SUPRA 55 SAPPHIRE) and transmission electron microscopy (TEM; JEM2100F). The chemical compositions and surface electronic states were investigated by X-ray photoelectron spectroscopy (XPS) on a PerkinElmer PHI 1600 ESCA system equipped with an Al Kα X-ray source.2.3 Electrochemical measurement
The carbon anodes were prepared by formulating uniform slurries containing activated carbon (AC), conductive carbon, and a PVDF binder in an optimized weight ratio (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) using NMP as the processing solvent. These slurries were precisely coated onto a carbon felt, followed by drying at 80 °C, achieving controlled active material loadings of ∼20 mg cm−2. The cathodes were prepared by combining V2O5@PEDOT composites with carbon nanotubes (CNTs) at a ratio of 7
1) using NMP as the processing solvent. These slurries were precisely coated onto a carbon felt, followed by drying at 80 °C, achieving controlled active material loadings of ∼20 mg cm−2. The cathodes were prepared by combining V2O5@PEDOT composites with carbon nanotubes (CNTs) at a ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and dispersed in DMF. The mixed solution underwent ultrasonic treatment for 30 min to ensure homogeneous dispersion. Then, the V2O5@PEDOT composites were obtained via filtration. The weight loading of V2O5@PEDOT was ∼1.5 mg cm−2. For the cell assembly, 2032-type coin cells were configured using either polished Mn metal sheets or carbon felt as anodes, with multiple filter paper separators (diameter = 16 mm) and an aqueous MnSO4 electrolyte (3 M). The electrochemical performance was comprehensively evaluated via cyclic voltammetry (0.5–2.5 mV s−1 scan rates) and galvanostatic charge–discharge tests within appropriate voltage windows (−1.4 to 0.8 V for AC‖V2O5; 0.4–1.9 V for Mn‖V2O5@PEDOT) using analysis equipment (CHI 660E workstation and LAND CT2001A system, respectively).
3 and dispersed in DMF. The mixed solution underwent ultrasonic treatment for 30 min to ensure homogeneous dispersion. Then, the V2O5@PEDOT composites were obtained via filtration. The weight loading of V2O5@PEDOT was ∼1.5 mg cm−2. For the cell assembly, 2032-type coin cells were configured using either polished Mn metal sheets or carbon felt as anodes, with multiple filter paper separators (diameter = 16 mm) and an aqueous MnSO4 electrolyte (3 M). The electrochemical performance was comprehensively evaluated via cyclic voltammetry (0.5–2.5 mV s−1 scan rates) and galvanostatic charge–discharge tests within appropriate voltage windows (−1.4 to 0.8 V for AC‖V2O5; 0.4–1.9 V for Mn‖V2O5@PEDOT) using analysis equipment (CHI 660E workstation and LAND CT2001A system, respectively).
3. Results and discussion
To elucidate the structure–property relationship of the V2O5@PEDOT composite, SEM and TEM were employed for morphological investigations. As evidenced in Fig. 1a–c, the composite maintains a well-defined nanorod-like architecture, exhibiting uniform widths of approximately 30 nm with longitudinal dimensions extending to several micrometers. After composting with CNTs, V2O5@PEDOT is embedded within the 3D interconnected CNT network (Fig. 1d–f). The CNT matrix fully encapsulates V2O5 nanorods, forming a robust conductive framework that ensures stable interfacial contact between the active material and conductive substrate. This hierarchical architecture facilitates efficient charge transfer pathways, thereby optimizing electrochemical kinetics.27 Further SEM-EDS mapping (Fig. S1a and b†) reveals the homogeneous distribution of C, V, O, and S, confirming the uniform incorporation of PEDOT across the composite. The oxidative polymerization process is initiated when EDOT monomers come into contact with V2O5 in the aqueous medium, leading to the in situ formation of a conductive PEDOT layer on the V2O5 surface. As shown in Fig. S2,† the content of PEDOT is ∼16.3%. This interfacial reaction occurs via a well-defined redox mechanism, in which V2O5 serves as the oxidizing agent and structural template. Thus, bulk V2O5 is exfoliated into the nanorod-like morphology.28 XRD analysis was performed to investigate the structural evolution of the V2O5@PEDOT composite (Fig. 2a). The diffraction patterns confirm that the phase of V2O5 (JCPDS no. 41-1426) remains intact after PEDOT modification (Fig. S3†). Importantly, the (200) diffraction peak exhibits a notable shift from 15.3° in pristine V2O5 to 9.1° in the V2O5@PEDOT composite, accompanied by peak broadening and intensity reduction. According to Bragg's law calculations, this shift corresponds to an interlayer spacing expansion from 5.8 Å to 9.7 Å, providing direct evidence of the successful PEDOT intercalation and the resulting interlayer expansion effect.24 Furthermore, intercalated PEDOT molecules serve as structural pillars that effectively prevent the collapse of the layered structure during electrochemical cycling.29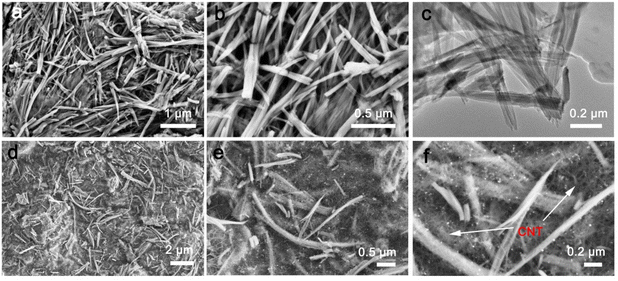 | ||
| Fig. 1 (a and b) SEM image and (c) TEM image of V2O5@PEDOT. (d–f) SEM images of V2O5@PEDOT composites with CNTs. | ||
 | ||
| Fig. 2 (a) XRD pattern, (b) XPS spectrum, (c) V 2p spectrum and (d) S 2p spectrum of the V2O5@PEDOT composite. | ||
The XPS spectrum of the V2O5@PEDOT composite is presented in Fig. 2b. Survey scan analysis reveals distinct characteristic peaks corresponding to C 1s, S 2p, V 2p, and O 1s core levels, confirming the successful incorporation of PEDOT into the composite. The high-resolution V 2p spectrum (Fig. 2c) exhibits a doublet structure with binding energies of 516.2 eV (V 2p3/2) and 525.0 eV (V 2p1/2), which demonstrates the coexistence of V5+/V4+ mixed valence states in the composite.25 This valence state analysis indicates that the interfacial interaction between V2O5 and PEDOT induces the partial reduction of V5+ to V4+, and the resulting mixed valence configuration significantly enhances the Mn2+ transport kinetics during electrochemical processes.30 The S 2p spectrum (Fig. 2d) displays characteristic features of the thiophene-based PEDOT polymer. These spectroscopic signatures provide compelling evidence of the successful polymerization of EDOT and its effective integration into the V2O5 matrix. Based on the XRD and XPS results of the V2O5@PEDOT composite, the reaction mechanism between V2O5 and PEDOT is discussed in detail. This study proposes a simple in situ polymerization method based on the redox reaction between V2O5 and the EDOT monomer. V2O5 acts as an oxidizing agent, initiating electron transfer and the deprotonation of EDOT monomers. This redox process involves oxygen extraction from the V2O5 lattice at the interface, leading to the reduction of V5+ to V4+ (as confirmed by XPS V 2p analysis in Fig. 2c) while simultaneously promoting EDOT polymerization into PEDOT on V2O5. After PEDOT polymerization, the (200) diffraction peak exhibits a notable shift to a high degree in the V2O5@PEDOT composite (Fig. 2a), providing direct evidence of the successful PEDOT intercalation and the resulting interlayer expansion effect.24 The resulting PEDOT coating exhibits strong adhesion to the V2O5 substrate at the organic–inorganic interface, creating a unique hybrid architecture with enhanced charge transfer properties.31
Coin cells were assembled using the AC anodes and 3 M MnSO4 electrolyte for comparing the performance of V2O5@PEDOT composite cathodes. As shown in Fig. 3a, the composite cathode exhibits similar charge–discharge voltage plateaus at 0.5 A g−1, demonstrating excellent electrochemical reversibility. Rate capability analysis (Fig. 3b) reveals that the optimized V2O5@PEDOT cathode delivers superior specific capacities of 340.3, 297.4, 242.3, and 211.8 mAh g−1 at various current densities. The capacity significantly outperforms unmodified V2O5 (143.1 mAh g−1 at 5 A g−1). Remarkably, when the current density is returned to 0.5 A g−1 after high-rate testing, V2O5@PEDOT maintains 291.2 mAh g−1, demonstrating exceptional Mn2+ storage reversibility and rapid ion transport kinetics. This was further confirmed by the well-defined charge–discharge plateaus and reduced polarization observed at various current densities (Fig. 3c). Long-term cycling tests at 1 A g−1 (Fig. 3d) show that the V2O5@PEDOT nanocomposite cathode achieves an initial capacity of 328.9 mAh g−1, which is much higher than that of V2O5. Furthermore, the V2O5@PEDOT nanocomposite maintains 176.4 mAh g−1 at 5 A g−1 after 1000 cycles (Fig. 3e), demonstrating outstanding structural stability in the MnSO4 electrolyte. The stable cycle performance can also be proven by EIS measurements. After 100 cycles at 1.0 A g−1, the Rct of the V2O5@PEDOT electrodes is 7.2 Ω (Fig. S4†). The Rct after cycling is similar to that before cycling (7.5 Ω). This means that ion transport is stable during cycling.
 | ||
| Fig. 3 (a) Initial three charge–discharge cycles, (b) rate performance, (c) charge–discharge curves, (d) cycle life at 1 A g−1 and (e) cycle life at 5 A g−1 of the V2O5@PEDOT composite. | ||
To elucidate the electrochemical kinetics of the V2O5@PEDOT composite, CV measurements were systematically performed across a range of scan rates (0.5–2.5 mV s−1), as presented in Fig. 4a and S5.† The CV profiles exhibit well-defined redox peaks with maintained shape integrity across scan rates, demonstrating the electrochemical reversibility of the V2O5@PEDOT nanocomposite. The kinetic behavior was quantitatively analyzed using the relationship between the peak current (i) and scan rate (ν):32,33
| i = aνb |
The quantitative separation of capacitive- and diffusion-controlled contributions can be achieved using the following equation:34,35
Electrochemical kinetic analysis reveals a distinct evolution in the charge storage behavior of the V2O5@PEDOT cathode with increasing scan rates (0.5–2.5 mV s−1). As shown in Fig. 4c, the capacitive contribution percentage demonstrates a progressive increase from 55.4% to 74.2%, reaching its maximum at a scan rate of 2.5 mV s−1 (Fig. 4d). This trend contrasts markedly with the V2O5 cathode, which exhibits low b-values (Fig. S6†) and decreased capacitive contributions (Fig. S7†). These findings demonstrate that the inorganic@organic V2O5@PEDOT nanocomposite architecture effectively promotes surface-controlled charge storage processes. The Mn2+ diffusion coefficient was quantitatively analyzed using the galvanostatic intermittent titration technique (GITT). As illustrated in Fig. 4e, the linear correlation between the voltage change (ΔEτ) and τ1/2, where τ is pulse duration, enabled the calculation of Mn2+ diffusion coefficients (DMn2+) using the equation:
Using a combination of ex situ XRD and XPS analyses, the structural dynamics and charge storage mechanism of the V2O5@PEDOT cathode during the electrochemical process were systematically elucidated (Fig. 5a). As depicted in Fig. 5b, the (200) diffraction peak shifts toward lower angles during the discharging process, corresponding to an interlayer expansion induced by Mn2+ intercalation. Notably, the peak fully reverts to its initial position upon charging to 0.8 V, demonstrating exceptional structural reversibility and lattice stability (Fig. 5c).36 Complementary XPS analysis (Fig. 5d) reveals the redox chemistry underlying this process: the pristine electrode exhibits mixed V5+ (517.6/525.2 eV) and V4+ (516.2/523.6 eV) states due to partial reduction during PEDOT polymerization. Upon discharging to −1.4 V, the emergence of V3+ species and the enhancement of V4+ signals confirm Mn2+ intercalation, while recharging restores the dominant V5+ state, verifying the presence of the highly reversible vanadium redox (Fig. 5e). Concurrently, the Mn 2p spectra show clear Mn2+ signals in the discharged state, with residual intensity persisting in the charged state, corroborating the XRD observations of incomplete Mn2+ extraction. These findings collectively establish a Mn2+ intercalation/deintercalation mechanism with minor irreversibility (Fig. 5f). The spatial distribution and electrochemical evolution of elemental constituents in the V2O5@PEDOT cathode were thoroughly investigated using SEM-EDS. As illustrated in Fig. S8,† EDS elemental mapping demonstrates that V, O, and S exhibit nearly identical spatial distribution patterns in discharged (Fig. S8a†) and charged (Fig. S8b†) states, confirming exceptional structural stability during electrochemical cycling. Mn exhibits pronounced state-dependent behavior, with strong characteristic signals appearing during discharging and a significantly attenuated intensity appearing upon charging, which provides direct evidence of the Mn2+ intercalation/deintercalation mechanism. The Mn2+ intercalation/deintercalation mechanism is schematically shown in Fig. 6. According to ex situ XRD and XPS analyses, Mn2+ ions are intercalated into the V2O5@PEDOT electrodes in the discharged state. After the charging process, Mn2+ ions are deintercalated from the V2O5@PEDOT electrodes.
Building on the outstanding performance of AC‖V2O5@PEDOT cells, we further investigated the practical application potential of V2O5@PEDOT cathodes in manganese-ion full battery systems by pairing them directly with Mn metal anodes. The Mn‖V2O5@PEDOT full cell demonstrates remarkable electrochemical properties, delivering an exceptional specific capacity of 424.3 mAh g−1 at 0.2 A g−1, which is a significant improvement compared to V2O5 cathodes (Fig. 7a). More importantly, the cell maintains superior rate capability, exhibiting 122.5 mAh g−1 even at a high current density of 1.0 A g−1 (Fig. 7a). The galvanostatic charge–discharge profiles (Fig. 7b) reveal distinct voltage plateaus, indicating well-defined redox reactions during Mn2+ insertion/extraction processes. To validate practical applicability, we successfully powered light-emitting diodes (LEDs) using two serially connected Mn‖V2O5@PEDOT cells (Fig. 7c), demonstrating the real-world viability of this battery configuration. These results collectively highlight that the inorganic@organic V2O5@PEDOT nanocomposite is a promising cathode material for high-performance AMIBs.
 | ||
| Fig. 7 (a) Capacity and (b) discharge–charge curves of the Mn‖V2O5@PEDOT full cell at different current densities. (c) Optical image of the red LED lighted using two Mn‖V2O5@PEDOT cells. | ||
4. Conclusions
The inorganic@organic V2O5@PEDOT nanocomposite was successfully synthesized via a facile in situ polymerization method by simply introducing the EDOT monomer into a V2O5 solution at room temperature, eliminating the need for additional oxidants or complex processing steps. The conformal PEDOT coating significantly enhanced the electrochemical performance by simultaneously suppressing vanadium dissolution and improving electronic conductivity, resulting in exceptional cycling stability after 1000 cycles at 5 A g−1 in the MnSO4 electrolyte and an exceptional rate capability (211.8 mAh g−1 at 5 A g−1). Systematic mechanism characterization confirmed the structural stability and high reversibility of Mn2+ insertion/extraction. The practical applicability was further demonstrated in a full-cell configuration (Mn‖V2O5@PEDOT), which maintained high capacity. This study not only presents a high-performance cathode material but also provides new insights into the design principles for advanced AMIBs, potentially expanding the research scope in sustainable energy storage systems.Data availability
All data supporting this research are included in the main article and ESI.†Author contributions
Xianyu Liu: conceptualization, writing original draft, and funding acquisition. Jianan Zhao: methodology, data curation, and validation. Zhigang Fan: writing – review & editing. Yingchun Xiao: supervision, investigation, and funding acquisition. Yande Zhao: formal analysis and resources. Qing Guo: investigation and validation.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work is supported by the Natural Science Foundation of Gansu Province (No. 24JRRA538 & No. 25CXGA090), the Youth Doctoral Fund of Education Department of Gansu (No. 2025QB-085), the Discipline Construction Project of Lanzhou City University, the Talent Project of Rewi Alley and the Scientific Research Project of Lanzhou City University (No. LZCU-KJ/2024-017 & No. LZCU-KJ/2025-002).References
- H. Wang, R. Tan, Z. Yang, Y. Feng, X. Duan and J. Ma, Stabilization Perspective on Metal Anodes for Aqueous Batteries, Adv. Energy Mater., 2021, 11, 2000962 CrossRef CAS.
- S. Chen, M. Zhang, P. Zou, B. Sun and S. Tao, Historical development and novel concepts on electrolytes for aqueous rechargeable batteries, Energy Environ. Sci., 2022, 15, 1805–1839 RSC.
- X. Zhou, T. Ruan, J. Xu, C. Li, S. Huang, J. Zhou, S. Lu, R. Song and R. Li, Host-design strategies of zinc anodes for aqueous zinc-ion batteries, RSC Adv., 2024, 14, 23023–23036 RSC.
- J. Pyun, H. Lee, S. Baek, S. Lee, H. Kwon, H. Lee, C. Y. Yoo and M. S. Chae, Nonaqueous Electrolyte Rechargeable Manganese Batteries with Potassium Manganese Hexacyanoferrate Cathodes, Adv. Sci., 2025, 2500132 CrossRef CAS PubMed.
- S. Bi, Y. Zhang, S. Deng, Z. Tie and Z. Niu, Proton-Assisted Aqueous Manganese-Ion Battery Chemistry, Angew. Chem., Int. Ed., 2022, 61, e202200809 CrossRef CAS PubMed.
- Q. Yang, X. Qu, H. Cui, X. He, Y. Shao, Y. Zhang, X. Guo, A. Chen, Z. Chen, R. Zhang, D. Kong, Z. Shi, J. Liu, J. Qiu and C. Zhi, Rechargeable Aqueous Mn-Metal Battery Enabled by Inorganic–Organic Interfaces, Angew. Chem., Int. Ed., 2022, 61, e202206471 CrossRef CAS PubMed.
- Z. Pan, T. Qin, W. Zhang, X. Chu, T. Dong, N. Yue, Z. Wang and W. Zheng, Non-layer-transformed Mn3O4 cathode unlocks optimal aqueous magnesium-ion storage via synergizing amorphous ion channels and grain refinement, J. Energy Chem., 2022, 68, 42–48 CrossRef CAS.
- Z. Fan, Z. Hou, W. Lu, H. Zheng, N. Chen, M. Yao, C. Wang, H. Jiang, D. Zhang and F. Du, Combination Displacement/Intercalation Reaction of Ag0.11V2O5 Cathode Realizes Efficient Manganese Ion Storage Properties, Small, 2025, 21, 2406501 CrossRef CAS PubMed.
- A. Nimkar, M. S. Chae, S. Wee, G. Bergman, B. Gavriel, M. Turgeman, F. Malchik, M. D. Levi, D. Sharon, M. R. Lukatskaya, N. Shpigel and D. Mandler, What About Manganese? Toward Rocking Chair Aqueous Mn-Ion Batteries, ACS Energy Lett., 2022, 7, 4161–4167 CrossRef CAS.
- S. Bi, S. Wang, F. Yue, Z. Tie and Z. Niu, A rechargeable aqueous manganese-ion battery based on intercalation chemistry, Nat. Commun., 2021, 12, 6991 CrossRef CAS PubMed.
- V. Soundharrajan, S. Nithiananth, J. Lee, K. Sakthiabirami, D. T. Pham, J. H. Kim, J.-Y. Hwang and J. Kim, Manganese ion batteries: LiV3O8 nanorods as a robust and long-life cathode module, J. Power Sources, 2023, 558, 232542 CrossRef CAS.
- Z. Feng, J. Sun, Y. Liu, H. Jiang, T. Hu, M. Cui, F. Tian, C. Meng and Y. Zhang, Polypyrrole-intercalation tuning lamellar structure of V2O5·nH2O boosts fast zinc-ion kinetics for aqueous zinc-ion battery, J. Power Sources, 2022, 536, 231489 CrossRef CAS.
- X. Zhang, F. Xue, X. Sun, T. Hou, Z. Xu, Y. Na, Q. An, Z. Chen, S. Cai and C. Zheng, High-capacity zinc vanadium oxides with long-term cyclability enabled by in-situ electrochemical oxidation as zinc-ion battery cathode, Chem. Eng. J., 2022, 445, 136714 CrossRef CAS.
- S. Cao, Y. Xiang, Q. Zou, Y. Jiang, H. Zeng, J. Li, J. Wu, X. Wu, X. Wu and L. Xiong, Preparation of Li3V2(PO4)3 as cathode material for aqueous zinc ion batteries by a hydrothermal assisted sol–gel method and its properties, RSC Adv., 2023, 13, 24385–24392 RSC.
- C. Li, X. Yun, Y. Chen, D. Lu, Z. Ma, S. Bai, G. Zhou, P. Xiao and C. Zheng, Unravelling the proton hysteresis mechanism in vacancy modified vanadium oxides for High-Performance aqueous zinc ion battery, Chem. Eng. J., 2023, 477, 146901 CrossRef CAS.
- Z. Li, L. Yang, S. Wang, K. Zhu and H. Li, Co-insertion of K+ and Ca2+ in vanadium oxide as high-performance aqueous zinc-ion battery cathode material, J. Alloys Compd., 2024, 992, 174589 CrossRef CAS.
- Y. Du, Y. Wang, B. Yang, X. Liu, C. Li, W. Li, P. Zhang, H. Lu, D. Bin and Y. Xia, Mott–Schottky Heterojunction Modulating Iron–Vanadium Oxide for High-Performance Aqueous Zinc Battery Cathodes, Nano Lett., 2025, 25, 1002–1009 CrossRef CAS PubMed.
- W. Tang, Q. Li, H. Ren, Z. Gong, Q. Liu, J. Liang and W. Wu, Printed zinc ion battery with excellent rate performance utilizing carbon-intercalated vanadium oxide cathode for flexible wearable electronics, J. Power Sources, 2025, 640, 236744 CrossRef CAS.
- S. Ye, S. Sheng, Q. Chen, L. Meng, W. Yao, H. Yao, Z. Wu and F. Zhang, Layer-by-layer assembled binder-free hydrated vanadium oxide-acetylene black electrode for flexible aqueous zinc ion battery, J. Electroanal. Chem., 2024, 964, 118334 CrossRef CAS.
- G. Yoo, Y.-G. Lee, B. Im, D. G. Kim, Y.-R. Jo and G. H. An, Integrated solution for a stable and high-performance zinc-ion battery using an electrolyte additive, Energy Storage Mater., 2023, 61, 102845 CrossRef.
- C. Jia, X. Zhang, S. Liang, Y. Fu, W. Liu, J. Chen, X. Liu and L. Zhang, Environmentally adaptable hydrogel electrolyte with the triple interpenetrating network in the flexible zinc-ion battery with ultralong stability, J. Power Sources, 2022, 548, 232072 CrossRef CAS.
- M. Wang, Y. Meng, Y. Xu, N. Chen, M. Chuai, Y. Yuan, J. Sun, Z. Liu, X. Zheng, Z. Zhang, D. Li and W. Chen, Aqueous all-manganese batteries, Energy Environ. Sci., 2023, 16, 5284–5293 RSC.
- D. Bin, W. Huo, Y. Yuan, J. Huang, Y. Liu, Y. Zhang, F. Dong, Y. Wang and Y. Xia, Organic-Inorganic-Induced Polymer Intercalation into Layered Composites for Aqueous Zinc-Ion Battery, Chem, 2020, 6, 968–984 CAS.
- Y. Du, X. Wang and J. Sun, Tunable oxygen vacancy concentration in vanadium oxide as mass-produced cathode for aqueous zinc-ion batteries, Nano Res., 2020, 14, 754–761 CrossRef.
- S. Li, X. Wei, C. Wu, B. Zhang, S. Wu and Z. Lin, Constructing Three-Dimensional Structured V2O5/Conductive Polymer Composite with Fast Ion/Electron Transfer Kinetics for Aqueous Zinc-Ion Battery, ACS Appl. Energy Mater., 2021, 4, 4208–4216 CrossRef CAS.
- T. Liu, Y. Liao, S. Liu, D. Tang, L. Chen and Q. Zhang, Understanding the Organic Intercalation for Aqueous Zinc-Ion Battery: From Interlayer Structure to Properties and Future Perspectives, ACS Sustain. Chem. Eng., 2024, 12, 15344–15369 CrossRef CAS.
- H. Qin, Z. Yang, L. Chen, X. Chen and L. Wang, A high-rate aqueous rechargeable zinc ion battery based on the VS4@rGO nanocomposite, J. Mater. Chem. A, 2018, 6, 23757–23765 RSC.
- S. Zafar, M. Sharma, S. N. Mahapatra and B. Lochab, An aqueous zinc-ion battery with an organic–inorganic nanohybrid cathode featuring high operating voltage and long-term stability, Chem. Commun., 2025, 61, 3151–3154 RSC.
- D. Xu, H. Wang, F. Li, Z. Guan, R. Wang, B. He, Y. Gong and X. Hu, Conformal Conducting Polymer Shells on V2O5 Nanosheet Arrays as a High-Rate and Stable Zinc-Ion Battery Cathode, Adv. Mater. Interfaces, 2019, 6, 1801506 CrossRef.
- A. Liu, F. Wu, Y. Zhang, Y. Jiang, C. Xie, K. Yang, J. Zhou and M. Xie, Ultralarge layer spacing and superior structural stability of V2O5 as high-performance cathode for aqueous zinc-ion battery, Nano Res., 2023, 16, 9461–9470 CrossRef CAS.
- M. Liu, X. Li, M. Cui, F. Chen, J. Li, W. Shi, Y. Liu, X. Li, Y. Wang, W. Zhang, C. Shao and Y. Liu, Amorphous organic-hybrid vanadium oxide for near-barrier-free ultrafast-charging aqueous zinc-ion battery, Nat. Commun., 2024, 15, 10769 CrossRef PubMed.
- Y. Ren, S. Chen, M. Odziomek, J. Guo, P. Xu, H. Xie, Z. Tian, M. Antonietti and T. Liu, Mixing Functionality in Polymer Electrolytes: A New Horizon for Achieving High-Performance All-Solid-State Lithium Metal Batteries, Angew. Chem., Int. Ed., 2025, 64, e202422169 CrossRef CAS PubMed.
- Y. De Luna, Z. Mohamed, A. Dawoud and N. Bensalah, Innovative 2D dioxonium vanadium oxide: enhancing stability in aqueous zinc-ion battery cathodes, RSC Adv., 2024, 14, 39193–39203 RSC.
- J. Xu, N. Han, S. Chen, Y. Zhang, Y. Jing, Z. Chen, S. Wang, R. Chen, P. Bing and Z. Li, The optimal integrating state of VOx with the synergistic effect of Cu2+ cation and polyaniline for high performance flexible fiber zinc-ion battery, J. Energy Storage, 2025, 120, 116415 CrossRef.
- H. Ding, Y. He, X. Yu, L. Chen, M. Chen, Y. Luo, J. Li and S. Wei, A novel 3D framework loaded with MnO2 for high-performance aqueous zinc-ion battery cathode, J. Electroanal. Chem., 2025, 986, 119101 CrossRef CAS.
- Y. Sha, J. Wang, Z. Sun, Z. Guo, J. Bi, H. Wang, C. Wang, Z. Liu and L. Qian, Sodium ion intercalated NH4V4O10 with adjustable interlayer-spacing as an advanced cathode for aqueous zinc ion battery, J. Energy Storage, 2025, 114, 115825 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra03230j |
| This journal is © The Royal Society of Chemistry 2025 |





