 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Single target formulation of thin film 58S bioactive glasses via magnetron sputtering with custom targets from spray-dried bioactive glass powders
Yao-Chong Fana,
Henni Setia Ningsiha,
Masatsugu Oishi b and
Yu-Jen Chou
b and
Yu-Jen Chou *a
*a
aDepartment of Mechanical Engineering, National Taiwan University of Science and Technology, No. 43, Sec. 4, Keelung Road, Taipei 10607, Taiwan. E-mail: yu-jen.chou@mail.ntust.edu.tw; Tel: +886-2-2737-6492
bGraduate School of Technology, Industrial and Social Sciences, Tokushima University, Josanjima-cho, Tokushima 770-8506, Japan
First published on 9th June 2025
Abstract
Due to the bioactive properties that stimulate the growth of new bones, bioactive glasses (BGs) have been widely used in fields of medical science. Yet, the lack of mechanical properties limits the application of BGs. Therefore in this study, thin film 58S BGs were prepared via magnetron sputtering with customed targets from spray-dried powders. The preparation method of radio-frequency magnetron sputtering was used with the advantages of excellent uniformity, high adherence, and precise thickness control over other deposition methods. The crystallographic information, thin film morphology, and elemental composition of the as-deposited thin film BGs were characterized via X-ray diffraction, scanning electron microscopy, and energy dispersive spectroscopy, respectively. Furthermore, the in vitro bioactivity was examined using simulated body fluid according to Kokubo's protocol, whereas the in vitro cell viability was assessed following the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Finally, the results showed the successful preparation of thin film 58S BG specimens with bioactivity and non-toxicity, and their corresponding formation mechanisms and property correlations were discussed.
Introduction
Since Hench's original report in 1969, bioactive glasses (BGs) have attracted tremendous attention and have shown their potential as promising materials with their superior properties such as bioactivity, biocompatibility, and biodegradability.1 Therefore in the past few decades, there have been extensive developments in the fields of biomedical engineering,2,3 with practical applications including bone implants, dental fillers, and drug carriers.4–6 To date, most research on BGs has primarily focused on the conventional melt-quenching method, with secondary attention given to the sol–gel preparation.7 Emerging alternative glass preparation technologies, such as spray pyrolysis,8,9 or spray drying,10,11 are also noteworthy. These methods produce BG specimens in bulk or powder form. BGs, whether in bulk, powder, or scaffold form-often fabricated by freeze drying12 and various 3D printing techniques13 have demonstrated positive in vitro and in vivo feedback, though their applications are currently limited to low-load bearing scenarios.14,15 In contrast, the functionalization of BG coatings onto metal implants has been considered as an alternative approach to obtain rapid integration with living tissue, which could help bone regeneration and offer support for bone growth.16 Hence, there has been enormous attention given to the study of bioactive coatings onto metal implants, while various deposition methods have demonstrated promising biological results.17,18To obtain metallic implants with good osteointegration, various thin film deposition methods, such as pulsed laser deposition,19 pulse electron deposition,20 magnetron sputtering,21 etc., have been used for the preparation of thin film BGs. Among these methods, plasma spraying is the most widely used and the only method that has received approval from the US Food and Drug Administration (FDA).22 It offers numerous advantages, including a wide range of substrates that can be coated with various coatings (polymers, metals, and ceramics) and cost-effective surface treatment for a variety of applications.23 However, it has a number of drawbacks, such as poor adhesion between the substrate and coating, phase change caused by exposure to high temperature, deviations in the coating density, poor microstructural control, and microcracks.24 Additionally, studies have employed pulsed laser deposited to fabricate thin film BGs onto Ti alloys,25,26 while Bellucci et al. demonstrated the preparation of 45S5 by pulse electron deposition method showing its high efficiency and optimized stoichiometry conservation.20 However, the high laser power might lead to micro-cracks and internal pores as well as damage the substrate, whereas the pulse electron source may not be widely available. In brief, although each deposition method has its own pros and cons, all these researches indicated the importance of BG coating for implant application development.
Apart from the deposition methods mentioned, magnetron sputtering is a potential technique that has been applied in fields of thin film technology owing to its versatility in automation, great uniformity and adherence, and precise control of specimen thicknesses.27 For instance, Stan et al. reported a series of optimization with their deposition system and demonstrated the prospect of adjusting the structure, morphology, and bioactivity of BG films.28,29 Moreover, Popa et al. functionalized dental screws with BG and showed positive effects on the adhesion and proliferation of human dental pulp stem cells.30 To date, the preparation of thin film BGs using the magnetron sputtering technique has employed only melt-quenched BG materials as cathode targets. The use of sol–gel, spray pyrolysis, or spray drying BGs for the thin film fabrication by magnetron sputtering remains unexplored. Exploring the sputtering deposition mechanisms when employing these latter types of BGs, along with the resulting structural, compositional, and biological features, could be significantly important for the future development of implant coatings.
Therefore, in the present work, we aimed to prepare thin film 58S BGs via magnetron sputtering based on a single target formulation. Among the various BG compositions, 58S stands out as particularly advantageous due to its low tendency to crystallize and its ability to maintain a high rate of HA formation, leading to enhanced cell mineralization.31 By fabricating the BG powders with the spray drying method, which is considered the most efficient method for converting the suspension into a free-flowing powder, the resulting powders were cold pressed into green compacts and sintered into custom targets. Then, sputtering of thin film 58S BGs was conducted, and the crystallographic information, surface morphology, and elemental composition were examined via X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy dispersive spectroscopy (EDS), respectively. For the evaluation of in vitro bioactivity tests, both SEM and EDS were used to observe the formation of hydroxyapatite, while the in vitro cell viability tests were assessed following a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Finally, formation mechanisms and their related properties of all specimens were discussed.
Experimental details
Synthesis
First, 58S BG powder (SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CaO
CaO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2O5 = 60
P2O5 = 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 in mol%) was fabricated using the spray drying method.10 Initially, a precursor solution was prepared by adding 238.1 g of tetraethyl orthosilicate (Si(OC2H5)4, 99.9%, Showa, Japan), as the source of SiO2, into 400.0 g of ethanol (C2H5OH, 99.5%, Echo, Taipei, Taiwan), and followed by adding 157.4 g of calcium nitrate tetrahydrate (Ca(NO3)2·4H2O, 98.5%, Showa, Japan) and 34.7 g of triethyl phosphate ((C2H5)3PO4, 99.0%, Alfa Aesar, UK) as the source of CaO and P2O5. Note that all precursors were obtained in analytical grade. Additionally, 0.5 M hydrochloric acid (HCl, 37.0%, Honeywell, United States) was used to adjust the pH of the solution to 2.5 for the optimization of precursor dissolution. Finally, by mixing the precursor solution with de-ionized water till 1000 mL, the final solution was stirred for a day to achieve homogeneity. For the spray drying method, the prepared precursor solution was atomized via a rotary disc atomizer operated at 20
5 in mol%) was fabricated using the spray drying method.10 Initially, a precursor solution was prepared by adding 238.1 g of tetraethyl orthosilicate (Si(OC2H5)4, 99.9%, Showa, Japan), as the source of SiO2, into 400.0 g of ethanol (C2H5OH, 99.5%, Echo, Taipei, Taiwan), and followed by adding 157.4 g of calcium nitrate tetrahydrate (Ca(NO3)2·4H2O, 98.5%, Showa, Japan) and 34.7 g of triethyl phosphate ((C2H5)3PO4, 99.0%, Alfa Aesar, UK) as the source of CaO and P2O5. Note that all precursors were obtained in analytical grade. Additionally, 0.5 M hydrochloric acid (HCl, 37.0%, Honeywell, United States) was used to adjust the pH of the solution to 2.5 for the optimization of precursor dissolution. Finally, by mixing the precursor solution with de-ionized water till 1000 mL, the final solution was stirred for a day to achieve homogeneity. For the spray drying method, the prepared precursor solution was atomized via a rotary disc atomizer operated at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm into fine droplets. The droplets were then directed into the spray drying equipment (SDD0-03, IDTA Machinery Co. Ltd, Taiwan) via a peristaltic pump with a flow rate of 20 mL min−1. Then, the inlet and outlet temperatures were set at 200 and 80 °C for the spray drying chamber, while the spray-dried powder was collected using an attached cyclone collector. Next, for the preparation of the target, the spray-dried BG powder was filtered with 140 mesh to acquire uniform particle sizes. Then, a 2.15-inch cylinder mold was filled with 17 g of BG powder and cold pressed with 150 MPa to obtain the green compact with a thickness of 3 mm. At last, heat treatment of 900 °C was carried out for impurity calcination and target sintering.
000 rpm into fine droplets. The droplets were then directed into the spray drying equipment (SDD0-03, IDTA Machinery Co. Ltd, Taiwan) via a peristaltic pump with a flow rate of 20 mL min−1. Then, the inlet and outlet temperatures were set at 200 and 80 °C for the spray drying chamber, while the spray-dried powder was collected using an attached cyclone collector. Next, for the preparation of the target, the spray-dried BG powder was filtered with 140 mesh to acquire uniform particle sizes. Then, a 2.15-inch cylinder mold was filled with 17 g of BG powder and cold pressed with 150 MPa to obtain the green compact with a thickness of 3 mm. At last, heat treatment of 900 °C was carried out for impurity calcination and target sintering.
For the deposition of thin film 58S BG specimens, pure Ti plates (Circle & Cycle Ltd, Taiwan) of 10 × 10 mm2 were used as substrates (Polished, with surface roughness of around 150 nm). All substrates were cleaned via ultrasonic for 5 min in ethanol, then fixed onto a stainless-steel holder in the chamber, with a target–substrate distance of 10 cm, and dried in a flow of nitrogen. During the sputtering process, the vacuum chamber was evacuated to 1 × 10−2 mTorr (0.0013 Pa) using a diffusion pump. The target was pre-sputtered for 5 min before each deposition to eliminate possible surface impurities. For all thin film BG specimens, the RF power (L200A01FM, MeiVac Inc, USA) and Ar gas flow were set at 100 W and 30 sccm (standard cubic centimeters per minute), with three working pressures of 2, 4, and 6 mTorr (0.266, 0.533, and 0.799 Pa), and the duration of deposition was kept at 12 h.
Characterizations
First, characterizations of crystallographic information were conducted with XRD (D8 Discover, Bruker, Germany) for the custom target and all as-deposited thin film BG specimens. The instrument is equipped with a Cu-Kα source with Ni-filter, giving a wavelength of 1.54 Å. Note that for these characterizations only, thin film BG specimens were sputtered onto (100) Si substrates (Semiconductor Wafer Inc., Taiwan) in order to remove the strong diffraction of the pure Ti substrate. The diffraction patterns were acquired with an incident angle of 0.5°, while the collection angle was ranging from 10 to 80° with step size set at 0.05° and a collection time of 2.5 s per step.Next, the surface morphology was examined by SEM (FEI Quanta 3D FEG, FEI, United States). Initially, SEM micrographs were captured via a field-emission probe for the observation of surface morphology. In addition, cross-sectional specimens were prepared for the measurements of specimen thicknesses. In addition, EDS spectra were obtained for the analysis of elemental compositions. For the statistical measurements of average thickness and elemental compositions, the measurements were carried out by sampling more than three regions across the whole specimen to ensure its reliability. Further, Fourier transform infrared spectroscopy (FTIR) was performed using a SHIMADZU Tracer-100 spectrometer in reflection mode with an Attenuated Total Reflectance (ATR) suite equipped with a Ge crystal prism to identify the functional groups in the thin film BG specimens. The analysis was conducted with a resolution of 4 cm−1 across a range of 4000 to 600 cm−1.
Evaluation of in vitro bioactivity of all thin film BG specimens were conducted based on Kokubo's protocol.32 By immersing the specimen into 10 mL of simulated body fluid (SBF) following the formula vSBF = 100 mm Sa, which has ion concentrations similar to human blood plasma (as shown in Table 1), the specimen was held at 37 °C for 14 d in a thermostatic orbital shaker (S300R, Firstek Scientific, Taiwan). Meanwhile, it is worth mentioning that the SBF solution was refreshed every 24 h to simulate our body condition. The resulting specimen was gathered and cleaned three times using both acetone and de-ionized water to clean any remaining salts, then were placed into an oven set at 70 °C to dry for 24 h. Lastly, for the observation of bioactivity, SEM and EDS were employed for direct observation and the Ca/P ratio of the formation of HA.
| Ion | Simulated body fluid | Human blood plasma |
|---|---|---|
| Na+ | 142.0 | 142.0 |
| K+ | 5.0 | 5.0 |
| Mg2+ | 1.5 | 1.5 |
| Ca2+ | 2.5 | 2.5 |
| Cl− | 147.8 | 103.0 |
| HCO3− | 4.2 | 27.0 |
| HPO42− | 1.0 | 1.0 |
| SO42− | 0.5 | 0.5 |
| Unit: mmol L−1 |
Nano-indentation tests were performed using a nano-indenter (TI-980 TriboIndenter, Hysitron Inc., United States). During indentation, a diamond Berkovich indenter with a 5-5-5 loading function was utilized. A loading/unloading rate was set to 200 μN s−1, reaching a maximum load of 1000 μN to evaluate the hardness and elastic modulus of each sample. It's important to ensure that the indentation depth remains below 10% of the specimen's thickness to avoid the influence from the substrate.
Finally, in vitro cell viability was assessed via MTT assay according to the ISO protocol 10993-5.33 To start with, osteoblast cells (MC3T3-E1, Taipei Medical University) were cultivated in a minimum essential medium (MEM) and were then transferred into several 24-well plates at a density of 2 × 104 cells per well. In the meantime, thin film BG specimens were sterilized in an autoclave at 130 °C and soaked in MEM for 24 h to form the test solutions. The solutions were incubated with cells at 37 °C for 72 h, the media were then removed, and followed by adding the MTT reagents. Then, all plates were transferred into a CO2 incubator set at 37 °C and incubated for another 2 h, while formazan crystals were formed by the healthy osteoblast cells. Finally, by adding dimethyl sulfoxide to dissolve the formazan crystals, the optical densities of all test solutions were measured via a microplate spectrophotometer (Cytation 3, Bio TEK, USA) at a wavelength of 550 nm.
Results
To start with, Fig. 1 shows the information on the prepared custom target. Firstly, the photograph of the custom target is shown in Fig. 1(a), indicating a successful preparation of a diameter of 2-inch. Additionally, the SEM image and EDS spectrum are shown in Fig. 1(b) and (c), showing the surface morphology of the sintered spray-dried BG powders with the corresponding Si-Kα, P-Kα, and Ca-Kα peaks observed at 1.74, 2.06, and 3.69 keV, respectively. Further, elemental compositions were computed and shown in Table 2, indicating the ratio of Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca: P is 56.01 ± 0.82
Ca: P is 56.01 ± 0.82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36.39 ± 2.22
36.39 ± 2.22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.60 ± 1.51, which shows a similar ratio as compared to the nominal 58S (Si
7.60 ± 1.51, which shows a similar ratio as compared to the nominal 58S (Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca: P = 57.14
Ca: P = 57.14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.33
33.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.52). Lastly, the XRD patterns of the as-prepared powder and the custom target are shown in Fig. 1(d). The results showed that the as-prepared powder exhibited an amorphous structure with a broad band around 20 to 40°, which was reported in our previous study.34 In contrast, after the heat treatment of 900 °C, multiple phases of calcium silicate (JCPDF 49-1672), calcium phosphate (JCPDF 09-0348), and phosphorus oxide (JCPDF 38-0932) could be found. In addition, the crystallinity of the custom target was computed as 28.0%.
9.52). Lastly, the XRD patterns of the as-prepared powder and the custom target are shown in Fig. 1(d). The results showed that the as-prepared powder exhibited an amorphous structure with a broad band around 20 to 40°, which was reported in our previous study.34 In contrast, after the heat treatment of 900 °C, multiple phases of calcium silicate (JCPDF 49-1672), calcium phosphate (JCPDF 09-0348), and phosphorus oxide (JCPDF 38-0932) could be found. In addition, the crystallinity of the custom target was computed as 28.0%.
 | ||
| Fig. 1 Information on the custom target from spray dried BG powders. (a) photograph, (b) SEM image, (c) EDS spectrum, and (d) XRD pattern. | ||
| Specimen | Si | Ca | P |
|---|---|---|---|
| Nominal | 57.14 | 33.33 | 9.52 |
| Target | 56.01 ± 0.82 | 36.39 ± 2.22 | 7.60 ± 1.51 |
| 2 mTorr | 59.03 ± 0.38 | 33.98 ± 0.23 | 6.99 ± 0.62 |
| 4 mTorr | 59.43 ± 0.36 | 32.98 ± 0.54 | 7.59 ± 0.38 |
| 6 mTorr | 58.78 ± 0.56 | 33.55 ± 0.23 | 7.67 ± 0.77 |
| Unit: at% |
Next, Fig. 2 shows the XRD patterns of the as-deposited thin film BG specimens with working pressures of 2, 4, and 6 mTorr (0.266, 0.533, and 0.799 Pa). Initially, it could be seen from the graph that there were no distinct diffraction intensities could be observed from angles of 10° to 80° for the thin film BG specimen sputtered with 2 mTorr (0.266 Pa). In contrast, a broad hump could be observed between angles of 20° to 30°, indicating the amorphous structure. In addition, as the working pressure increased, thin film BG specimens sputtered with 4 and 6 mTorr (0.533, and 0.799 Pa) showed similar results with no distinct diffractions. In brief, the XRD results suggested that all thin film BG specimens were successfully deposited with amorphous structures.
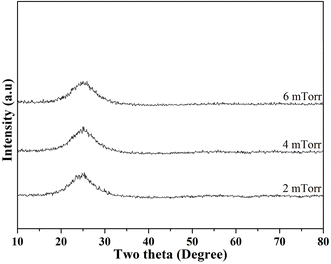 | ||
| Fig. 2 XRD patterns of as-deposited thin film 58S BG specimens with working pressures of 2, 4, and 6 mTorr. | ||
Further, Fig. 3 shows the SEM images of all thin film BG specimens. Initially, Fig. 3(a) showed the undeposited Ti substrate without any in-growths or precipitation. Next, for the thin film BG specimen sputtered with 2mTorr (0.266 Pa), a smooth surface morphology could be observed in Fig. 3(b), indicating a uniform growth of BG. In addition, with the increased working pressure, some spherical in-growths on the surface could be found (Fig. 3(c) and (d)). The measured in-growths diameters vary between 0.4 to 2.1 μm, showing a rise as the working pressure increases. Furthermore, thicknesses of thin film BG specimens were measured via the cross-sectional images as shown in Fig. 3(e)–(g). By sampling five regions across the whole specimen, the averaged film thicknesses were statistically measured, which resulted in 651 ± 29, 807 ± 17, and 906 ± 15 nm for the thin film BG specimens deposited with 2, 4, and 6 mTorr (0.266, 0.533, and 0.799 Pa), respectively.
The elemental compositions of the target and as-deposited thin film BG specimens were characterized via EDS, and the recorded spectra are shown in Fig. 4. The results showed that the peaks of C-Kα, O-Kα, Ti-Lα, Si-Kα, P-Kα, Cl-Kα, Ca-Kβ, Ca-Kα, and Ti-Kα could be found at 0.28, 0.55, 0.51, 1.74, 2.03, 2.65, 3.69, 4.01, and 4.51 keV, respectively, from all thin film BG specimens. Further, elemental compositions were computed and shown in Table 2. It could be seen from the table that the composition of the target showed a similar ratio as compared to the nominal 58S composition (Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca: P = 57.14
Ca: P = 57.14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.33
33.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.52). Then, for the thin film BG specimens, the Si
9.52). Then, for the thin film BG specimens, the Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca: P ratios are 59.03 ± 0.38
Ca: P ratios are 59.03 ± 0.38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.98 ± 0.23
33.98 ± 0.23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.99 ± 0.62, 59.43 ± 0.36
6.99 ± 0.62, 59.43 ± 0.36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32.98 ± 0.54
32.98 ± 0.54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.59 ± 0.38, and 58.78 ± 0.56
7.59 ± 0.38, and 58.78 ± 0.56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.55 ± 0.23
33.55 ± 0.23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.67 ± 0.77 for the thin film BG specimens with working pressures of 2, 4, and 6 mTorr (0.266, 0.533, and 0.799 Pa), respectively. Increased concentrations of around 2 to 4% were observed on both Si and Ca, whereas the concentrations of P were decreased by around 5% among all thin film BG specimens. In brief, it could be observed that the composition of all thin film BG specimens was stoichiometrically transferred to the specimens with around 5% variance.
7.67 ± 0.77 for the thin film BG specimens with working pressures of 2, 4, and 6 mTorr (0.266, 0.533, and 0.799 Pa), respectively. Increased concentrations of around 2 to 4% were observed on both Si and Ca, whereas the concentrations of P were decreased by around 5% among all thin film BG specimens. In brief, it could be observed that the composition of all thin film BG specimens was stoichiometrically transferred to the specimens with around 5% variance.
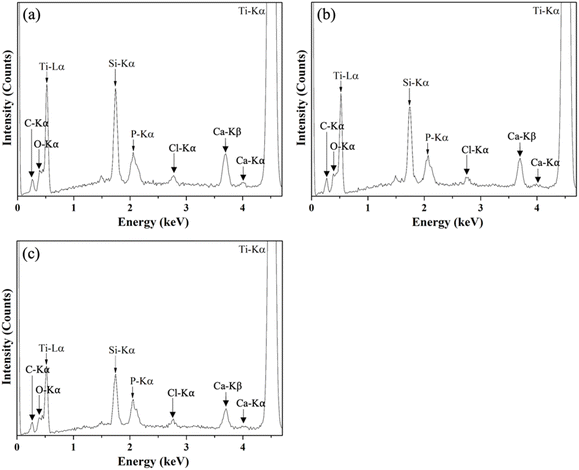 | ||
| Fig. 4 EDS spectra of as-deposited thin film BG specimens with working pressures of (a) 2 mTorr, (b) 4 mTorr, and (c) 6 mTorr. | ||
Additionally, the hardnesses and elastic modulus of all specimens were determined using nano-indentation. To start with, by sampling five areas from each specimen, the average hardness of the pure Ti substrate was measured as 2.01 ± 0.40 GPa. Further, significant increases in specimen hardnesses could be observed from the thin film BG specimens, showing 4.68 ± 0.57, 4.98 ± 0.26, and 4.55 ± 0.10 GPa for the thin film BG specimens prepared at working pressures of 2, 4, and 6 mTorr (0.266, 0.533, and 0.799 Pa), respectively. Further, the elastic moduli were measured as 68.4 ± 0.4, 67.6 ± 0.6, and 69.6 ± 0.4 GPa for the thin film BG specimens prepared at working pressures of 2, 4, and 6 mTorr (0.266, 0.533, and 0.799 Pa), respectively.
For the in vitro bioactivity, evaluations were conducted after SBF immersion for 14 d, and the resulting SEM images are shown in Fig. 5, along with their magnified images of the marked area. Initially, it could be observed that the formation of needle-shaped hydroxyapatite deposits on the surface of all the thin film BG specimens. In contrast to the as-deposited thin film BG specimens with working pressures of 4 and 6 mTorr (0.533, and 0.799 Pa), the hydroxyapatite deposits had increased, as compared to the as-deposited thin film BG specimens with working pressures of 2 mTorr (0.266 Pa). These results evidenced the interaction between the surface of the thin film BG specimens and the SBF solution, culminating in hydroxyapatite formation. Meanwhile, the results from EDS analysis showed that the Ca/P ratios for the thin film BG specimens with working pressures of 2, 4, and 6 mTorr (0.266, 0.533, and 0.799 Pa) are 1.37, 1.52, and 1.45, respectively. In addition, FTIR spectra were employed to examine the chemical bonds of the formation of HA, and the results are shown in Fig. 6. To start with, the main bands related to the silica network could be observed at around 1050 cm−1 (corresponding to Si–O stretching). While peaks around 810 cm−1 were found, which are corresponding to the symmetric stretching vibrations of Si–O–Si groups. Further, the peaks around 610 cm−1 are characteristic of P–O bending of PO43− groups, which correspond to the calcium phosphate-based compounds. Meanwhile, a supplementary band around 700 to 750 cm−1 was observed, which could be attributed to bands involving ν4 bending of orthophosphates and/or pyrophosphates. In summary, the calcium phosphate compound deposits on the surface of all thin film BG specimens were observed after 14 d of SBF immersion, indicating potential bioactivity.
 | ||
| Fig. 6 FTIR spectra of hin film BG specimens with working pressures of 2 mTorr, 4 mTorr, and 6 mTorr after SBF immersion for 14 d. | ||
Finally, Fig. 7 shows the in vitro cell viability results of all thin film BG specimens. Based on the ISO protocol 10993-5, the cell viability was computed against the control sample as a percentage, while the computed value must pass the level of 70% (marked in the dashed line) in order to be regarded as a non-toxic material. As shown in Fig. 7, the results revealed that all percentages of cell viabilities were above 70%, satisfying the ISO standard requirement. In addition, the cell viabilities of thin film BG specimens deposited with 2, 4, and 6 mTorr (0.266, 0.533, and 0.799 Pa) are 130.8 ± 6.2, 142.2 ± 3.8, and 180.2 ± 5.9%, respectively, showed higher viability as compared to the control sample (100%), and could be considered as an evident proliferation effect. In brief, the in vitro cell viability results indicated that all thin film BG specimens exhibit non-toxicity and could help the proliferation of osteoblast cells.
Discussion
First, we discuss the formation details of the custom target. To start with, it is worth noting that a target shrinkage of around 7–8% was measured after target sintering. Thus, a custom mold of 2.15-inch was used to counter the shrinkage. Additionally, based on the SEM images shown in Fig. 1(b), the target porosity was calculated by ImageJ software as 28.1 ± 2.5%. This might not be ideal and could be further improved by employing other processes, such as cold isostatic pressing (CIP) and hot isostatic pressing (HIP). Additionally, other approaches of using finer powders could enhance packing density, while applying sintering aids could promote better densification. Regarding the crystallization, Hadem et al. reported that the crystallization temperature of the 58S BG was measured as 825 °C.35 Meanwhile, the sintering temperature of 900 °C used in this work is higher than the crystallization temperature, thus resulting in the formation of various calcium and phosphorus phases.Next, for the thin film BG specimens, the surface morphology was discussed. Based on the SEM images of the as-deposited thin film BG specimens as shown in Fig. 2, specimens that sputtered with higher working pressure resulted in a rough surface with increased agglomeration found on its surface. The formation of these aggregates could be described as the results of the surface energy minimization process which was reported by Stan et al.28 Owing to the short mean free path of the sputtered atoms under the higher working pressure, which have higher tendency to collide with other atoms in the chamber, the atoms lost their energy during the collision and could not be deposited onto the substrate effectively. In addition, the transverse diffusion ability of the atoms is decreased, resulting in the formation mechanism of island growth, therefore leading to rough surface morphology. Regarding the EDS results, apart from the primary elements of Ti, Si, Ca, and P, a Cl-Kα intensity at 2.65 keV was observed in the spectra of all films. This could be attributed to the residual chlorine remaining from the hydrochloric acid used to adjust the pH of the precursor solution.
Next, for the thin film thicknesses, the deposition rates in this study were computed as 54.2, 67.2, and 75.5 nm h−1, showing slower rates as compared to previous studies.36,37 This is owing to the long target–substrate distance (10 cm) in the current sputtering system. Following Meng et al.,38 when the mean free path of the sputtering atoms is smaller than the target–substrate distance, the energy of the atoms is not sufficient for an effective deposition, which led to low deposition rate and low film density. Additionally, with the increasing working pressure, increased specimen thicknesses could be obtained, this was reported by Stuart et al.,39 demonstrated that the number of backscattered atoms would increase at a higher rate than the number of ejected atoms as the working pressure increased, thus leading to an increase in deposition rate. Meanwhile, one can observe the positive effect of thin film BG deposition on hardness. The results showed an increased hardness of up to 5 GPa as compared to pure Ti of 2 GPa, showing a similar trend with Popa et al.40 In addition, a study by Teghil et al. showed that 45S5® Bioglass coatings by pulsed laser deposition (PLD) possess hardnesses of around 2 to 4 GPa,41 Kawagichi et al. investigated the surface modification through electrophoretic deposition (EPD) of 45S5® Bioglass coatings, demonstrating hardnesses of 0.5 to 2.0 GPa and elastic moduli between 70 and 130 GPa, using either direct current (DC) or alternating current (AC),42 and Tang et al. prepared 6P57 BG coatings via spray deposition and examined their hardnesses and elastic moduli under different load levels (50, 200, 500 μN), showing hardnesses ranging from 3.4 to 7.8 GPa and elastic moduli from 80 to 90 GPa.43 These reports are comparable to the current study, indicating its readiness for biomedical applications. Moreover, Miki-Yoshida and Aandrade44 reported that the deposition rate decreases as the film thickness increases, suggesting that the deposition rate dependent on the film thickness. The mechanism that explain the thickness-dependent growth rate is similar to that of inhibited particle growth.45 The growth rate should be thickness-dependent, as the crystallite faces necessitate repeated nucleation events in order to progress to the subsequent stages of growth.
Finally, we discussed the in vitro bioactivity and the in vitro cell viability of the thin film BG specimens. Initially, the SEM results showed significant differences in the surface morphology after SBF immersion. The as-prepared thin film BG specimen has a smoothed surface with some distribution of small-sized in-growths. In contrast, the specimen after SBF immersion showed a dense layer with uniformly distributed precipitations, such surface morphology is commonly observed as the Ca-deficient apatite. Additionally, the EDS analysis showed a significant difference in Ca/P ratio as compared to as-deposited thin film BG (from 4.34–4.86 to 1.37–1.52). Although the Ca/P ratios fell short of the nominal Ca/P ratio of 1.67 for hydroxyapatite, possibly due to the effect of the BG thin films underneath, the results in this study demonstrated the preferential development of the apatite layer following an extended immersion in SBF.
Conclusions
In this study, thin film BG specimens were successfully prepared via magnetron sputtering technique. The resulting crystallographic composition, thin film morphologies, specimen thicknesses, atomic compositions, and hardness were characterized by XRD, SEM, EDS, and nano-indentation. The results demonstrated the successful preparation of custom targets from spray drying powders and its ability to adjust the morphology, composition, and thickness of thin film BG specimens via altering the working pressure, and the formation mechanism was discussed. Finally, for the in vitro bioactivity and cell viability, the SEM, EDS, and MTT results confirmed the non-toxic and potential bioactivity of all thin film BG specimens after 14 d of SBF immersion. With advantages such as chemical flexibility, controllable morphology, and rapid kinetic process of the spray drying technique over melt-quench and sol–gel methods, these findings reveal the potential development of thin film BG coatings and could be considered as a possible candidate for bone implant applications.Data availability
All data underlying the results are available as part of the article and no additional source data are required.Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.Acknowledgements
The authors acknowledge the financial support from the Tokushima University and the National Taiwan University of Science and Technology (Grant Numbers TU-NTUST-2024-06) and National Science and Technology Council (Grant number: NSTC 112-2221-E-011-049).References
- L. L. Hench, R. J. Splinter, W. C. Allen and T. K. Greenlee, Bonding mechanisms at the interface of ceramic prosthetic materials, J. Biomed. Mater. Res., 1971, 5, 117–141 CrossRef.
- L. L. Hench, Bioceramics: From concept to clinic, J. Am. Ceram. Soc., 1991, 74, 1487–1510 CrossRef CAS.
- W. Cao and L. L. Hench, Bioactive materials, Ceram. Int., 1996, 22, 493–507 CrossRef CAS.
- W. Xia and J. Chang, Well-ordered mesoporous bioactive glasses (mbg): A promising bioactive drug delivery system, J. Controlled Release, 2006, 110, 522–530 CrossRef CAS.
- M. Vallet-Regi, Ceramics for medical applications, J. Chem. Soc., Dalton Trans., 2001, 97–108 RSC.
- X. Chatzistavrou, O. Tsigkou, H. D. Amin, K. M. Paraskevopoulos, V. Salih and A. R. Boccaccini, Sol–gel based fabrication and characterization of new bioactive glass–ceramic composites for dental applications, J. Eur. Ceram. Soc., 2012, 32, 3051–3061 CrossRef CAS.
- K. Deshmukh, T. Kovarik, T. Krenek, D. Docheva, T. Stich and J. Pola, Recent advances and future perspectives of sol–gel derived porous bioactive glasses: A review, RSC Adv., 2020, 10, 33782–33835 RSC.
- C.-K. Kuo, L.-G. Chen, C.-F. Tseng and Y.-J. Chou, Influences of acid catalysts on the microstructure, bioactivity and cytotoxicity of bioactive glass nanoparticles prepared by spray pyrolysis, J. Non-Cryst. Solids, 2021, 560, 120710 CrossRef CAS.
- A. B. Workie and E. M. Sefene, Ion-doped mesoporous bioactive glass: Preparation, characterization, and applications using the spray pyrolysis method, RSC Adv., 2022, 12, 1592–1603 RSC.
- Y.-J. Chou, C.-W. Hsiao, N.-T. Tsou, M.-H. Wu and S.-J. Shih, Preparation and in vitro bioactivity of micron-sized bioactive glass particles using spray drying method, Appl. Sci., 2018, 9, 19 CrossRef.
- G. Vecchio, V. Darcos, S. L. Grill, F. Brouillet, Y. Coppel, M. Duttine, A. Pugliara, C. Combes and J. Soulie, Spray-dried ternary bioactive glass microspheres: Direct and indirect structural effects of copper-doping on acellular degradation behavior, Acta Biomater., 2024, 181, 453–468 CrossRef CAS.
- D. Santos, T. Martins, S. Carvalho, M. Pereira, M. Houmard and E. Nunes, Simple preparation of 58s bioactive glass/polycaprolactone composite scaffolds by freeze-drying under ambient conditions, Mater. Lett., 2019, 256, 126647 CrossRef CAS.
- K. Leong, C. Cheah and C. Chua, Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs, Biomaterials, 2003, 24, 2363–2378 CrossRef.
- A. Shearer, M. Montazerian, J. J. Sly, R. G. Hill and J. C. Mauro, Trends and perspectives on the commercialization of bioactive glasses, Acta Biomater., 2023, 160, 14–31 CrossRef PubMed.
- A. A. El-Rashidy, J. A. Roether, L. Harhaus, U. Kneser and A. R. Boccaccini, Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models, Acta Biomater., 2017, 62, 1–28 CrossRef PubMed.
- S. Foppiano, S. J. Marshall, G. W. Marshall, E. Saiz and A. P. Tomsia, Bioactive glass coatings affect the behavior of osteoblast-like cells, Acta Biomater., 2007, 3, 765–771 CrossRef PubMed.
- R. Sergi, D. Bellucci and V. Cannillo, A comprehensive review of bioactive glass coatings: State of the art, challenges and future perspectives, Coatings, 2020, 10, 757 CrossRef.
- G. E. Stan, M. Montazerian, A. Shearer, B. W. Stuart, F. Baino, J. C. Mauro and J. M. F. Ferreira, Critical advances in the field of magnetron sputtered bioactive glass thin-films: An analytical review, Appl. Surf. Sci., 2024, 646, 158760 CrossRef.
- P.-H. Kuo, S. S. Joshi, X. Lu, Y.-H. Ho, Y. Xiang, N. B. Dahotre and J. Du, Laser coating of bioactive glasses on bioimplant titanium alloys, Int. J. Appl. Glass Sci., 2019, 10, 307–320 CrossRef.
- D. Bellucci, M. Bianchi, G. Graziani, A. Gambardella, M. Berni, A. Russo and V. Cannillo, Pulsed electron deposition of nanostructured bioactive glass coatings for biomedical applications, Ceram. Int., 2017, 43, 15862–15867 CrossRef.
- G. E. Stan, S. Pina, D. U. Tulyaganov, J. M. F. Ferreira, I. Pasuk and C. O. Morosanu, Biomineralization capability of adherent bio-glass films prepared by magnetron sputtering, J. Mater. Sci.: Mater. Med., 2010, 21, 1047–1055 CrossRef PubMed.
- Fda website - characterization of metallic coatings and/or calcium phosphate coatings on orthopedic devices, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/characterization-metallic-coatings-andor-calcium-phosphate-coatings-orthopedic-devices, 2024,.
- G. Davis, G. Groff and R. Zatorski, Plasma spray coatings as treatments for aluminum, titanium and steel adherends, Surf. Interface Anal., 1997, 25, 366–373 CrossRef.
- K. S. Raja, M. Misra and K. Paramguru, Deposition of calcium phosphate coating on nanotubular anodized titanium, Mater. Lett., 2005, 59, 2137–2141 CrossRef CAS.
- Y. Zhao, M. Song, C. Chen and J. Liu, The role of the pressure in pulsed laser deposition of bioactive glass films, J. Non-Cryst. Solids, 2008, 354, 4000–4004 CrossRef CAS.
- J. Ma, C. Z. Wang, C. L. Ban, C. Z. Chen and H. M. Zhang, Pulsed laser deposition of magnesium-containing bioactive glass film on porous ti–6al–4v substrate pretreated by micro-arc oxidation, Vacuum, 2016, 125, 48–55 CrossRef CAS.
- K. Wasa, M. Kitabatake and H. Adachi, Thin Film Materials Technology: Sputtering of Control Compound Materials, Springer Science & Business Media, ( 2004) Search PubMed.
- G. E. Stan, D. A. Marcov, I. Pasuk, F. Miculescu, S. Pina, D. U. Tulyaganov and J. M. F. Ferreira, Bioactive glass thin films deposited by magnetron sputtering technique: The role of working pressure, Appl. Surf. Sci., 2010, 256, 7102–7110 CrossRef CAS.
- G. E. Stan, C. O. Morosanu, D. A. Marcov, I. Pasuk, F. Miculescu and G. Reumont, Effect of annealing upon the structure and adhesion properties of sputtered bio-glass/titanium coatings, Appl. Surf. Sci., 2009, 255, 9132–9138 CrossRef CAS.
- A. C. Popa, G. E. Stan, M. Enculescu, C. Tanase, D. U. Tulyaganov and J. M. F. Ferreira, Superior biofunctionality of dental implant fixtures uniformly coated with durable bioglass films by magnetron sputtering, J. Mech. Behav. Biomed. Mater., 2015, 51, 313–327 CrossRef CAS PubMed.
- W.-L. Huang, F. Vietanti, D.-R. Li, T.-W. Wang and Y.-J. Chou, Enhancement of in vitro bioactivity and cytotoxicity of mesoporous bioactive glass through template-free spray drying synthesis, J. Non-Cryst. Solids, 2024, 641, 123135 CrossRef CAS.
- T. Kokubo, H. Kushitani, C. Ohtsuki, S. Sakka and T. Yamamuro, Chemical reaction of bioactive glass and glass-ceramics with a simulated body fluid, J. Mater. Sci.: Mater. Med., 1992, 3, 79–83 CrossRef.
- R. F. Wallin and E. Arscott, A practical guide to iso 10993-5: Cytotoxicity, Med. Device Diagn. Ind., 1998, 20, 96–98 Search PubMed.
- Y.-J. Chou, C.-W. Hsiao, N.-T. Tsou, M.-H. Wu and S.-J. Shih, Preparation and in vitro bioactivity of micron-sized bioactive glass particles using spray drying method, Appl. Sci., 2019, 9, 19 CrossRef.
- H. Hadem, A. K. Ojha, A. Mitra, R. Rajasekaran, P. V. Vaidya, S. Dhara, S. Das and K. Das, Mixed alkali effect on the mechanical, thermal and biological properties of 58s bioactive glass, Ceram. Int., 2024, 50, 31925–31936 CrossRef CAS.
- C. Berbecaru, H. V. Alexandru, G. E. Stan, D. A. Marcov, I. Pasuk and A. Ianculescu, First stages of bioactivity of glass-ceramics thin films prepared by magnetron sputtering technique, Mater. Sci. Eng., B, 2010, 169, 101–105 CrossRef CAS.
- G. E. Stan, A. C. Popescu, I. N. Mihailescu, D. A. Marcov, R. C. Mustata, L. E. Sima, S. M. Petrescu, A. Ianculescu, R. Trusca and C. O. Morosanu, On the bioactivity of adherent bioglass thin films synthesized by magnetron sputtering techniques, Thin Solid Films, 2010, 518, 5955–5964 CrossRef CAS.
- L.-J. Meng and M. P. dos Santos, Influence of the target-substrate distance on the properties of indium tin oxide films prepared by radio frequency reactive magnetron sputtering, J. Vac. Sci. Technol. A, 2000, 18, 1668–1671 CrossRef CAS.
- B. Stuart, M. Gimeno-Fabra, J. Segal, I. Ahmed and D. M. Grant, Preferential sputtering in phosphate glass systems for the processing of bioactive coatings, Thin Solid Films, 2015, 589, 534–542 CrossRef CAS.
- A. C. Popa, V. M. F. Marques, G. E. Stan, M. A. Husanu, A. C. Galca, C. Ghica, D. U. Tulyaganov, A. F. Lemos and J. M. F. Ferreira, Nanomechanical characterization of bioglass films synthesized by magnetron sputtering, Thin Solid Films, 2014, 553, 166–172 Search PubMed.
- R. Teghil, L. D'Alessio, D. Ferro and S. Barinov, Hardness of bioactive glass film deposited on titanium alloy by pulsed laser ablation, J. Mater. Sci. Lett., 2002, 21, 379–382 CrossRef.
- K. Kawaguchi, M. Iijima, K. Endo and I. Mizoguchi, Electrophoretic deposition as a new bioactive glass coating process for orthodontic stainless steel, Coatings, 2017, 7, 199 CrossRef.
- C. Y. Tang, C. P. Tsui, D. Janackovic and P. Uskokovic, Nanomechanical properties evaluation of bioactive glass coatings on titanium alloy substrate, J. Optoelectron. Adv. Mater., 2006, 8, 1194 Search PubMed.
- M. Miki-Yoshida and E. Andrade, Growth and structure of tin dioxide thin films obtained by an improved spray pyrohydrolysis technique, Thin Solid Films, 1993, 224, 87–96 Search PubMed.
- P. Wynblatt and N. Gjostein, Particle growth in model supported metal catalysts—i. Theory, Acta Metall., 1976, 24, 1165–1174 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |



