Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Investigation of the physical, chemical and thermal properties of a novel lignocellulosic fiber extracted from the Ravenala madagascariensis leaf stalk
Mohammad Abul Hasan Shibly *ab,
Mohammad Mohsin Ul Hoquea,
Prosenjit Senac,
Khandaker Akil Mahadi Ohia,
Md. Maruf Hossaina,
Md. Masum Miaa,
Md. Abdus Sabur
*ab,
Mohammad Mohsin Ul Hoquea,
Prosenjit Senac,
Khandaker Akil Mahadi Ohia,
Md. Maruf Hossaina,
Md. Masum Miaa,
Md. Abdus Sabur d,
Mohammad Junaebur Rashide and
Mohammad Mahbubur Rahman
d,
Mohammad Junaebur Rashide and
Mohammad Mahbubur Rahman d
d
aNational Institute of Textile Engineering and Research, Dhaka 1350, Bangladesh. E-mail: hasanduet002@gmail.com
bJahangirnagar University, Dhaka 1342, Bangladesh
cBangladesh University of Textiles, Dhaka-1208, Bangladesh
dBangladesh Council of Scientific and Industrial Research, Dhaka 1205, Bangladesh
eUniversity of Dhaka, Dhaka, 1000, Bangladesh
First published on 16th July 2025
Abstract
Natural plant fibers are inexpensive, lightweight, renewable, and environmentally friendly, making them sustainable substitutes for synthetic materials. This study aims to identify alternative, eco-friendly replacements for nonbiodegradable fibers used in polymer composites. To achieve this goal, the fibers from Ravenala madagascariensis leaf stalks were thoroughly characterized, with a focus on their physical, mechanical, thermal, and morphological properties. The hygroscopic properties (moisture content and regain), density, and chemical composition of the fibers were evaluated following ASTM D2654, ASTM D1909, ASTM D891-18, and TAPPI standards, respectively. Chemical composition analysis revealed that the fiber contained 54.25 wt% cellulose, 20.12 wt% hemicellulose, and 15.17 wt% lignin, contributing to its enhanced mechanical properties. The crystallinity, surface structure, chemical bonds, and thermal behavior of the fibers were analyzed via XRD, SEM, FTIR, and TGA techniques. This novel fiber has a moisture content and regain percentages of 9.17% and 10.1%, respectively. Its average tensile strength is 151 MPa for a 20 mm gauge length (GL) and 136.8 MPa for a 30 mm gauge length (GL), with a crystallinity index of 67.37%, in which the size of the crystals is 15.64 nm. The fiber degradation begins at a maximum temperature of 550 °C. This original fiber holds potential for applications in the production of cellulose nanoparticles, fiber-reinforced composites, biomaterials and so on.
1. Introduction
Over the past three decades, the development of fiber materials has flourished substantially because of their wide variety of applications. For instance, the utilization of synthetic fibers in composite products is remarkable due to their useful contributions to supplementary products as well as their high-strength material qualities. However, the high carbon emissions while producing such fibers as well as the awareness of ‘zero carbon emissions’ in recent years have shifted the focus to produce eco-friendly composite materials.Environmental threats, along with protective regulations, have served as catalysts for the use of natural resources across various production sectors.1,2 As a result, interest in finding new materials to replace traditional materials is increasing, with natural fibers emerging as promising options. Note that natural fibers are biodegradable, recyclable, and lightweight. Moreover, its natural polymers do have appealing fiber properties, such as a wide stiffness range and a high strength-to-weight ratio.3 Owing to their natural abundance, exploring more opportunities to find sources of natural fibers is essential.4
Recent studies suggest that natural fibers are well suited for reinforcing polymeric composite materials; thus, natural fibers are replacing synthetic fibers in the composite industry.5–12 The utilization of natural fibers in industry and agriculture, however, results in the production of significant amounts of waste.13–15 Therefore, developing an efficient method to convert biomass waste into usable reinforcement materials may provide a solution for producing economical and environmentally friendly composites. The “green composites”16,17 are made from natural fiber reinforcements, and several researchers have already produced such composites.18,19 For example, banana fibers20 are extensively used in textiles to provide a lustrous appearance. Furthermore, nontraditional fibers such as hemp and flax21,22 have become popular and are gradually replacing synthetic fibers.23
In fact, natural fibers consist of cellulose, hemicellulose, lignin, and pectin, with the proportions of these components varying between different fibers.24 A higher cellulose content increases fiber flammability, whereas a greater lignin content tends to lower the fiber degradation temperature.21,22,25 As a result, the presence of lignin and cellulose enhances the thermal stability of natural fibers and their functionalized materials, making them suitable for use in various polymer matrices for diverse functional applications.23,26 Additionally, natural fibers offer an alternative energy source for biodegradable reinforcement materials.27 The strong chemical and electrical resistance, effective thermal and acoustic insulation, and high fracture resistance of these materials make them an attractive area of research for various potential applications.28 Nearly every industry is moving toward a greener, eco-friendly approach, aiming to replace synthetic materials with natural alternatives.29 Many automotive manufacturers now use biofiber-based composites, such as headliners, trunk liners, dashboards, seat backs, and door panels, to produce various car parts and accessories.30,31 Additionally, fiber-reinforced composites are also used in the shipbuilding, aerospace, and construction industries.32 Table 1 lists the applications and sources of different plant-based natural fibers.
| Fiber name | Source | Scientific name | Applications | Reference |
|---|---|---|---|---|
| Hemp | Hemp plant | Cannabis sativa | Textiles, paper, ropes, and skincare products | 33–36 |
| Ramie | Ramie plant | Boehmeria nivea | Apparel, home furnishings, and fishing nets | 37, 38 |
| Pineapple leaf fiber | Pineapple leaves | Ananas comosus | Textiles, upholstery, accessories | 39, 40 |
| Typha fiber | Typha leaves | Typha Australis | Composites | 41 |
| Hogla | Hogla plant | Typha elephantina Roxb | Textiles, and composites | 42 |
| Coconut tree | Primary flower leaf stalks, husks | Cocos nucifera | Composites, rope, doormats, gardening products | 43, 44 |
| Palm tree | Leaf stalks | Livistona rotundifolia | Composites, packaging materials | 45 |
| Corn leaf fiber | Corn plant | Zea mays | Composites | 46, 47 |
| Kenaf | Kenaf plant leaf stalks | Hibiscus cannabinus | Packaging materials, insulation | 48, 49 |
| Stinging nettle fiber | Stinging nettle plant | Urtica dioica | Clothing, cordage, twine | 50, 51 |
| Water hyacinth fiber | Water hyacinth plant | Pontederia crassipes | Polymer composites | 52–54 |
| Banana fiber | Banana plant stems | Musa spp. | Textiles, handicrafts, papermaking | 55–57 |
| Soy silk | Soybean residue | Glycine max | Clothing, accessories | 58 |
| Piñatex | Pineapple leaves | Ananas comosus | Textiles, footwear, bags, accessories | 59 |
| Sisal | Sisal plant leaves | Agave sisalana | Ropes, twine, carpets, geotextiles | 60 |
| Abaca | Abaca plant (Manila hemp) | Musa textilis | Fiber craft, tea bags, specialty papers | 61 |
| Lotus fiber | Lotus plant rhizomes | Nelumbo nucifera | Luxury textiles, traditional asian garments | 62 |
| Kapok | Kapok tree seeds | Ceiba pentandra | Pillow stuffing, insulation, and hydrogel | 63 |
| Spider silk | Produced by spiders | Various spider species | Lightweight but strong textiles, medical uses | 64, 65 |
A wide variety of natural fibers are derived from various parts of plants, including the stem, root, bark, leaf stalk, husk, fruit, etc.66 The literature review highlighted the importance of characterizing new natural fibers for composite materials, as such analysis determines their potential for various applications. During this process, a previously uncharacterized cellulosic natural fiber was identified in the leaf stalk of the Ravenala (R.) madagascariensis plant. Since no prior studies on the characterization of this specific fiber were found in the existing literature, the present study conducted its characterization based on established methodologies used for other natural fibers The main objective of this study was to examine the physical, chemical, thermal, and mechanical properties of Ravenala madagascariensis fibers (RMFs). The investigations included the linear density, moisture properties, chemical composition, surface morphology, functional characteristics, crystallinity index, crystallite size, mechanical properties, and thermal behavior of the RMFs. These properties were analyzed via chemical analysis, scanning electron microscopy (SEM), Fourier transform-infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), tensile testing, and thermogravimetric analysis (TGA).
2. Materials and methods
2.1 Materials
R. madagascariensis is a tree that can grow at a height of 30–60 feet (9–18 m). Its leaves are green, oblong, and feature a broad, pinnate margin with evergreen venation, measuring over 2.4 meters in length. Globally, R. madagascariensis is native to Madagascar, where it grows both in the wild and in cultivated settings. It is also widely cultivated in tropical and subtropical regions—including Bangladesh, India, and parts of North America—mainly for ornamental landscaping due to its striking appearance.67 In this study, lignocellulosic fibers were extracted from the leaf stalk of the R. madagascariensis plant through an eco-friendly extraction process. Currently, there is no comprehensive data on the global annual harvest of Ravenala madagascariensis for fiber use. While native to Madagascar and occasionally traded for ornamental purposes, it is not commercially harvested at scale for its fiber. Notably, 90 kg of its seeds were exported to Pakistan in July 2021, indicating limited international trade. This study focuses on the basic fiber characterization of the plant, aiming to support future research into its commercial viability and sustainable fiber applications.682.2 Extraction of the fibers
Ravenala madagascariensis is an abundant ornamental plant widely cultivated in tropical and subtropical regions, including Bangladesh. Its large leaf stalks are regularly pruned to maintain the aesthetic appearance of the plant, resulting in a substantial amount of biomass waste. Instead of discarding these stalks, they were collected and chopped into 10′′–12′′ pieces and prepared for fiber extraction. This approach offers a sustainable solution for waste valorization while contributing to the development of environmentally friendly, renewable materials for composite applications. This study presents the first detailed characterization of fibers derived from this plant species, revealing promising attributes for utilization in textile and biocomposite applications.A variety of natural fibers are obtained from different parts of plants through methods such as water retting, mechanical processes, chemical extraction and enzymatic retting. The selection of an extraction method is influenced by factors such as the type of fiber needed, the method's efficiency, the time available, the intended application of the fibers, and the cost of extraction.69 The leaf stalks of R. madagascariensis were cut, and the hollow sections inside them were removed. The fibers were then extracted from the leaf stalks via a mechanical combing process, as shown in Fig. 1. The extracted fibers were washed at a temperature of 50 °C for 2 hours to remove excess gummy substances, and their surfaces were thoroughly cleaned with fresh water. After washing, the fibers were dried under direct sunlight for approximately two days.
 | ||
| Fig. 1 RMF extraction process: (a) R. madagascariensis plant, (b) collected leaf stalks, (c) removed spongy parts of leaf stalks, (d) mechanical retting, and (e) extracted fibers. | ||
2.3 Methods of fiber characterization
 | (1) |
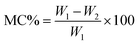 | (2) |
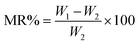 | (3) |
 | (4) |
 | (5) |
 | (6) |
Differential scanning calorimetry (DSC) was performed to complement the thermogravimetric analysis. A 10 mg sample was placed in sealed pans to prevent contamination. The sealed pan was then positioned inside the calorimeter and heated under an inert nitrogen atmosphere up to 45 °C. Significant melting peak temperatures were recorded at a consistent heating rate of 10 °C min−1.74
Energy dispersive X-ray spectroscopy (EDX) is a widely used method for identifying surface elements, such as oxygen, nitrogen, and carbon, in natural fibers. EDX analysis, which was conducted via the TEAM™ EDS system integrated with SEM, was employed to identify the elemental composition of the R. madagascariensis fibers.
| Hemicellulose% = Holocellulose% − Cellulose% | (7) |
3. Results and discussion
3.1 Physical characterization and fiber density
The R. madagascariensis leaf stalk fibers had an average length of 26.4 cm. Accurately measuring the diameter of natural fibers is challenging because of their irregular thickness, which varies along their length as a result of environmental factors and growth conditions. To determine the diameter of R. madagascariensis fibers, measurements were taken at three random points on each fiber. As shown in Fig. 2, the diameters at the first, second, and third points were 0.170192 mm, 0.172115 mm, and 0.181731 mm, respectively. The average single-fiber weight was calculated as 0.008455 g on the basis of 20 fibers of varying lengths. The fineness of the fiber was 33.70 ± 11.72 Tex. Table 2 shows the fineness of Rosa hybrida bark fiber (14.51 Tex), Hylocereus undatus stem fiber (14.82 Tex), and Saccharum bengalense grass fiber (18.63 Tex) which are all lower than RMFs. The specific density was 1.08 g cm−3. This low density makes it suitable for lightweight applications as an alternative to synthetic fibers. A comparison of the physical properties of RMFs with those of other natural fibers is shown in Table 2.| Fiber | Diameter (μm) | Density (gm per cc) | Count (Tex) | Reference |
|---|---|---|---|---|
| RMFs | 140–303 | 1.08 | 33.70 ± 11.72 | Current work |
| Rosa hybrida bark fiber | 214–238 | 1.194 | 14.51 | 66 |
| Coconut tree leaf | 140–990 | 1.2 | — | 72 |
| Hylocereus undatus stem fiber | 173.53 | 1.08 | 14.82 | 76 |
| Eleusine indica grass | 315.4 ± 10 | 1.14 | — | 77 |
| Cyperus pangorei fiber | 133.3 | 1.10 | 11–14 | 78 |
| Saccharum Bengalense grass | 320.47 | 1.17 | 18.63 ± 6.28 | 79 |
| Ziziphus mauritiana fiber | 570.2 | 1.13 | — | 80 |
3.2 FTIR analysis
The Fourier transform infrared (FTIR) spectrum shown in Fig. 3 presents the transmittance (%) as a function of wavenumber (cm−1). The spectrum exhibited several significant absorption bands corresponding to specific molecular vibrations. Key peaks are observed at 3336 cm−1, 2914 cm−1, 1728 cm−1, 1600 cm−1, 1507 cm−1, 1370 cm−1, 1238 cm−1, 1034 cm−1, and 892 cm−1. The prominent peak at approximately 3390 cm−1 is typically associated with O–H stretching vibrations, indicating the presence of hydroxyl groups or water molecules. The peak regions of the fibers corresponding to the specified functional groups are presented in Table 3.| Wavenumber (cm−1) | Allocations | References |
|---|---|---|
| 3336 | A prominent absorption peak at 3336 cm−1 is observed in RMFs, attributed to the O–H and C–H stretching in cellulose. This peak corresponds to the presence of alpha-cellulose, polysaccharide, and monosaccharide molecules | 81 |
| 2914 | The small peak at 2914 cm−1 is associated with the C–H stretching vibrations of CH and CH2 groups in cellulose and hemicellulose | 82 |
| 1728 | The small peak observed at 1728 cm−1 is attributed to the carbonyl group of carboxylic acid present in lignin | 83 |
| 1600 | A small peak in 1600 cm−1 is indicating water absorption in natural cellulose | 84 |
| 1507 | The vibrational activity at 1507 cm−1 is attributed to the stretching of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in aromatic lignin structures C bonds in aromatic lignin structures |
85 |
| 1370 | The C–O groups in the aromatic rings of hemicellulose and lignin | 86 |
| 1238 | The absorbance peak at 1238 cm−1 corresponds to the C–O stretching vibration of an acetyl group in lignin | 87 |
| 1034 | The absorption peak at 1034 cm−1 is attributed to the C–O vibration in cellulose | 88 |
| 892 | Hemicellulose exhibits characteristic peaks at 896 cm−1, corresponding to the stretching vibrations and deformations of C–C–H, C–O–C, and C–C–O bonds in cellulose | 89 |
3.3 Moisture content and regain analysis
The composition of a fiber is significantly affected by its moisture content. The ability of a textile to retain body heat under varying climatic conditions greatly influences its comfort level, making moisture regulation a critical aspect of performance. Changes in moisture content impact textile properties such as elasticity, friction, fiber diameter, and tensile strength. A decrease in equilibrium relative humidity can cause a textile to become weaker, more brittle, and fragile. To minimize moisture loss to the environment, maintaining air humidity during fiber processing is essential.90 The average moisture content and moisture regain of the fibers are 9.172% and 10.102%, respectively, which are similar to those of bamboo fibers (9.16%).91 The results were obtained via the use of five distinct fiber samples, each weighing five grams. The standard deviations for the two measurements were 0.601 and 0.726, with coefficients of variation of 6.56% and 7.18%, respectively, indicating consistent moisture contents and regain values among the samples.3.4 Fiber mechanical property analysis
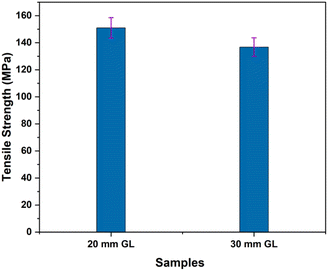
The mechanical properties of the RMFs were measured through tensile tests conducted for two different gauge lengths (GLs), as detailed in Table 4. Three key tensile properties were analyzed: tensile strength, Young's modulus and elongation at break. As illustrated in Fig. 4, the tensile strength of the 20 mm GL was significantly greater than that of the 30 mm GL. Factors such as the fiber extraction method, leaf age, climate conditions, microstructure, and defects caused by cracks influence the tensile properties of RMFs. The presence and accumulation of defects in the longer GL (30 mm) led to more rapid failure. Additionally, the tensile test results are impacted by the GL, instrument precision, grips, and compliance of the testing device.66| GL (mm) | Mean diameter (mm) | Tensile strength (MPa) | Young's modulus (GPa) | Elongation at break (%) |
|---|---|---|---|---|
| 20 | 0.2191 ± 0.05 | 151 ± 19.99 | 4.325 ± 1.36 | 6.475 ± 3.63 |
| 30 | 0.1933 ± 0.05 | 136.8 ± 26.64 | 5.70 ± 1.15 | 5.178 ± 2.89 |
The results in Fig. 5 demonstrate that the Young's modulus for the 30 mm GL is 5.70 GPa greater than that for the 20 mm GL. This trend is expected, as the arrangement of defects relative to the fiber length and volume may result in an increase in the Young's modulus with increasing GL.66 Fig. 6 presents the Weibull distribution plots for the diameter, tensile strength, and elongation at break of the RMFs. The data show that the values for diameter, tensile strength, and elongation at break fall within the expected range and align well with the fitted curves. This study concludes that the mechanical properties determined using the two-parameter Weibull distributions closely match the average experimental results.
3.5 XRD analysis
Natural cellulose exists in two forms, Iα and Iβ. Certain plant fibers, such as cotton, jute, flax, and hemp, tend to have a relatively high proportion of Iβ. Beta cellulose is formed by eliminating water and forming oxygen bridges between C-1 and C-4, with the stacking of parallel hydrogen-bonded sheets partially stabilized by van der Waals interactions.92 The X-ray diffraction (XRD) pattern of natural fibers extracted from R. madagascariensis is shown in Fig. 7. The x-axis, labeled “2 theta (degree),” ranges from 5 to 60°, whereas the y-axis represents “intensity (counts).” The pattern reveals crystalline regions with peaks at specific 2θ angles. Two prominent peaks are observed: a broad and intense peak at 2θ = 15.56° and a sharp, tall peak at 2θ = 22.02°, indicative of a strong crystalline phase corresponding to cellulose-I diffraction.The crystallinity index (CI) of the R. madagascariensis fibers was 67.37%, which was higher than that of the Dracaena reflexa fibers (57.32%), Coir fibers (57%), Calotropis gigantea fibers (56.08%), Grewia tilifolia fibers (41.7%) and Palmyra seed sprout fibers (PSSFs) (38%). Fibers with more crystalline regions exhibit enhanced mechanical stability and stiffness. However, the moderate CI of this fiber suggests that its mechanical strength and stiffness are also moderate. The CI implies that the fiber is thermally stable and can withstand a broad temperature range before degradation. The amorphous fraction (32.63%) makes the fibers more susceptible to swelling and disintegration in certain solvents. The higher crystallinity index indicates that the fiber may be suitable for biocomposite materials requiring a specific amorphous-to-crystalline ratio.93,94
The average size of a single crystal, referred to as the crystalline size (CS), was calculated to be 15.64 nm. In materials science, the crystalline size is commonly used to describe nanoparticles, colloids, gels, and spray-dried agglomerates. A smaller crystallite size enhances the sintering process, allowing for lower sintering temperatures.95
3.6 TGA along with DSC analysis
Fig. 8 shows the thermogravimetric behavior of the RMF sample, along with the corresponding differential scanning calorimetry (DSC) data. The graph depicts the mass change of the sample as a function of temperature or time. The percentage weight reduction, relative to the sample's initial weight, is plotted on the right y-axis, whereas the heat flow in watts per gram (W g−1) is shown on the left y-axis. The x-axis represents the temperature in degrees Celsius (°C). The data points on the graph are marked with brackets and connected by lines. As the temperature increased, the sample weight decreased, indicating disintegration. A more complex heat flow curve is also observed.The weight reduction begins significantly at 85.6 °C, reaching 93.8%, likely due to the evaporation of moisture or volatiles. Dehydration is suggested by the first endothermic peak at 58.01 °C, with a heat flow of −0.003 W g−1. Effective decomposition occurs at 275 °C, with a weight loss of 90.5%. The most prominent thermal event is an exothermic peak at 380 °C (0.037 W g−1), indicating further degradation. At 400 °C, a substantial weight reduction of 19.5% was observed, indicating the onset of decomposition. Finally, the material continues to degrade or transform at 550 °C, as shown by a weight loss of 13.96% and a heat flow of 0.05 W g−1.
3.7 Morphological analysis (SEM and EDX)
SEM was used to examine the surface morphology of the untreated R. madagascariensis fibers. As represented in Fig. 9a, the fibers exhibited a textured and uneven surface, primarily due to the presence of hemicellulose, lignin, and other impurities covering the fibers. These natural surface irregularities contribute to poor surface wet-out and insufficient fiber–matrix bonding. The surface is believed to contain oils, waxes, and dirt. To ensure strong interfacial interactions between the fiber and the polymer matrix, the surface of the fiber must be optimized. Chemical treatment is necessary to remove organic matter and contaminants before incorporating the fibers into the polymer matrix.96,97 The cross-sectional analysis shown in Fig. 9b of R. madagascariensis fibers reveals that each single fiber is composed of multiple hollow structures of varying shapes. These structures consist of thick microstructures and are enveloped by microfibrils along their longitudinal surface. The covering of microfibrils adds texture and possibly contributes to the durability and adhesion properties of the fibers, which could improve their performance in composite materials or textiles.98Fig. 10 shows the elemental quantitative analysis of R. madagascariensis fibers, expressed in terms of atomic and mass percentages. In Table 5, quantitative analysis of elements was performed on the basis of atomic percentage and weight. The EDS analysis indicates the absence of nitrogen and sulfur in the fibers.
| Element | Atomic (%) | Weight (%) |
|---|---|---|
| Aluminum | 9.20 | 13.58 |
| Carbon | 56.73 | 37.28 |
| Chlorine | 16.86 | 32.71 |
| Oxygen | 13.64 | 11.94 |
| Sodium | 3.57 | 4.4 |
3.8 Chemical composition analysis
Identifying the chemical behavior of a substance is essential for understanding its properties, structure, characteristics, and processing capabilities. The composition of a fiber significantly influences these factors. The R. madagascariensis fibers were found to contain 15.17% lignin, 20.12% hemicellulose, and 54.25% cellulose. The high cellulose content enhances its suitability for high-value applications and facilitates processing for various purposes. This contributes to improved crystallinity, mechanical properties, biodegradability, hydrolysis resistance, and thermal stability, which are in good agreement with the results shown earlier. Hemicellulose and lignin present in relatively small amounts help regulate the stiffness and bundling of fibers, making them suitable for high-performance composite applications. Additionally, the fiber was found to have 2.10% extractive content. The percentage distributions of these chemical components are illustrated in the pie chart in Fig. 11. A comparison of the chemical composition, mechanical properties, moisture content and thermal stability of the samples is shown in Table 6.| Name of fiber | Chemical composition | Tensile strength (MPa) | Moisture content (%) | Thermal stability (°C) | References | ||
|---|---|---|---|---|---|---|---|
| Cellulose (%) | Hemicellulose (%) | Lignin (%) | |||||
| RMF | 54.25 | 20.12 | 15.17 | 151 | 9.172 ± 0.601 | 275 | Current work |
| Coconut tree leaf | 27 | 14 | 27.7 | 119.8 | 4.7 | 280.1 | 99 |
| Tamarind | 59 | 22 | 19 | 61.16 | 9.64 | 264 | 100 |
| Rosa hybrid bark fiber | 52.99 | 18.49 | 17.34 | 352.01 | 11.60 | 290 | 66 |
| Sisal | 60–78 | 10–14.2 | 8–14 | 320 | 20–22 | 234 | 101 |
| Pineapple leaf fiber | 73.4 | 7.1 | 10.5 | 210–695 | 9.8 | 236.6 | 102, 103 |
| Ficus racemosa | 72.36 | 11.21 | 10.45 | 270 | 6.13 | 200 | 104 |
4. Conclusion and outlook
A novel natural fiber was successfully extracted from the leaf stalks of Ravenala madagascariensis using environmentally friendly retting methods, including mechanical processing and sun-drying.X-ray diffraction (XRD) and Fourier-transform infrared spectroscopy (FTIR) confirmed the fiber's structural similarity to other lignocellulosic fibers, reinforcing its classification as a typical plant-derived material.
Chemical composition analysis showed a high cellulose content of 54.25%, making the fiber a strong candidate for applications that benefit from cellulose-rich materials, such as biocomposites and cellulose-based products.
The fineness of the extracted fiber was measured at 33.70 ± 11.72 Tex, which is within the typical range for natural fibers used in various industrial applications.
Scanning Electron Microscopy (SEM) revealed a rough surface with voids and cracks, which may improve fiber-matrix adhesion when used as reinforcement in composite materials.
Thermogravimetric analysis demonstrated that the fiber remains thermally stable up to 275 °C, indicating its suitability for low-to medium-temperature processing conditions.
The fiber exhibited moderate mechanical properties in terms of density, tensile strength, and elongation, supporting its potential use in lightweight composite structures.
The ability to employ RMFs for a variety of purposes, including composite reinforcements and the assessment of their mechanical, chemical, and physical properties, is generally enhanced by this study.
Data availability
The data will be made available upon request.Author contributions
Mohammad Abul Hasan Shibly – conceptualized, developed the methodology, analyzed the data, and revised the paper. Mohammad Mohsin Ul Hoque – designed the experiments and investigated. Prosenjit Sen – analyzed the data and wrote the draft manuscript. Khandaker Akil, Mahadi Ohi, Md. Maruf Hossain and Md. Masum Mia performed the experiments. Md. Abdus Sabur – analyzed and interpreted the data. Mohammad Junaebur Rashid – experiments, reviewed and edited the manuscript. Mohammad Mahbubur Rahman – contributed reagents and data curation.Conflicts of interest
The authors declare that they have no known conflicts of interest or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors are grateful to the Bangladesh Council of Scientific and Industrial Research, Dhaka-1205, and the Microsystem and Nanoengineering Lab, Department of Electrical & Electronic Engineering, University of Dhaka, for support in the characterization of the fibers.References
- A. V. Rajulu, G. B. Rao, B. R. P. Rao, A. M. S. Reddy, J. He and J. Zhang, Properties of ligno-cellulose fiber Hildegardia, J. Appl. Polym. Sci., 2002, 84(12), 2216–2221 CrossRef CAS.
- S. Zhu, S. K. Biswas, Z. Qiu, Y. Yue, Q. Fu, F. Jiang and J. Han, Transparent wood-based functional materials via a top-down approach, Prog. Mater. Sci., 2023, 132, 101025 CrossRef CAS.
- N. M. Nurazzi, M. R. M. Asyraf, S. Fatimah Athiyah, S. S. Shazleen, S. A. Rafiqah, M. M. Harussani, S. H. Kamarudin, M. R. Razman, M. Rahmah, E. S. Zainudin, R. A. Ilyas, H. A. Aisyah, M. N. F. Norrrahim, N. Abdullah, S. M. Sapuan and A. Khalina, A Review on Mechanical Performance of Hybrid Natural Fiber Polymer Composites for Structural Applications, Polymers, 2021, 13(13), 2170 CrossRef CAS PubMed.
- K. K. Yun, M. S. Hossain, S. Han and C. Seunghak, Rheological, mechanical properties, and statistical significance analysis of shotcrete with various natural fibers and mixing ratios, Case Stud. Constr. Mater., 2022, 16, e00833 Search PubMed.
- A. Belaadi, A. Bezazi, M. Bourchak, F. Scarpa and C. Zhu, Thermochemical and statistical mechanical properties of natural sisal fibres, Composites, Part B, 2014, 67, 481–489 CrossRef CAS.
- A. Atiqah, M. Jawaid, M. R. Ishak and S. M. Sapuan, Effect of alkali and silane treatments on mechanical and interfacial bonding strength of sugar palm fibers with thermoplastic polyurethane, J. Nat. Fibers, 2018, 15(2), 251–261 CrossRef CAS.
- V. P. Kommula, K. O. Reddy, M. Shukla, T. Marwala and A. V. Rajulu, Physico-chemical, tensile, and thermal characterization of Napier grass (native African) fiber strands, Int. J. Polym. Anal. Charact., 2013, 18(4), 303–314 CrossRef CAS.
- M. Jawaid, A. Othman, N. Saba, Y. A. Shekeil, M. T. Paridah and H. P. S. Abdul Khalil, Effect of chemical modifications of fibers on tensile properties of epoxy hybrid composites, Int. J. Polym. Anal. Charact., 2014, 19(5), 391–403 CrossRef CAS.
- K. O. Reddy, M. Shukla, C. U. Maheswari and A. V. Rajulu, Evaluation of mechanical behavior of chemically modified Borassus fruit short fiber/unsaturated polyester composites, J. Compos. Mater., 2012, 46(23), 2987–2998 CrossRef.
- P. Sudhakara, A. P. Kamala Devi, C. Venkata Prasad, K. Obi Reddy, L. Dong Woo, B. S. Kim and J. I. Song, Thermal, mechanical, and morphological properties of maleated polypropylene compatibilized Borassus fruit fiber/polypropylene composites, J. Appl. Polym. Sci., 2013, 128(2), 976–982 CrossRef CAS.
- A. Ali, K. Shaker, Y. Nawab, M. Ashraf, A. Basit, S. Shahid and M. Umair, Impact of hydrophobic treatment of jute on moisture regain and mechanical properties of composite material, J. Reinf. Plast. Compos., 2015, 34(24), 2059–2068 CrossRef CAS.
- S. H. Mahmud, M. W. Akram, S. M. R. Ferdous, D. Islam, K. Fatema, M. S. A. Chowdhury, A. Das and S. M. Ovi, Fabrication and mechanical performance investigation of jute/glass fiber hybridized polymer composites: Effect of stacking sequences, Next Mater., 2024, 5, 100236 CrossRef.
- T. Väisänen, A. Haapala, R. Lappalainen and L. Tomppo, Utilization of agricultural and forest industry waste and residues in natural fiber-polymer composites: A review, Waste Manag., 2016, 54, 62–73 CrossRef PubMed.
- N. Reddy and Y. Yang, Biofibers from agricultural byproducts for industrial applications, Trends Biotechnol., 2005, 23(1), 22–27 CrossRef CAS PubMed.
- Y. G. Thyavihalli Girijappa, S. Mavinkere Rangappa, J. Parameswaranpillai and S. Siengchin, Natural fibers as sustainable and renewable resource for development of eco-friendly composites: a comprehensive review, Front. Mater., 2019, 6, 226 CrossRef.
- F. P. La Mantia and M. Morreale, Green composites: A brief review, Composites, Part A, 2011, 42(6), 579–588 CrossRef.
- E. Zini and M. Scandola, Green composites: an overview, Polym. Compos., 2011, 32(12), 1905–1915 CrossRef CAS.
- A. Varada Rajulu, A. Venu Nadhan and R. Rama Devi, Properties of ligno-cellulosic bilayered vegetable fabric from ridge gourd, J. Appl. Polym. Sci., 2006, 102(3), 2338–2342 CrossRef.
- H. R. Anik, S. I. Tushar, S. Mahmud, A. H. Khadem, P. Sen and M. Akter, Into the Revolution of NanoFusion: Merging High Performance and Aesthetics by Nanomaterials in Textile Finishes, Adv. Mater. Interfaces, 2024, 2400368 Search PubMed.
- R. Bhatnagar, G. Gupta and S. Yadav, A review on composition and properties of banana fibers, Cellulose, 2015, 60, 65 Search PubMed.
- L. B. Manfredi, E. S. Rodríguez, M. Wladyka-Przybylak and A. Vázquez, Thermal degradation and fire resistance of unsaturated polyester, modified acrylic resins and their composites with natural fibres, Polym. Degrad. Stab., 2006, 91(2), 255–261 CrossRef CAS.
- N. P. G. Suardana, M. S. Ku and J. K. Lim, Effects of diammonium phosphate on the flammability and mechanical properties of bio-composites, Mater. Des., 2011, 32(4), 1990–1999 CrossRef CAS.
- A. Ramachandran, S. Mavinkere Rangappa, V. Kushvaha, A. Khan, S. Seingchin and H. N. Dhakal, Modification of Fibers and Matrices in Natural Fiber Reinforced Polymer Composites: A Comprehensive Review, Macromol. Rapid Commun., 2022, 43(17), 2100862 CrossRef CAS PubMed.
- P. B A, A. Buradi, S. N, V. K. Vasu, J. Hatgundi and H. D, Study on characterization of mechanical, thermal properties, machinability and biodegradability of natural fiber reinforced polymer composites and its Applications, recent developments and future potentials: A comprehensive review, Mater. Today: Proc., 2022, 52, 1255–1259 CAS.
- D. B. Dittenber and H. V. S. GangaRao, Critical review of recent publications on use of natural composites in infrastructure, Composites, Part A, 2012, 43(8), 1419–1429 CrossRef.
- P. P. Das, V. Chaudhary, F. Ahmad, A. Manral, S. Gupta and P. Gupta, Acoustic performance of natural fiber reinforced polymer composites: Influencing factors, future scope, challenges, and applications, Polym. Compos., 2022, 43(3), 1221–1237 CrossRef CAS.
- M. Z. Islam, M. E. Sarker, M. M. Rahman, M. R. Islam, A. T. M. F. Ahmed, M. S. Mahmud and M. Syduzzaman, Green composites from natural fibers and biopolymers: A review on processing, properties, and applications, J. Reinf. Plast. Compos., 2022, 41(13–14), 526–557 CrossRef CAS.
- M. M. Kabir, H. Wang, K. T. Lau and F. Cardona, Chemical treatments on plant-based natural fibre reinforced polymer composites: An overview, Composites, Part B, 2012, 43(7), 2883–2892 CrossRef CAS.
- A. Ashothaman, J. Sudha and N. Senthilkumar, A comprehensive review on biodegradable polylactic acid polymer matrix composite material reinforced with synthetic and natural fibers, Mater. Today: Proc., 2023, 80, 2829–2839 CAS.
- M. Akter, M. H. Uddin and H. R. Anik, Plant fiber-reinforced polymer composites: a review on modification, fabrication, properties, and applications, Polym. Bull., 2023, 81, 1–85 CrossRef.
- M. Akter, M. H. Uddin and I. S. Tania, Biocomposites based on natural fibers and polymers: A review on properties and potential applications, J. Reinf. Plast. Compos., 2022, 41(17–18), 705–742 CrossRef CAS.
- M. Syduzzaman, M. A. Al Faruque, K. Bilisik and M. Naebe, Plant-Based Natural Fibre Reinforced Composites: A Review on Fabrication, Properties and Applications, Coatings, 2020, 10(10), 973 CrossRef CAS.
- S. Arango, R. Greco, N. Guzzo, E. Raffrenato, M. Montanari and L. Bailoni, Physical Characterization of Ten Hemp Varieties to Use as Animal Bedding Material, Animals, 2023, 13(2), 284 CrossRef PubMed.
- A. Bourdot, T. Moussa, A. Gacoin, C. Maalouf, P. Vazquez, C. Thomachot-Schneider, C. Bliard, A. Merabtine, M. Lachi, O. Douzane, H. Karaky and G. Polidori, Characterization of a hemp-based agro-material: Influence of starch ratio and hemp shive size on physical, mechanical, and hygrothermal properties, Energy Build., 2017, 153, 501–512 CrossRef.
- E. Sassoni, S. Manzi, A. Motori, M. Montecchi and M. Canti, Novel sustainable hemp-based composites for application in the building industry: Physical, thermal and mechanical characterization, Energy Build., 2014, 77, 219–226 CrossRef.
- S. Musio, J. Müssig and S. Amaducci, Optimizing hemp fiber production for high performance composite applications, Front. Plant Sci., 2018, 9, 1702 CrossRef PubMed.
- P. Jagadeesh, M. Puttegowda, Y. G. Thyavihalli Girijappa, P. Shivanna, S. Mavinkere Rangappa and S. Siengchin, Investigations on physical, mechanical, morphological and water absorption properties of ramie/hemp/kevlar reinforced vinyl ester hybrid composites, J. Vinyl Addit. Technol., 2023, 29(3), 555–567 CrossRef CAS.
- S. S. Munawar, K. Umemura and S. Kawai, Characterization of the morphological, physical, and mechanical properties of seven nonwood plant fiber bundles, J. Wood Sci., 2007, 53(2), 108–113 CrossRef.
- A. R. G. de Azevedo, M. T. Marvila, M. L. P. Antunes, E. C. Rangel and R. Fediuk, Technological Perspective for Use the Natural Pineapple Fiber in Mortar to Repair Structures, Waste Biomass Valorization, 2021, 12(9), 5131–5145 CrossRef CAS.
- P. H. F. Pereira, H. L. Ornaghi Júnior, L. V. Coutinho, B. Duchemin and M. O. H. Cioffi, Obtaining cellulose nanocrystals from pineapple crown fibers by free-chlorite hydrolysis with sulfuric acid: physical, chemical and structural characterization, Cellulose, 2020, 27(10), 5745–5756 CrossRef CAS.
- M. K. Moghaddam and S. M. Mortazavi, Physical and Chemical Properties of Natural Fibers Extracted from Typha Australis Leaves, J. Nat. Fibers, 2016, 13(3), 353–361 CrossRef CAS.
- U. N. Haq, A. Huraira and M. A. Uddin, Physical characteristics of Typha elephantina Roxb. fiber (Hogla) for textile application, J. Text. Inst., 2022, 113(11), 2328–2334 CrossRef CAS.
- R. Thirumurugan, M. Jayaraj, D. Shanmugam and T. Ramkumar, Characterization of new natural cellulosic fiber from coconut tree primary flower leaf stalk fiber (CPFLSF), J. Nat. Fibers, 2021, 18(11), 1844–1856 CrossRef CAS.
- H. Gupta, H. Kumar, A. K. Gehlaut, S. K. Singh, A. Gaur, S. Sachan and J.-W. Park, Preparation and characterization of bio-composite films obtained from coconut coir and groundnut shell for food packaging, J. Mater. Cycles Waste Manage., 2022, 24(2), 569–581 CrossRef CAS.
- A. K. Rout, J. Kar, D. K. Jesthi and A. K. Sutar, Effect of surface treatment on the physical, chemical, and mechanical properties of palm tree leaf stalk fibers, BioResources, 2016, 11(2), 4432–4445 CrossRef CAS.
- G. Singh, S. Jose, D. Kaur and B. Soun, Extraction and Characterization of Corn Leaf Fiber, J. Nat. Fibers, 2022, 19(5), 1581–1591 CrossRef CAS.
- Z. Luo, P. Li, D. Cai, Q. Chen, P. Qin, T. Tan and H. Cao, Comparison of performances of corn fiber plastic composites made from different parts of corn stalk, Ind. Crops Prod., 2017, 95, 521–527 CrossRef CAS.
- P. Ramesh, B. Durga Prasad and K. L. Narayana, Characterization of kenaf fiber and its composites: A review, J. Reinf. Plast. Compos., 2018, 37(11), 731–737 CrossRef CAS.
- S. F. A. S. Abdullah; N. Z. M. Zuhudi; K. D. M. Aris; M. N. Roslan; M. D. Isa, Physical and Mechanical Characterization of Kenaf Fiber Filament Wound Composite Produced Using Vacuum-Bagging and Heat-Shrink Tube Method, In Proceedings of International Conference of Aerospace and Mechanical Engineering 2019, Singapore, Eds. Rajendran, P., Mazlan, N. M., Rahman, A. A. A., Suhadis, N. M., Razak, N. A., Abidin, M. S. Z., Springer, Singapore: Singapore, 2020; pp pp 519–527 Search PubMed.
- M. Raj, S. Fatima and N. Tandon, An experimental and theoretical study on environment-friendly sound absorber sourced from nettle fibers, J. Build. Eng., 2020, 31, 101395 CrossRef.
- M. P. Mudoi, S. Sinha and V. Parthasarthy, Polymer composite material with nettle fiber reinforcement: A review, Bioresour. Technol. Rep., 2021, 16, 100860 CrossRef CAS.
- H. Abral, D. Kadriadi, A. Rodianus, P. Mastariyanto, Ilhamdi, S. Arief, S. M. Sapuan and M. R. Ishak, Mechanical properties of water hyacinth fibers – polyester composites before and after immersion in water, Mater. Des., 2014, 58, 125–129 CrossRef CAS.
- H. Abral, M. H. Dalimunthe, J. Hartono, R. P. Efendi, M. Asrofi, E. Sugiarti, S. M. Sapuan, J.-W. Park and H.-J. Kim, Characterization of Tapioca Starch Biopolymer Composites Reinforced with Micro Scale Water Hyacinth Fibers, Starch – Stärke, 2018, 70(7–8), 1700287 CrossRef.
- S. Chonsakorn, S. Srivorradatpaisan and R. Mongkholrattanasit, Effects of different extraction methods on some properties of water hyacinth fiber, J. Nat. Fibers, 2019, 16(7), 1015–1025 CrossRef CAS.
- J. Ronald Aseer, K. Sankaranarayanasamy, P. Jayabalan, R. Natarajan and K. Priya Dasan, Morphological, Physical, and Thermal Properties of Chemically Treated Banana Fiber, J. Nat. Fibers, 2013, 10(4), 365–380 CrossRef CAS.
- S. K. Paramasivam, D. Panneerselvam, D. Sundaram, K. N. Shiva and U. Subbaraya, Extraction, Characterization and Enzymatic Degumming of Banana Fiber, J. Nat. Fibers, 2022, 19(4), 1333–1342 CrossRef CAS.
- M. Z. I. Mollah, M. S. Miah, M. W. Akram, S. H. Mahmud, M. R. I. Faruque and K. S. Al-mugren, Thermoplastic-polymer matrix composite of banana/betel nut husk fiber reinforcement: Physico-mechanical properties evaluation, e-Polymers, 2024, 24(1), 20230158 CrossRef CAS.
- H. Pang, S. Zhao, L. Mo, Z. Wang, W. Zhang, A. Huang, S. Zhang and J. Li, Mussel-inspired bio-based water-resistant soy adhesives with low-cost dopamine analogue-modified silkworm silk Fiber, J. Appl. Polym. Sci., 2020, 137(23), 48785 CrossRef CAS.
- S. Basak, D. B. Shakyawar, K. K. Samanta, S. Debnath, M. Bhowmick and N. Kumar, Development of natural fibre based flexural composite: A sustainable mimic of natural leather, Mater. Today Commun., 2022, 32, 103976 CrossRef CAS.
- A. Pappu, M. Saxena, V. K. Thakur, A. Sharma and R. Haque, Facile extraction, processing and characterization of biorenewable sisal fibers for multifunctional applications, J. Macromol. Sci., Part A:Pure Appl. Chem., 2016, 53(7), 424–432 CrossRef CAS.
- Y. Liu, Y. Ma, J. Yu, J. Zhuang, S. Wu and J. Tong, Development and characterization of alkali treated abaca fiber reinforced friction composites, Compos. Interfaces, 2019, 26(1), 67–82 CrossRef CAS.
- Y. Pan, G. Han, Z. Mao, Y. Zhang, H. Duan, J. Huang and L. Qu, Structural characteristics and physical properties of lotus fibers obtained from Nelumbo nucifera petioles, Carbohydr. Polym., 2011, 85(1), 188–195 CrossRef CAS.
- D. Sartika, K. Syamsu, E. Warsiki, F. Fahma and I. W. Arnata, Nanocrystalline Cellulose from Kapok Fiber (Ceiba pentandra) and its Reinforcement Effect on Alginate Hydrogel Bead, Starch – Stärke, 2021, 73(9–10), 2100033 CrossRef CAS.
- M. Humenik; T. Scheibel; A. Smith, Spider Silk: Understanding the Structure–Function Relationship of a Natural Fiber, In Progress in Molecular Biology and Translational Science, Ed. Howorka, S., Academic Press, 2011, Vol. 103, pp 131–185 Search PubMed.
- C. Y. Hayashi, N. H. Shipley and R. V. Lewis, Hypotheses that correlate the sequence, structure, and mechanical properties of spider silk proteins, Int. J. Biol. Macromol., 1999, 24(2), 271–275 CrossRef CAS PubMed.
- M. A. H. Shibly, M. I. Islam, M. N. H. Rahat, M. M. Billah, M. M. Rahman, M. S. Bashar, B. Abdul and H. S. Alorfi, Extraction and characterization of a novel cellulosic fiber derived from the bark of Rosa hybrida plant, Int. J. Biol. Macromol., 2024, 257, 128446 CrossRef CAS.
- S. Suroowan; F. Mahomoodally, R. Madagascariensis, In Underexplored Medicinal Plants from Sub-Saharan Africa, Elsevier, 2020, pp 247–252 Search PubMed.
- T. Haevermans, A. Hladik, C.-M. Hladik, J. Razanatsoa, A. Haevermans, V. Jeannoda and P. Blanc, Description of five new species of the Madagascan flagship plant genus Ravenala (Strelitziaceae), Sci. Rep., 2021, 11(1), 21965 CrossRef CAS PubMed.
- W. Konczewicz, M. Zimniewska and M. A. Valera, The selection of a retting method for the extraction of bast fibers as response to challenges in composite reinforcement, Text. Res. J., 2018, 88(18), 2104–2119 CrossRef CAS.
- Y. Huang, F. Meng, R. Liu, Y. Yu and W. Yu, Morphology and supramolecular structure characterization of cellulose isolated from heat-treated moso bamboo, Cellulose, 2019, 26, 7067–7078 CrossRef CAS.
- P. Senthamaraikannan and M. Kathiresan, Characterization of raw and alkali treated new natural cellulosic fiber from Coccinia grandis. L, Carbohydr. Polym., 2018, 186, 332–343 CrossRef CAS PubMed.
- S. Indran and R. E. Raj, Characterization of new natural cellulosic fiber from Cissus quadrangularis stem, Carbohydr. Polym., 2015, 117, 392–399 CrossRef CAS PubMed.
- Y. Liu, J. Xie, N. Wu, Y. Ma, C. Menon and J. Tong, Characterization of natural cellulose fiber from corn stalk waste subjected to different surface treatments, Cellulose, 2019, 26, 4707–4719 CrossRef CAS.
- M. Kathirselvam, A. Kumaravel, V. P. Arthanarieswaran and S. S. Saravanakumar, Isolation and characterization of cellulose fibers from Thespesia populnea barks: A study on physicochemical and structural properties, Int. J. Biol. Macromol., 2019, 129, 396–406 CrossRef CAS PubMed.
- A. D. O. Betene, F. E. Betene, F. Martoïa, P. J. J. Dumont, A. Atangana and P. M. A. Noah, Physico-chemical and thermal characterization of some lignocellulosic fibres: Ananas comosus (AC), Neuropeltis acuminatas (NA) and Rhecktophyllum camerunense (RC), J. Miner. Mater. Charact. Eng., 2020, 8(4), 205–222 CAS.
- M. A. H. Shibly, M. I. Islam, M. M. U. Hoque, M. Sabit, M. M. Rahman, Z. Islam and M. J. Rashid, Hylocereus undatus plant's stem agro-waste: A potential source of natural cellulosic fiber for polymer composites, Sustainable Chem. Pharm., 2024, 41, 101692 CrossRef CAS.
- A. Khan, R. Vijay, D. L. Singaravelu, M. R. Sanjay, S. Siengchin, F. Verpoort, K. A. Alamry and A. M. Asiri, Extraction and characterization of natural fiber from Eleusine indica grass as reinforcement of sustainable fiber reinforced polymer composites, J. Nat. Fibers, 2021, 18(11), 1742–1750 CrossRef CAS.
- K. Mayandi, N. Rajini, P. Pitchipoo, J. T. W. Jappes and A. V. Rajulu, Extraction and characterization of new natural lignocellulosic fiber Cyperus pangorei, Int. J. Polym. Anal. Charact., 2016, 21(2), 175–183 CrossRef CAS.
- R. Vijay, D. L. Singaravelu, A. Vinod, I. D. F. P. Raj, M. R. Sanjay and S. Siengchin, Characterization of novel natural fiber from saccharum bengalense grass (Sarkanda), J. Nat. Fibers, 2020, 17(12), 1739–1747 CrossRef CAS.
- A. Vinod, R. Vijay, D. Lenin Singaravelu, A. Khan, M. R. Sanjay, S. Siengchin, F. Verpoort, K. A. Alamry and A. M. Asiri, Effect of alkali treatment on performance characterization of Ziziphus mauritiana fiber and its epoxy composites, J. Ind. Text., 2022, 51(2_suppl), 2444S–2466S CrossRef CAS.
- P. Narayanasamy, P. Balasundar, S. Senthil, M. R. Sanjay, S. Siengchin, A. Khan and A. M. Asiri, Characterization of a novel natural cellulosic fiber from Calotropis gigantea fruit bunch for ecofriendly polymer composites, Int. J. Biol. Macromol., 2020, 150, 793–801 CrossRef CAS PubMed.
- V. P. Arthanarieswaran, A. Kumaravel and S. S. Saravanakumar, Characterization of new natural cellulosic fiber from Acacia leucophloea bark, Int. J. Polym. Anal. Charact., 2015, 20(4), 367–376 CrossRef CAS.
- R. Vijay, D. L. Singaravelu, A. Vinod, M. R. Sanjay, S. Siengchin, M. Jawaid, A. Khan and J. Parameswaranpillai, Characterization of raw and alkali treated new natural cellulosic fibers from Tridax procumbens, Int. J. Biol. Macromol., 2019, 125, 99–108 CrossRef CAS PubMed.
- P. Senthamaraikannan, M. R. Sanjay, K. S. Bhat, N. H. Padmaraj and M. Jawaid, Characterization of natural cellulosic fiber from bark of Albizia amara, J. Nat. Fibers, 2019, 16(8), 1124–1131 CrossRef CAS.
- R. Dalmis, G. B. Kilic, Y. Seki, S. Koktas and O. Y. Keskin, Characterization of a novel natural cellulosic fiber extracted from the stem of Chrysanthemum morifolium, Cellulose, 2020, 27, 8621–8634 CrossRef CAS.
- R. Gopinath, P. Billigraham and T. P. Sathishkumar, Characterization studies on new natural cellulosic fiber extracted from the bark of erythrina variegata, J. Nat. Fibers, 2022, 19(14), 8246–8265 CrossRef CAS.
- N. Sumrith, L. Techawinyutham, M. R. Sanjay, R. Dangtungee and S. Siengchin, Characterization of alkaline and silane treated fibers of ‘water hyacinth plants’ and reinforcement of ‘water hyacinth fibers’ with bioepoxy to develop fully biobased sustainable ecofriendly composites, J. Polym. Environ., 2020, 28, 2749–2760 CrossRef CAS.
- D. Cheng, B. Weng, Y. Chen, S. Zhai, C. Wang, R. Xu, J. Guo, Y. Lv, L. Shi and Y. Guo, Characterization of potential cellulose fiber from Luffa vine: a study on physicochemical and structural properties, Int. J. Biol. Macromol., 2020, 164, 2247–2257 CrossRef CAS PubMed.
- K. Senthilkumar, N. Rajini, N. Saba, M. Chandrasekar, M. Jawaid and S. Siengchin, Effect of alkali treatment on mechanical and morphological properties of pineapple leaf fibre/polyester composites, J. Polym. Environ., 2019, 27, 1191–1201 CrossRef CAS.
- A. Célino, S. Fréour, F. Jacquemin and P. Casari, Characterization and modeling of the moisture diffusion behavior of natural fibers, J. Appl. Polym. Sci., 2013, 130(1), 297–306 CrossRef.
- J. Yuan, Q. Chen, C. Fang, S. Zhang, X. Liu and B. Fei, Effect of chemical composition of bamboo fibers on water sorption, Cellulose, 2021, 28(11), 7273–7282 CrossRef CAS.
- A. N. Balaji and K. J. Nagarajan, Characterization of alkali treated and untreated new cellulosic fiber from Saharan aloe vera cactus leaves, Carbohydr. Polym., 2017, 174, 200–208 CrossRef CAS PubMed.
- A. Uddin, M. R. Islam and S. Islam, Splitting and authentication of the newest retrieved cellulose-rich organic fiber from the exterior layer of Bangladeshi palmyra seed sprouts, RSC Adv., 2024, 14(41), 30336–30345 RSC.
- J. Jayaramudu, B. R. Guduri and A. V. Rajulu, Characterization of new natural cellulosic fabric Grewia tilifolia, Carbohydr. Polym., 2010, 79(4), 847–851 CrossRef CAS.
- A. Ruys, Processing, structure, and properties of alumina ceramics, Alumina Ceramics, 2019, 4, 71–121 Search PubMed.
- K. O. Reddy, K. R. N. Reddy, J. Zhang, J. Zhang and A. Varada Rajulu, Effect of alkali treatment on the properties of century fiber, J. Nat. Fibers, 2013, 10(3), 282–296 CrossRef CAS.
- L. Boopathi, P. S. Sampath and K. Mylsamy, Investigation of physical, chemical and mechanical properties of raw and alkali treated Borassus fruit fiber, Composites, Part B, 2012, 43(8), 3044–3052 CrossRef CAS.
- R. Vijay, A. Vinod, D. L. Singaravelu, M. R. Sanjay and S. Siengchin, Characterization of chemical treated and untreated natural fibers from Pennisetum orientale grass-A potential reinforcement for lightweight polymeric applications, Int. J. Lightweight Mater. Manuf., 2021, 4(1), 43–49 CAS.
- K. Obi Reddy, G. Sivamohan Reddy, C. Uma Maheswari, A. Varada Rajulu and K. Madhusudhana Rao, Structural characterization of coconut tree leaf sheath fiber reinforcement, J. For. Res., 2010, 21(1), 53–58 CrossRef.
- C. U. Maheswari, B. R. Guduri and A. V. Rajulu, Properties of lignocellulose tamarind fruit fibers, J. Appl. Polym. Sci., 2008, 110(4), 1986–1989 CrossRef CAS.
- A. R. Martin, M. A. Martins, O. R. R. F. da Silva and L. H. C. Mattoso, Studies on the thermal properties of sisal fiber and its constituents, Thermochim. Acta, 2010, 506(1–2), 14–19 CrossRef CAS.
- C. H. Dong, Z. Lv, L. Zhang, H. J. Shen, N. N. Li and P. Zhu, Structure and characteristics of pineapple leaf fibers obtained from pineapple leaves, Adv. Mater. Res., 2014, 998, 316–319 Search PubMed.
- A. R. S. Neto, M. A. M. Araujo, F. V. D. Souza, L. H. C. Mattoso and J. M. Marconcini, Characterization and comparative evaluation of thermal, structural, chemical, mechanical and morphological properties of six pineapple leaf fiber varieties for use in composites, Ind. Crops Prod., 2013, 43, 529–537 CrossRef.
- P. Manimaran, S. P. Saravanan and M. Prithiviraj, Investigation of physico chemical properties and characterization of new natural cellulosic fibers from the bark of Ficus Racemosa, J. Nat. Fibers, 2021, 274–284 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |