Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Extraction of protein from oilseed meal residues of sesame, hazelnut, almond and pumpkin produced in the oil industry for application in human and animal food
Narges Rahemizadeh and
Ali Gholami *
*
Department of Analytical Chemistry, Faculty of Chemistry, University of Kashan, P.O. Box 87317-51167, Kashan, Islamic Republic of Iran. E-mail: agholami@kashanu.ac.ir
First published on 16th July 2025
Abstract
In recent years, seeds and oils derived from oilseeds have become increasingly popular additives to food, so the cultivation of oil plants worldwide is growing rapidly. Alkaline extraction remains the preferred technique for protein extraction from oilseed meal in commercial processes. This study presents the current status of knowledge on the protein and amino acid composition of common oilseed meals and the nutrition, health benefits and food applications of oilseed meals containing sesame, hazelnut, almond, and pumpkin. Additionally, the amount of amino acid L-alanine in the oil seeds was determined using the ninhydrin method. The amount of L-alanine in the oilseed meal was then measured using UV-Vis spectroscopy at a wavelength of 570 nm. The percentages of protein in the isolates containing sesame, hazelnut, almond, and pumpkin were 76.20, 79.05, 81.04, and 80.22, respectively. The amount of protein in oilseed meal was determined using the Kjeldahl method for the validity test. Oilseeds are widely applied in the food industry for human and animal consumption owing to their health benefits and good functional properties.
1. Introduction
Proteins are the most important macronutrients for humans.1 The global demand for protein is increasing; consequently, there is a need for new sources of dietary protein. Animal proteins are expensive in terms of market price, land requirement and environmental impact.2 In addition, consumer confidence in animal proteins has decreased owing to food safety concerns.3 The vegetable oil industry produces a lot of waste since disposal of this waste is costly and problematic from an economic and environmental viewpoint. This waste also has high nutritional and medicinal value.4 Therefore, waste can be used in various processes that lead to the production of high-value products and reduce the resulting production waste to a minimum.5,6The animal production industry provides quality food proteins for one of the most basic human needs. This is achieved through a synergistic relationship with other segments of agriculture. The increasing global demand for protein as human and animal feed has led to increased interest in other protein sources, such as oilseed meal. The resulting meal from the oil extraction process shows promise as animal feed owing to its high crude protein content.7,8 The production of edible oil from oilseeds is a major sector in the global food industry. However, this prominence comes from the generation of substantial amounts of waste. The primary byproduct of the oil extraction process is oil cake. Out of the 23 million tons increase in total oil-cake production, 17 million tons are produced in developing nations, such as India, Brazil, and Argentina. Oil cake primarily comprises highly lignified husk lignocellulosic fibers (40%), proteins (35%), and phenolic compounds (5.7%).9
Proteins used in food processing have different origins and can be classified into animal and vegetable proteins.10 Oilseed meal is a good source of nutrients, such as sesame, hazelnut, almond, pumpkin and safflower seeds,11 especially those that contribute to human health. Many nutrients and peptides in oilseed meal are biologically active. These peptides show various activities, such as anti-blood pressure, antioxidant, anti-cancer, anti-disease, immune system modulator, and cholesterol reduction.12 Oilseed meal is a good source of nutritional components, especially those that contribute to human health. Many nutrients and peptides in oilseed meals are biologically active. These peptides show different activities, such as anti-hypertensive, antioxidant, anti-cancer, anti-disease, immune-modulating and cholesterol-lowering.13 In this research, the residue protein of sesame oilseed meal (SOM),14,15 protein of hazelnut oilseed meal (HOM),16,17 protein of almond oilseed meal (AOM),18 and protein of pumpkin oilseed meal (POM),19,20 which are rich in protein sources, were used. SOM protein is rich in methionine, cysteine and tryptophan. SOM can be added to supplements to improve the nutritional balance. SOM, an inexpensive vegetable protein source, can be used to fortify infant formula.21
Studies have shown that the protein of HOM can be a protein source for functional foods and, to some extent, replace existing sources in a more environmentally friendly, cheap and sustainable way. The amino acid composition of the HOM protein was comparable to that of some animal proteins and superior to that of many vegetable proteins. Additionally, the results demonstrated that hazelnut protein isolate could be an alternative and sustainable source for vegetable protein production.22 Almonds contain valuable sources of nutrients, such as lipids, proteins, dietary fibers, vitamins, minerals and phenolic compounds. AOM is a fat-free and high-protein meal. This meal can be valued and used as a human or animal food. Using the two-dimensional electrophoresis method, approximately 188 different proteins have been identified in almond seeds.16,18
Protein of POM has the highest level between 60% and 65%, which makes it an attractive and promising breeding source of plant origin. Biochemical descriptions of cultivations and functional and nutritional evaluations of POM are carried out, which can lead to the innovative use of pumpkin as a resource in different food systems as a functional element or as a complementary food.23
There are three ways to prepare protein isolate containing chemical, enzymatic and physical methods. One of the chemical methods is alkaline extraction, which is the most common conventional method used to extract vegetable proteins. Structural changes in an amino acid or protein that occur at different pH values change the relative solubility of the molecule. The pH value at which the concentrations of the anionic and cationic groups are equal is the isoelectric point of the protein. Amino acids and proteins have the lowest solubility at their isoelectric points.24 Additionally, the quantitative analysis of proteins depends on the type and nature of the protein, the nature of other components of the protein sample, and the speed, accuracy, and sensitivity of the test. Proteins contain almost the same percentage of nitrogen about 16%. The most common method for determining the amount of nitrogen in organic materials, which is based on neutralization with titration, is the Kjeldahl method.25 The quantitative analysis of amino acids, specific functional groups in amino acids, can react to produce specific colored products. The color intensity of the product formed by a specific group of amino acids varies according to the number of active or free groups in the reaction and their accessibility to the reagent. Methods such as ninhydrin, xanthoproteic, Hapkinsol, sodium nitroprusside, Milon, lead acetate, Pauli, and Sakaguchi have been used for the quantitative determination of amino acids.26
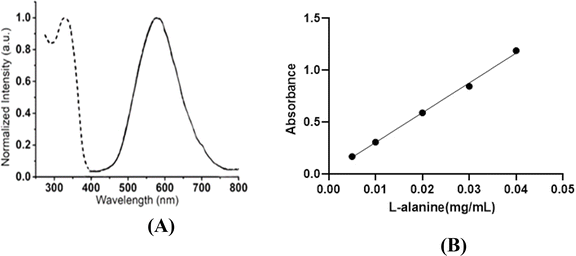
Amino acids consist of two parts: amines and carboxylic acids. Although instrumental techniques, such as HPLC, are currently used to determine compounds containing amino acid groups, the simple and convenient ninhydrin method still has several advantages because it does not require expensive equipment and is suitable for the routine analysis of large numbers of samples.27 In the ninhydrin method, amino acids pair two ninhydrin molecules through the nitrogen atom, and the amino acid is separated as an aldehyde according to Scheme 1. In this method, through the free electron pair, nitrogen reacts with the amino acid and creates a purple color. Type I and II amines respond to the ninhydrin test. According to the characteristics of amino acids, most amino acids are type 1 and type 2 amines. The absorption is checked using a spectrophotometer at a wavelength of 570 nm. One of the investigated amino acids is L-alanine. The calibration curve can be obtained using the Beer–Lambert law and the appropriate linear equation of the amino acid L-alanine and used in calculations.26,27
In the present study, the oilseed meal was prepared at Barij Essence Company (Kashan, Iran). The sample was produced by the cold pressing method, and it was completely crushed and powdered using a grinding machine. Then, the oil was extracted using a Soxhlet machine with an n-hexane solvent, and protein isolation was prepared.28,29 Then, using the ninhydrin test and the L-alanine absorption curve by applying the spectrophotometric method, the investigation of the quantitative measurement of sesame, hazelnut, pumpkin, and almond amino acids was performed.
2. Materials and methods
2.1. Materials
Sesame, hazelnut, almond, and pumpkin oilseed meal were obtained from Barij Essence Company, Kashan City (Iran), defatted using a Soxhlet apparatus with n-hexane solvent, and then turned into powder using a grinder. The sieve was passed through 20 meshes to obtain a homogeneous particle size. All the chemicals, including normal hexane, soda solution, neutral hydrochloric acid, tin(II) chloride dehydrate, glacial acetic acid, sodium acetate trihydrate, ninhydrin, dimethyl sulfoxide, and ethanol, were obtained from Merck, Germany.2.2. Measurement of some physical and chemical properties of oilseed meal
Oilseed meals should exhibit characteristics according to existing standards. In terms of appearance, they should be free of visible insects, animal droppings, pests and molds and be in the form of oilseed meal, lumps or feathers, with a good smell. The chemical composition of isolated proteins of SOM, HOM, AOM and POM was measured using the AOAC standard methods of 1990. A 105 C oven was used for moisture determination, the Soxhlet method for fat determination, the Kjeldahl method for protein determination, and a 550 C oven for ash determination.29 For statistical measurement, the samples were analyzed 3 times, and the measurement error was calculated.2.3. Preparation of protein isolate
The protein isolate of the oilseed meal was prepared according to the method with modifications.282.4. Preparation of oilseed meal
An oilseed meal was prepared at Barij Essence Pharmaceutical Company. After removing the foreign substances, the oilseed meal was made into powder by a mill and passed through a sieve with 20 meshes. Then, a portion of the sample was mounted on a Soxhlet apparatus with the solvent of n-hexane, and oil extraction was performed for 4 hours.302.5. Oilseed protein extraction
5 g of oilseed meal was degreased by n-hexane solvent and Soxhlet apparatus and poured into 100 mL of water; the pH was adjusted to 10.00 with soda solution and stirred with a magnetic stirrer at room temperature for 1 hour. The aqueous extract was overflowed, filtered with cotton and then centrifuged. The resulting extract was diluted with a neutral hydrochloric acid solution, and its pH was adjusted to 4.00. When the aqueous extract was neutralized, a light white sediment was separated from the extract. The remaining solution and sediment were centrifuged. The sediment separated in the test tube was washed twice with water and then dried in an oven at a temperature of 50 °C,30 as depicted in Fig. 1.2.6. Ninhydrin test method
24 mg of tin(II) chloride was carefully weighed with a scale (±0.0001) and then dissolved in 10 mL of sodium acetate 4.0 M. Then, 0.2 g of ninhydrin was weighed, and in 10 mL of dimethyl sulfoxide, one volume of tin(II) chloride solution was mixed with three times the volume of ninhydrin solution to make the reagent.A 100 mL volumetric flask was made up of volume with distilled water, and 0.5, 1.0, 2.0, 3.0, and 4.0 mL of this solution were transferred by pipette to 10 mL flasks and made up to volume with distilled water to obtain solutions with concentrations of 0.005, 0.01, 0.02, 0.03 and 0.04 (mg mL−1) of L-alanine should be obtained.26,27
2.7. L-Alanine amino acid calibration curve
1 mL of the newly made reagent was transferred to the Pyrex test tubes with a volumetric pipette, and 1.00 mL of each of the standard solutions was slowly added to the reagent using a volumetric pipette. The lid of the tubes was closed, and the solutions were mixed well for one minute. Then, the tubes were placed in a boiling water bath simultaneously and exactly for 15 minutes. After this time, the tubes were removed from the bath and cooled in a cold-water bath until they reached the ambient temperature. Then, with a volume pipette, 5.0 mL of 50% ethanol was added to each tube and mixed well until it was uniform. The absorbance of the solutions was read at a wavelength of 570 nm with a spectrophotometer against the control solution containing 50% ethanol.26,272.8. Optimization of several variables (pH–temperature–addition of salt)
To optimize the process of protein extraction from oilseed meal, the effect of pH and optimal temperature, as well as the effect of salt on increasing the yield of protein isolate, was investigated.2.9. Amino acid composition analysis
The analysis of the amino acid composition of isolated protein was performed by applying the high performance liquid chromatography (HPLC) method. This instruction includes three work steps: preparation of the sample resulting from hydrolysis for chromatography, including derivatization, drying, etc.; isolation of the derivative obtained from the preparation stage by HPLC; and buffer for the mobile phase. Exactly 11.48 g of sodium acetate was weighed and poured into a one-liter flask. It was dissolved in 900 mL of water, and its pH was adjusted to 1.60 with acetic acid and sodium hydroxide. Then, the balloon was inflated with water. Then, 0.5 mL of triethylamine was added to this solution and mixed well. This solution was filtered with a special filter of 0.45 micrometers.Phase A: 940 mL of the buffer made in the previous step was mixed with 60 mL of acetonitrile and degassed. Phase B: 60% acetonitrile and 40% water were mixed and degassed.31,32
3. Results and discussion
3.1. Physical and chemical characteristics of oilseed meal
The results related to the test of some physical and chemical characteristics of oilseed meal containing fat percentage, protein percentage, moisture percentage, and total ash percentage were calculated based on the standards mentioned in Subsection 2.2, as shown in Table 1.| Oilseed meal | Ash% | Moisture% | Protein yield% | Fat% |
|---|---|---|---|---|
| Sesame | 6.16 ± 0.21 | 9.60 ± 0.10 | 76.20 ± 0.03 | 12.09 ± 1.02 |
| Hazelnut | 5.12 ± 0.32 | 8.50 ± 0.15 | 79.02 ± 0.04 | 10.10 ± 1.03 |
| Almonds | 6.27 ± 0.2 | 8.40 ± 0.10 | 81.4 ± 0.08 | 11.01 ± 0.92 |
| Pumpkin | 6.24 ± 0.10 | 8.90 ± 0.12 | 80.22 ± 0.05 | 10.22 ± 1.01 |
According to Table 1, the percentage of fat extracted by the Soxhlet apparatus from all the mentioned oil seeds is in the numerical range of 10.00–12.00. Additionally, the moisture level of the oilseed meal is between 8.00 and 9.00 percent relative humidity (%RH), which is acceptable according to the standard. Moreover, the amount of ash is between 5.0% and 7.00% of ash (%ash). According to the table, pumpkin oilseed meals have the most ash. Almond has the highest amount of protein among the oilseed meals considered in this project, and pumpkin, hazelnut, and sesame are ranked next in terms of protein content.33
3.2. L-Alanine absorption calibration curve
This experiment was repeated three times according to Subsubsection 2.3.4 for each concentration, and the average absorption was obtained. According to Table 2 and Fig. 2, it shows the curve of the absorption graph according to the concentration of L-alanine. Additionally, eqn (1) shows the absorption equation in terms of the L-alanine concentration.34| A = 28.685C + 0.0182 R2 = 0.997 | (1) |
| C (mg mL) | 0.005 ± 0.05 | 0.01 ± 0.05 | 0.02 ± 0.05 | 0.03 ± 0.05 | 0.04 ± 0.05 |
| A | 0.169 | 0.308 | 0.591 | 0.846 | 01.189 |
3.3. Optimum conditions in alkaline pH environment SOM
The results of different alkaline pH conditions on protein isolate percentage efficiency and total amino acid percentage in terms of L-alanine by the ninhydrin test in alkaline are presented in Fig. 3A1 and A2. The pH levels were 8.00, 9.00, 10.00, 11.00, and 12.00. Additionally, an acidic pH of equal to 5.00 at 25 °C was investigated. At pH 8.00, the amount of protein is higher than the other tests, but the efficiency percentage is very low. At pH 10.00, the amount of amino acid and its percentage efficiency are more suitable and affordable than the alkaline pH of 9.00–11.00–12.00. It was concluded that the lowest solubility can be found at an alkaline pH of 10.00, where the positive and negative charges are equal, and it is electrically neutral and completely precipitates; however, proteins have the lowest solubility at this point.353.4. Precipitation of proteins in acidic pH SOM
According to Fig. 3B1 and B2, the best neutralization pH, which is suitable in terms of efficiency and amino acid percentage, is pH 4.00. At pH lower than the isoelectric point, owing to the positive charge of the protein, the particles move away from each other with repulsive force, and at pH higher than the isoelectric point, due to the negative charge of the protein, they move away from each other and remain in solution. Therefore, at pH 4.00, most protein isolates is formed.3.5. Investigating the total amino acid percentage of SOM at different temperatures
Temperature is an important factor in measuring the amount of protein isolation; the maximum amount of protein can be extracted at ambient temperature, and in terms of energy consumption, it is more economical. According to Fig. 3C1 and C2, it can be concluded that the efficiency of percentage and amount of amino acid at 25 °C is better compared to temperatures of 35 °C, 50 °C and 60 °C. Since high temperature can cause protein denaturation, the alkaline solution can easily hydrolyze the protein, and the molecular weight decreases; the extraction temperature is 25 °C, which is the best protein yield rate.363.6. Investigating the extraction efficiency of adding NaCl to SOM
The percentage of total amino acids increased by about 4.4% in the presence of salt; consequently, a higher efficiency percentage was obtained. The presence of lateral ionized branches in the protein causes changes in solubility in the presence of salt. The interaction between salt ions and some charged groups of protein is one of the important reasons for increasing protein solubility in salt solutions. By increasing the ionic strength of the salt solution, the degree of protein dissolution in the source material increases initially until it reaches its maximum value.37,383.7. Investigating protein of SOM, HOM, AOM and POM
The optimum conditions for sesame protein isolates are an alkaline pH of 10.00 and a neutralization pH of 4.00 at 25 °C for other protein isolates. Using the method of ninhydrin, the yield percentage and its amino acid were measured by a UV-Vis device at a wavelength of 570 nm. The results for hazelnut, almond and pumpkin were examined, as illustrated in Table 3. In this comparison, the amino acid percentage of almond, pumpkin, sesame, and hazelnut was the maximum in order, and by adding salt to the degreasing powder of seeds at 25 °C, the percentage efficiency increased.| Row | pH alkaline | pH acidic | Temperature (°C) | Percentage yield% | Amino acid% | |
|---|---|---|---|---|---|---|
| 1 | Sesame | 10.00 ± 0.10 | 4.00 ± 0.13 | 25 ± 0.50 | 32.32 ± 0.11 | 75.60 ± 1.11 |
| 2 | Hazelnut | 10.00 ± 0.12 | 4.00 ± 0.15 | 25 ± 0.41 | 22.098 ± 0.10 | 76.00 ± 1.13 |
| 3 | Almond | 10.00 ± 0.11 | 4.00 ± 0.10 | 25 ± 0.52 | 25.12 ± 0.15 | 78.21 ± 0.99 |
| 4 | Pumpkin | 10.00 ± 0.10 | 4.00 ± 0.10 | 25 ± 0.55 | 26.83 ± 0.13 | 77.673 ± 1.14 |
3.8. Investigation of the amino acid profile of SOM, HOM, AOM and POM
A review of the amino acid profiles of SOM, HOM, AOM and POM is shown in Table 4. The results in Table 4 were obtained by HPLC. The abundance of amino acids in SOM and its protein percentages are very high. Consequently, it contains protein with an amino acid profile of good nutritional value. Considering the lack of animal protein sources, SOM can be a suitable substitute for daily protein consumption.39,40| Amino acids | SOM | HOM | AOM | POM |
|---|---|---|---|---|
| Glu | 21.2 ± 0.11 | 16.60 ± 0.14 | 20.34 ± 0.12 | 14.643 ± 0.11 |
| Gly | 5.1 ± 0.13 | 3.09 ± 0.15 | 3.89 ± 0.15 | 3.86 ± 0.14 |
| His | 3.3 ± 0.15 | 1.02 ± 0.20 | 2.06 ± 0.14 | 1.88 ± 0.27 |
| Arg | 19.5 ± 0.11 | 10.30 ± 0.24 | 3.06 ± 0.11 | 12.11 ± 0.24 |
| Ala | 5.2 ± 0.12 | 4.30 ± 0.11 | 4.19 ± 0.16 | 4.5 ± 0.18 |
| Tyr | 2.9 ± 0.21 | 2.37 ± 0.16 | 2.58 ± 0.26 | 2.9 ± 0.19 |
| Pro | 3.1 ± 0.15 | 3.21 ± 0.18 | 7.35 ± 0.11 | 2.75 ± 0.11 |
| Val | 2.8 ± 0.23 | 3.42 ± 0.21 | 2.42 ± 0.14 | 4.6 ± 0.12 |
| Ile | 2.6 ± 0.18 | 2.95 ± 0.11 | 2.57 ± 0.19 | 2.08 ± 0.19 |
| Lys | 2.4 ± 0.14 | 2.45 ± 0.14 | 2.50 ± 0.21 | 3.15 ± 0.17 |
| Leu | 5.1 ± 0.12 | 5.97 ± 0.16 | 5.38 ± 0.14 | 6.59 ± 0.18 |
| Phe | 3.9 ± 0.19 | 4.08 ± 0.19 | 2.72 ± 0.15 | 2.38 ± 0.19 |
| Met | 2.6 ± 0.17 | 0.78 ± 0.16 | 0.64 ± 0.20 | 3.14 ± 0.23 |
| ASP | 5.87 ± 0.20 | 9.79 ± 0.21 | 12.54 ± 0.14 | 6.41 ± 0.13 |
| Ser | 4.9 ± 0.22 | 4.12 ± 0.23 | 3.6 ± 0.11 | 3.28 ± 0.24 |
| Thr | 6.8 ± 0.16 | 2.82 ± 0.17 | 1.72 ± 0.17 | 3.4 ± 0.14 |
| Cys | 0.9 ± 0.17 | 0.99 ± 0.15 | 0.65 ± 0.11 | 1.58 ± 0.16 |
The abundance of amino acids in HOM and its protein percentages are very high. The amino acid composition in hazelnut protein isolates is comparable to that of some animal proteins and is used as a suitable protein composition. According to Table 4, the HOM protein contains a high nutritional value. It can be considered an alternative and sustainable source for the production of vegetable protein, and owing to the growth of the world population, and ethical and environmental concerns, it can respond to the increasing demand for vegetable protein sources instead of animal protein.16–41
The abundance of amino acids in AOM and its protein percentages are very high. Owing to the appropriate protein percentage in almond protein isolates, it has a high nutritional value. According to Table 4, the production protein of AOM can be used to develop effective and environmentally friendly strategies for extracting the remaining oil and protein from AOM. It also leads to the production of high-quality protein and oil that can be used for food programs.42
The abundance of amino acids in the protein of POM and its protein percentage are very high. According to Table 4, the POM protein is a suitable source of vegetable protein and has a high nutritional value. Therefore, it can respond to the increasing demand for vegetable protein sources instead of animal protein.19,20
The oil extraction industry produces a lot of waste, and the disposal of these wastes is costly and problematic from economic and environmental perspectives. These wastes also have high nutritional and medicinal value, so they were used in various processes that led to the production of a product with high nutritional value, and the resulting production waste was minimized. The general use of oilseed meals means valuing their protein part in the production of value-added products for the food, cosmetic and pharmaceutical industries. Among the protein sources used in livestock and poultry diets, oilseed meals are very important. The production of protein isolates of seeds for breeding and the rest of the animal generation as an effective feed is of great importance to the eater. Changing lifestyles in developed and developing countries have affected the eating habits of consumers and encouraged the use of ready-made and low-volume foods, and public awareness about the benefits of eating plant-based protein products, such as low-calorie and fiber-containing products. There is an increasing trend of avoiding animal fats. Common sources of protein include red meat, chicken, eggs, dairy products and fish.43
The rapid growth of the population, the limitation of food sources and the increase in demand for new and cheap protein sources with favorable functional characteristics have directed the attention of scientists to plant protein sources, especially oilseeds, rice bran, alfalfa, peas, walnuts, etc. Research investments in agriculture and agri-food technology should be considered to develop the production of protein-rich seeds, valorize previously consumed plant protein fractions and develop a new generation of protein extracts from agricultural sources, such as oilseed meal, for human nutrition.11
Several studies have been conducted on protein extraction methods, and alkaline extraction and isoelectric point precipitation are the most common methods for preparing protein isolates in the food industry.9–11
4. Conclusion
In this study, the alkaline extraction method for the preparation of sesame, hazelnut, almond and pumpkin protein isolate was investigated. The amounts of protein in the protein isolates containing sesame, hazelnut, almond and pumpkin obtained using the ninhydrin method were 76.20, 79.05, 81.04, and 80.22, respectively. Additionally, to test the validity of the protein content in an oilseed meal, the Kjeldahl method was used. To check the amino acid profile of an oilseed meal containing sesame, hazelnut, almond, and pumpkin, the HPLC technique was used. Favorable results were obtained, which show that it contains an abundance of amino acids in the protein isolate of oilseeds and its protein percentage is very high. The investigated temperature of 25 °C was chosen because the highest percentage of efficiency and the highest percentage of amino acid were obtained. In this investigation, the presence of NaCl salt at a rate of 0.1% presented favorable results. As the salt concentration increases, protein surface charges interact with salt instead of interacting with water. Consequently, the hydrophobic parts of the protein are placed on the surface, causing the proteins to stick together and precipitate.Data availability
The data supporting this article have been included as part of the manuscript. For requests about the original data, please do not hesitate to contact the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the University of Kashan for supporting this work with Grant Number 463609.References
- T. Liu, Y. Zhao, N. Wu, S. Chen, M. Xu, H. Du and Y. Tu, et al., Egg white protein-based delivery system for bioactive substances: A review, Crit. Rev. Food Sci. Nutr., 2024, 64(3), 617–637 CrossRef PubMed.
- L. Aljerf, N. AlMasri and U. Prince, Statistical relationship between milk constituents used in breeding programs during lactation: French case study, Int. J. Case Rep. Stud, 2018, 2(2), 88–91 Search PubMed.
- M. R. Komeroski and V. R. D. Oliveira, Influence of the Amount and Type of Whey Protein on the Chemical, Technological, and Sensory Quality of Pasta and Bakery Products, Foods, 2023, 12(14), 2801 CrossRef CAS PubMed.
- L. Aljerf and N. Aljerf, Food products quality and nutrition in relation to public. Balancing health and disease, Prog. Nutr., 2023, 25(1), 10–23751 Search PubMed.
- Y. M. Abdushaeva, The problem of vegetable protein and its solutions, in IOP Conference Series: Earth and Environmental Science, IOP Publishing, 2020, vol. 613, p. 012003 Search PubMed.
- A. S. Chandran, S. Suri and P. Choudhary, Sustainable plant protein: an up-to-date overview of sources, extraction techniques and utilization, Sustainable Food Technol., 2023, 1(4), 466–483 RSC.
- A. S. Chandran, S. Suri and P. Choudhary, Sustainable plant protein: an up-to-date overview of sources, extraction techniques and utilization, Sustainable Food Technol., 2023, 1(4), 466–483 RSC.
- T. Y. Negawoldes, Review on nutritional limitations and opportunities of using rapeseed meal and other rape seed by-products in animal feeding, J Nutr Health Food Eng, 2018, 8(1), 43–48 Search PubMed.
- F. Rodríguez-Félix, A. Z. Graciano-Verdugo, M. J. Moreno-Vásquez, I. Lagarda-Díaz, C. G. Barreras-Urbina, L. Armenta-Villegas and J. A. Tapia-Hernández, et al., Trends in Sustainable Green Synthesis of Silver Nanoparticles Using Agri-Food Waste Extracts and Their Applications in Health, J. Nanomater., 2022, 2022(1), 8874003 CrossRef.
- S. Langyan, P. Yadava, F. N. Khan, Z. A. Dar, R. Singh and A. Kumar, Sustaining protein nutrition through plant-based foods, Front. Nutr., 2022, 8, 772573 CrossRef PubMed.
- D. Lazdiņa, D. Segliņa, Z. A. Zvaigzne, A. Butlers and I. Ciproviča, Effect of Defatting Method on Japanese Quince (Chaenomeles japonica) Fruit Seed Protein Isolate Technological Properties, Foods, 2025, 14(2), 234 CrossRef PubMed.
- M. Kumar, M. Tomar, J. Potkule, R. Verma, S. Punia, A. Mahapatra and J. F. Kennedy, et al., Advances in the plant protein extraction: Mechanism and recommendations, Food Hydrocoll., 2021, 115, 106595 CrossRef CAS.
- D. Kadam and S. S. Lele, Value addition of oilseed meal: a focus on bioactive peptides, J. Food Meas. Charact., 2018, 12, 449–458 CrossRef.
- C. Nouska, M. Deligeorgaki, C. Kyrkou, A. M. Michaelidou, T. Moschakis, C. G. Biliaderis and A. Lazaridou, Structural and physicochemical properties of sesame cake protein isolates obtained by different extraction methods, Food Hydrocoll., 2024, 151, 109757 CrossRef CAS.
- Y. Mustafa and G. Hüseyin, Sesame seed protein: Amino acid, functional, and physicochemical profiles, Foods Raw Mater., 2023, 11(1), 72–83 Search PubMed.
- N. J. E. Gheise, A. Riasi, A. Z. Shahneh, V. Naseri, F. Kafilzadeh, A. Bolandnazar and F. Ahmadi, Comparative evaluation of nutrient composition, in vitro nutritional value, and antioxidant activity of de-oiled meals from walnut, hazelnut, almond, and sesame, Trop. Anim. Health Prod., 2023, 55(5), 333 CrossRef CAS PubMed.
- D. Sen and D. Kahveci, Production of a protein concentrate from hazelnut meal obtained as a hazelnut oil industry by-product and its application in a functional beverage, Waste Biomass Valorization, 2020, 11, 5099–5107 CrossRef CAS.
- Q. Yang, W. Qi, Y. Shao, X. Zhang, F. Wu and Z. Zhang, Stability and pH-Dependent Mechanism of Astaxanthin-Loaded Nanoemulsions Stabilized by Almond Protein Isolate, Foods, 2024, 13(24), 4067 CrossRef CAS PubMed.
- S. Vinayashree and P. Vasu, Biochemical, nutritional and functional properties of protein isolate and fractions from pumpkin (Cucurbita moschata var. Kashi Harit) seeds, Food Chem., 2021, 340, 128177 CrossRef CAS PubMed.
- D. Sert, H. Rohm and S. Struck, High-pressure-assisted protein isolation from pumpkin seed press cake, Int. J. Food Sci. Technol., 2024, 59(1), 368–379 CrossRef CAS.
- G. S. Olasunkanmi, F. T. Omolayo and O. T. Olusegun, Fatty acid profile, physico-chemical and functional properties of oil and protein isolate simultaneously extracted from sesame (Sesamum indicum) seed, Ann.:Food Sci. Technol., 2017, 18(1), 1–10 CAS.
- D. Sen and D. Kahveci, Production of a protein concentrate from hazelnut meal obtained as a hazelnut oil industry by-product and its application in a functional beverage, Waste Biomass Valorization, 2020, 11, 5099–5107 CrossRef CAS.
- A. G. A. Sá, M. T. B. Pacheco, Y. M. F. Moreno and B. A. M. Carciofi, Processing effects on the protein quality and functional properties of cold-pressed pumpkin seed meal, Food Res. Int., 2023, 169, 112876 CrossRef PubMed.
- M. Kumar, M. Tomar, J. Potkule, R. Verma, S. Punia, A. Mahapatra and J. F. Kennedy, et al., Advances in the plant protein extraction: Mechanism and recommendations, Food Hydrocoll., 2021, 115, 106595 CrossRef CAS.
- P. Sáez-Plaza, T. Michałowski, M. J. Navas, A. G. Asuero and S. Wybraniec, An overview of the Kjeldahl method of nitrogen determination. Part I. Early history, chemistry of the procedure, and titrimetric finish, Crit. Rev. Anal. Chem., 2013, 43(4), 178–223 CrossRef.
- W. Xu, C. Zhong, C. Zou, B. Wang and N. Zhang, Analytical methods for amino acid determination in organisms, Amino Acids, 2020, 52(8), 1071–1088 CrossRef CAS PubMed.
- H. Bouzid, C. M. Filho, J. R. C. Marques, A. J. Valente and L. M. Gando-Ferreira, A New Approach on the Amino Acid Lysine Quantification by UV-Visible Spectrophotometry, Rev. Fiz. Chim., 2020, 71(8), 159–175 CrossRef CAS.
- N. Thiex, Evaluation of analytical methods for the determination of moisture, crude protein, crude fat, and crude fiber in distillers dried grains with solubles, J. AOAC Int., 2009, 92(1), 61–73 CrossRef CAS PubMed.
- S. Singharaj and E. Onsaard, Production and characteristic of sesame proteins, J. Food Sci. Agric. Technol., 2015, 1, 188–192 Search PubMed.
- M. A. López-Bascón and M. L. De Castro, Soxhlet extraction, in Liquid-Phase Extraction, Elsevier, 2020, pp. 327–354 Search PubMed.
- M. T. Hearn, High–Performance Liquid Chromatography and Its Application to Protein Chemistry, in Advances in Chromatography, 2021, pp. 1–82 Search PubMed.
- C. Xiao, J. Xiao, Y. Wu, J. Pang, F. Chen, W. Zhang and D. Xu, Determination of Amino Acids of Novel Food in Food by HPLC Coupled with Pre-Column Derivatization, Foods, 2024, 13(24), 4012 CrossRef CAS PubMed.
- N. Thiex, Evaluation of analytical methods for the determination of moisture, crude protein, crude fat, and crude fiber in distillers dried grains with solubles, J. AOAC Int., 2009, 92(1), 61–73 CrossRef CAS PubMed.
- S. W. Sun, Y. C. Lin, Y. M. Weng and M. J. Chen, Efficiency improvements on ninhydrin method for amino acid quantification, J. Food Compos. Anal., 2006, 19(2–3), 112–117 CrossRef CAS.
- Z. Zhang, S. He, H. Liu, X. Sun, Y. Ye, X. Cao and H. Sun, et al., Effect of pH regulation on the components and functional properties of proteins isolated from cold-pressed rapeseed meal through alkaline extraction and acid precipitation, Food Chem., 2020, 327, 126998 CrossRef CAS PubMed.
- H. Khazaei, M. Subedi, M. Nickerson, C. Martínez-Villaluenga, J. Frias and A. Vandenberg, Seed protein of lentils: Current status, progress, and food applications, Foods, 2019, 8(9), 391 CrossRef CAS PubMed.
- R. Kaur, G. Ghoshal and S. Chauhan, Optimizing conditions for protein isolation from de-oiled sunflower meal using response surface methodology (RSM), J. Food Meas. Charact., 2024, 18(5), 3708–3719 CrossRef.
- A. G. A. Sá, D. C. da Silva, M. T. B. Pacheco, Y. M. F. Moreno and B. A. M. Carciofi, Oilseed by-products as plant-based protein sources: Amino acid profile and digestibility, Future Foods, 2021, 3, 100023 CrossRef.
- D. Melo, M. Álvarez-Ortí, M. A. Nunes, A. S. Costa, S. Machado, R. C. Alves and M. B. P. Oliveira, et al., Whole or defatted sesame seeds (Sesamum indicum L.)? The effect of cold pressing on oil and cake quality, Foods, 2021, 10(9), 2108 CrossRef CAS PubMed.
- B. G. Acan, O. S. Toker, I. Palabiyik, H. R. Pirouzian, K. Bursa, M. Kilicli and N. Konar, et al., Physicochemical properties of chocolate spread with hazelnut cake: Comparative study and optimization, LWT, 2021, 147, 111548 CrossRef CAS.
- T. P. Sari, A. H. Dhamane, K. Pawar, M. Bajaj, P. C. Badgujar, A. Tarafdar and S. Pareek, et al., High-pressure microfluidisation positively impacts structural properties and improves functional characteristics of almond proteins obtained from almond meal, Food Chem., 2024, 448, 139084 CrossRef CAS PubMed.
- R. Singh, S. Langyan, S. Sangwan, B. Rohtagi, A. Khandelwal and M. Shrivastava, Protein for human consumption from oilseed cakes: a review, Front. Sustain. Food Syst., 2022, 6, 856401 CrossRef.
- F. Ji, J. Xu, Y. Ouyang, D. Mu, X. Li, S. Luo and Z. Zheng, et al., Effects of NaCl concentration and temperature on fibrillation, structure, and functional properties of soy protein isolate fibril dispersions, LWT, 2021, 149, 111862 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |