DOI:
10.1039/D5RA00288E
(Review Article)
RSC Adv., 2025,
15, 20239-20267
A review of commercial plastic waste recycling into graphene materials†
Received
12th January 2025
, Accepted 15th May 2025
First published on 16th June 2025
Abstract
Since their discovery and application in human life, plastic has become the most popular materials on the planet, with applications in almost all fields. The fast growth of the world population and the remarkable expansion of the worldwide economy, along with increased global productivity, are the primary causes of the overproduction of plastic materials. Plastic waste poses a growing hazard to human life by contaminating the environment, particularly water and soil, which in turn leads to serious diseases and endangers human life. Thus, while discussing waste recycling in general, the topic of plastic waste recycling is always given priority. To maximize recycling, various ideas and discussions have been put forth over the years for turning plastic waste into other materials, such as carbonaceous materials, particularly graphene. Some top-down methods such as pyrolysis and flash Joule heating provide high conversion efficiencies of up to 70% and 90%, respectively, but require large energy supplies to reach extremely high temperatures from 600 °C to 3000 °C. In contrast, typical bottom-up methods such as chemical vapor deposition and microwave plasma provide remarkable efficiencies of up to 50% under specific conditions of inert gas environments. Thus, this review introduces some of the groundbreaking methods reported to date for recycling plastic waste into one of the materials of the century—graphene.
1. Introduction
Plastic plays a crucial role in daily activities and contemporary manufacturing processes. Plastic is everywhere—from small household items to large components such as machinery and equipment used in industry and construction. Plastic has enabled convenient consumer products that consume limited natural resources. Plastic is a great material and a driving force for economic growth and the modernization of industry and agriculture. However, a production output of more than 400 million tons per year and more than 300 million tons per year of plastic waste discharged into the environment are extremely alarming numbers for the current state of plastic waste.1–4 The risk is increasing as the annual plastic output has doubled in the past 20 years to 460 million tons and is on track to triple in four decades.3,4 An estimated 66% of plastic produced each year ends up in the environment after single or more uses, while less than 10% is recycled. In nature, plastic has been found in Arctic ice and microplastics in fish living in the deepest parts of the ocean.5,6 In humans, microplastics have been detected in the blood, breast milk and placentas.7–9 This is an actual worldwide issue that has been a burden for all nations for many years.
With the current trend of using plastic products, the amount of plastic produced in the coming decades will increase rapidly, considering the endless needs of life (Fig. 1).10 Fig. 1a illustrates the 10 specific regions of plastic consumption. All areas are expected to see a rise in plastic consumption. Especially, China and other Asian countries are expected to use the most plastic, with China accounting for the highest amount, approximately 235 million tons per year in 2050. India is expected to have the greatest rate of plastic consumption growth until 2050, with a 3.4-fold increase from 2020. This is a result of improving GDP and population growth, urbanization development, economic conditions, industrialization level, and inflation index.10
 |
| | Fig. 1 Forecasts from 1990 to 2050 of (a) ten region-specific plastic consumption, (b) plastic use by polymer type, and (c) application-based plastic usage over the decades. Figure has been reproduced with permission from ref. 10. Copyright 2024, Elsevier. | |
Fig. 1b illustrates various plastic types for diverse applications in many sectors over the next seven decades from now until 2050. Polyvinyl polypropylene (PP) and polyvinyl chloride (PVC) are the two most commonly used plastic materials, suggesting the emergence of a focus on recycling. Fig. 1c shows the application-based plastic usage over the decades between 1990 and 2050. The demand for plastic products in all sectors has been increasing every decade, and the total level in 2050 is predicted to be more than four times that of 1990. This is truly alarming in the context of the current urgent global climate and pollution issues.
1.1 Negative environmental impacts of plastic waste
Fig. 2 shows the pathways of the origins, modes of transit, and interactions among ecosystems of microplastics.11 Through this, we can see how dangerous microplastic waste is to the environment. To better visualize the dangers of plastic waste in the world, we must mention the harm it causes to the environment, specifically:12–16 (i) plastic products contain many toxic substances that are harmful to human health. Most disposable plastic products, when exposed to high temperatures, are at risk of releasing toxic substances into the product itself, such as heavy metals and microplastic particles that can cause a high risk of cancer; (ii) plastic waste when buried will seriously pollute groundwater and the risk of microplastic particles remaining in the water is very high. In addition, buried plastic waste causes serious soil pollution, making it almost impossible to cultivate or grow crops. Furthermore, when people use domestic water contaminated with plastic, they are at risk of many dangerous diseases, such as the digestive tract, cancer, etc.; (ii) plastic waste, when burned, will produce emissions containing styrene gas, dioxins, and chlorinated furans, which are extremely toxic to human health. It can cause us to have reduced immunity and digestive dysfunction and even lead to a high risk of cancer if exposed regularly; (iv) plastic waste when dumped into the sea will seriously affect the marine environment, affect the life of marine life, and destroy the ecological balance of the sea. More dangerously, recent studies have shown that tiny plastic particles exist in the bodies of marine life.
 |
| | Fig. 2 Diagram showing the origins and routes of transportation for waste plastics. Figure has been reproduced with permission from ref. 11. Copyright 2022, Royal Society of Chemistry. | |
1.2 Negative human impact of plastic waste
Plastic waste impacts the human body through various pathways, including its chemical and physical properties, many of which are not immediately visible. When plastic waste breaks down, it releases small micro and nanopieces, which can enter the body through our daily consumption routes, especially drinking water and food.17 Moreover, microplastic waste also contains many harmful chemicals that penetrate the body, causing difficult-to-detect complications and long-term diseases.18,19 Oral intake is the most common and direct pathway through which micro- and nano-plastic particles enter the human body through daily eating and drinking activities. From water bottles and plastic food containers to plastic nanoparticles found in agricultural products and livestock meat, these are the most direct and fastest sources of penetration.20–22 Microplastics from polymers that are difficult to decompose, such as polyethylene (PE), polystyrene (PS), and polyethylene oxide (PEO), have dangerous effects on the body, from external influences (causing allergies and skin irritation) to internal organs such as the lungs, stomach, liver, and kidneys, and even plastic nanoparticles can penetrate the blood and reach the cellular level (Fig. 3).23 Therefore, to limit the entry of microplastics into the human body, consumers should avoid using foods and drinks packaged in disposable plastic bags, nylon bags, or reheated in plastic containers.
 |
| | Fig. 3 Risk factors and routes of human exposure to microplastics. Figure has been reproduced with permission from ref. 23. Copyright 2022, Royal Society of Chemistry. | |
1.3 Importance of processing plastic waste and deriving it into carbon materials
Recycling plastic waste is essential for mitigating environmental pollution, conserving resources, and promoting a circular economy. Traditional disposal methods, such as landfilling and incineration, lead to greenhouse gas emissions, microplastic pollution, and ecosystem damage, making advanced recycling technologies more urgent. Transforming plastic waste into high-value carbon materials like carbon materials (graphene, carbon nanotubes, and activated carbon) offers a sustainable and economical solution. These materials have excellent electrical, thermal, and mechanical properties, enabling applications in energy storage, electronics, biology, environmental and wastewater treatment. Compared with traditional carbon materials, plastic waste-derived carbon materials support the development of sustainable materials and green technologies.
Among the various plastic waste-derived carbon materials, graphene is a highly efficient and sustainable solution for addressing global plastic pollution. Compared with other carbon materials derived from plastic, including carbon nanotubes and activated carbon, graphene exhibits superior electrical conductivity, high surface area, and exceptional thermal conductivity, making it the most promising candidate for use in the field. Beyond its technical advantages, graphene upcycling reduces environmental pollution and prevents non-recyclable plastics from accumulating in landfills. Integrating graphene recycling into a circular economy can reduce fossil fuel dependency, lower carbon emissions, and create a sustainable market for advanced carbon materials. Overcoming scalability challenges and improving waste collection systems will be key to making plastic-derived graphene a mainstream solution for the future of clean energy and sustainable materials. In this context, the purpose of this review is to provide readers with an improved comprehension of the methods being investigated and developed to recycle plastic waste into graphene materials.
2. Introduction of commercial plastic products
2.1 Seven popular plastic types
Plastics can be categorized according to several characteristics, including their origin, structure, molecular forces, manner of polymerization, behavior in response to temperature changes, and methods of preservation (Fig. 4).24 Plastics are classified into three main types: natural, semi-synthetic, and synthetic polymers, depending on their origin.24–28 Natural plastics are made from natural materials like starch, chitin, and lignin, which are natural polymers that have been around since the ancient era.26 Petrochemicals such as silicone, nylon, polyethylene, and polystyrene are the source of synthetic polymers.27 Natural polymeric materials, such as plastics based on cellulose, can be chemically altered to create semi-synthetic materials.28
 |
| | Fig. 4 Classification of plastic by (a) polymer classification, (b) physical chemistry and applications, and (c) material origins and biodegradability. Figure has been reproduced with permission from ref. 24. Copyright 2024, Elsevier. | |
Fig. 5 shows the seven popular plastic product types based on the standard number, which illustrates the unique classification of plastics based on physio-chemical qualities and uses, material source and biodegradability, lifespan, and international standards.24 The plastic object often has a triangle sign at the bottom with a matching number within. This symbol, which corresponds to digits from 1 to 7, is known as the plastic identification code published by the International Organization for Standardization (ASTM).29,30 Plastics are separated into two categories based on safety: safe and hazardous plastics. Plastic no. 1 is polyethylene terephthalate (PET), which is colorless, transparent, and lightweight. PET is often used to make beverage and food containers.31 This type of plastic should only be used once, not repeatedly, and should not be used to store hot food at high temperatures because of its low melting point (260 °C) and deformation at temperatures above 70 °C.31,32 High-density polyethylene (HDPE), a plastic derived from petroleum, is the no. 2 of the commercial plastic list. This polymer is a multipurpose plastic that finds employment in a wide range of applications, from the industry sector (piping, roofing, bottles) to household items (lids, liquid jugs, packaging), and playground equipment.33 HDPE has enhanced tensile strength and backbone intermolecular stresses because of its minimal branching and linear structure. HDPE's tensile strength and intermolecular forces are enhanced by its linear structure with minimal branching. Moreover, HDPE is easily recycled and provides excellent dimensional stability, durability, and resistance to weather and corrosion.34 Number 3 is polyvinyl chloride (PVC), a thermoplastic polymer that is extremely strong, flexible, and adaptable. PVC is frequently used in insulation, medical equipment, ducts, pipelines, and other applications. PVC is ranked as the third most produced or synthesized plastic substance. PVC is a brittle solid that is white and granular or powdered. It is frequently utilized in the home and building sectors as a cost-effective solution (door, window, water pipe, non-food packaging).35 PVC has been effectively used for a long time because of its low cost, high mechanical qualities, ease of processing, and lightweight nature. PVC is classified as a hazardous material even though it is widely used. Toxic chemicals like BPA, phthalates, lead, dioxins, mercury, and cadmium are used in the manufacture of PVC.36 Most notably, bisphenol A, which, when burned, has the potential to change human sex hormones or cause cancer.37,38 The no. 4 on the list is low-density polyethylene (LDPE), which is also a polyethylene product. Because its molecules have a lower density than HDPE, LDPE resin is more flexible and thinner. Consequently, compared to HDPE, this form of plastic is more elastic but has less hardness and impact resistance. Due to its most straightforward structure among all plastics, it is inexpensive and simple to manufacture, which makes it a popular material for use in packaging, waste bins, and liquid containers—plastic wraps being the most famous application.39 Apart from its benefits, it is a very combustible organic substance. Additionally, LDPE's gas permeability ratings fall short of expectations. They have very little resistance to UV light, making them prone to stress cracking. Their uneven main chain structure and large amorphous area give them less density and strength than HDPE. Polypropylene (PP), also referred to as polypropene, is no. 5 in the group. This is a product of the polymerization reaction from propylene monomers to form thermoplastic and finds usage in different fields. Although PP plastic is strong, flexible, and particularly heat-resistant, it is unable to eliminate chemicals. Currently, PP is a material that is frequently used to make plastic bottles and other products that hold liquids or heated food, especially microwave food containers.40 Experts advise using PP because of its chemical inertness, great mechanical strength, and good heat resistance. However, many studies have shown that PP food containers can release microplastics into food when microwaved.41–44 The no. 6 plastic on the list is polystyrene (PS), which is obtained in solid and foamed forms after the polymerization of aromatic hydrocarbon styrene monomers. In contrast to the other groups mentioned above, PS has poor physical characteristics, including being brittle and tearable, having a low melting point, and having a weak barrier to air and water vapor.45,46 Despite this, PS is one of the most extensively used plastics, and due to its extremely low cost of manufacture, it is produced at a scale of several million metric tons annually.41 However, styrene, a substance thought to be hazardous to the brain and neurological system, may have leached from PS when exposed to high temperatures.47–49 Genes, the liver, lungs, and immune system may be impacted. With such characteristics, PS is not recommended for repeated reuse and is currently not recommended for use in beverage and food containers. Number 7 is the symbol for a set of several types of polymers that are widely used today; some notable names include polycarbonate (PC), polymethyl methacrylate (PMMA), polylactic acid (PLA), Nylons, and Acrylonitrile Butadiene Styrene (ABS). Polycarbonates are the most well-known polymers in this category; they are used to make durable, robust items. Polycarbonates are frequently utilized in households, sports, electronic devices and especially compact discs.50 Polymethyl methacrylate, or acrylic, is another material in this category no. 7 that is well-known for its use as an extremely resilient transparent roofing sheet that may take the place of glass because of its heat capacity, mechanical strength and good optical properties.51 PMMA is included in the category of substitute thermoplastic materials. PMMA is utilized in aircraft canopies, skylights, broken windows, lit signage, and translucent roofing sheets.51,52 Within this category, an interesting biodegradable plastic is polylactic acid (PLA), a thermoplastic monomer sourced from organic renewable resources like sugarcane or corn starch. The process of producing PLA differs from that of conventional plastics in that it uses biomass sources instead of fossil fuels, namely petroleum distillation and polymerization. The same machinery used to create petrochemical plastics may also be used to produce PLA, making the production procedures for PLA extremely affordable despite the variations in precursors. PLA finds commercial use in a variety of products, such as biodegradable films, food and liquid containers.53,54 PLA also has a broader range of uses in biomedical engineering.55–59 One famous member in group no. 7 is Nylons. Nylons are a group of plastics that belong to the polyamide family. Nylons have a wide variety of material types, such as Nylon 6,6; Nylon 6,12; Nylon 4,6; Nylon 6; and Nylon 12, which are distinguished by their amide groups (CONH) and offer an unprecedented breadth of possible qualities.60–65 To create items with exact shapes, they may also be melted and remolded. Nylon can be easily processed into films, fibers, and various mold forms because of its adaptable structure. Nylon can be easily processed into films, fibers, and various mold forms because of its adaptable structure. Nylons are durable materials with strong heat and chemical resistance; most have a semi-crystalline structure. There is also a typical thermoplastic that needs to be mentioned in group no. 7, namely acrylonitrile butadiene styrene (ABS). This is an amorphous polymer formed after the polymerization process of 3 different monomers: acrylonitrile, butadiene, and styrene.66–68 ABS is a favored material for structural applications because of its good physical properties, such as high stiffness, resistance to impact, abrasion, and strain.68 ABS is a preferred material for the production of electrical housings, vehicle components, consumer goods, pipe fittings, and toys due to its comparatively low manufacturing cost.69–73
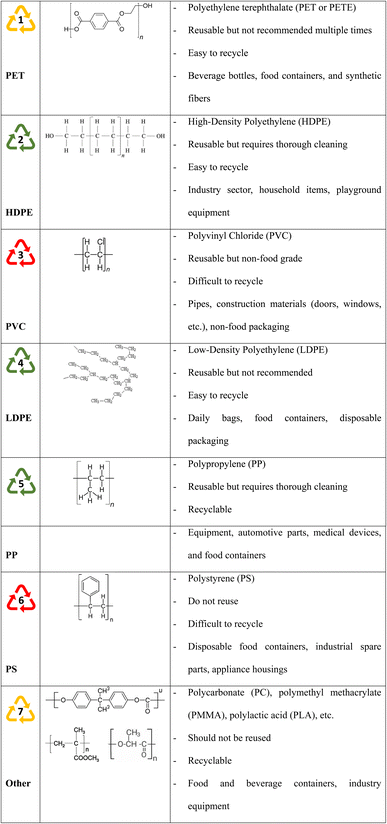 |
| | Fig. 5 Classification table of the seven most popular plastic types today and some general information. The green color represents types that are easy to recycle, the yellow color represents types that can be recycled, and the red color represents types that are very difficult to recycle. | |
2.2 Current progress of plastic waste recycling technology
Currently, there are two major technical strategies for plastic waste recycling: mechanical recycling and chemical recycling.74–76 Each has various advantages and limitations that are suitable for specific purposes, requirements of technology and input plastic waste conditions. Table 1 presents the key advantages and limitations of the two major plastic waste recycling technologies.
Table 1 Advantages and limitations of the two major plastic waste recycling technologies
| Methods |
Advantages |
Disadvantages |
| Mechanical technology |
- Use fewer resources and equipment, recycling facilities are straightforward and cost-effective |
- The quality of the output material is degraded and contaminated |
| - Suitable for plastic waste in contaminated form and composite with metals through cutting and grinding process |
- The output products depend on the quality of input plastic waste |
| - Currently being commercialized on a large-scale |
- Recycled products cannot be infinitely recyclable |
| - Does not involve toxic chemicals |
- Not suitable for all plastic waste types |
| Chemical technology |
- High quality output monomer and oligomer products |
- The systems and techniques are complicated and expensive |
| - This technology can recycle hard and expired plastics |
- The synthesis processes require specific temperatures and high energy consumption |
| - This technology can be recycled multiple times |
- Solvent requirements and possible negative environmental effects |
| - It is not yet determined if industrial scale is feasible |
Mechanical recycling is used in almost all plastic recycling facilities, and typical processes such as cutting, grinding, washing, flaking and pelletizing.77 The final product obtained can be an input material for the reuse process or can also be an input material for chemical recycling technology. This method currently provides the highest amount of recycled plastic materials and is widely used in most plastic recycling plants in the world, from small to large scales. This mechanical recycling technology provides a comprehensive solution to the problem of processing and classifying plastic waste before processing at higher and more complex levels, such as chemical recycling. For example, Fig. 6a and 6b show a general flow diagram of the recycling process of PET bottles and polyethylene (PE) plastic bags on an industrial scale.78–80
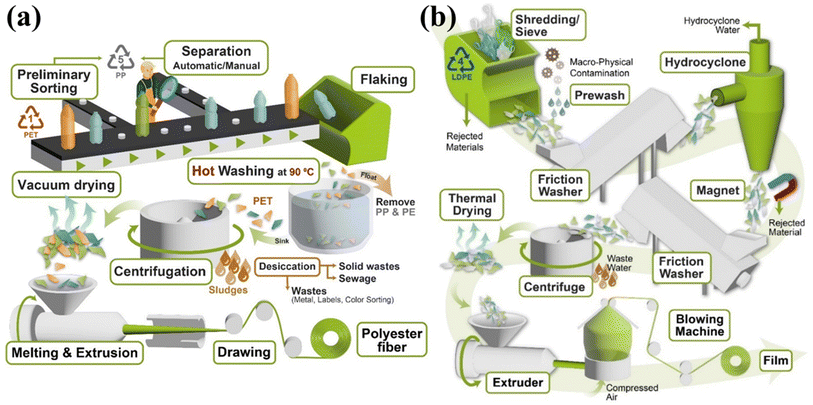 |
| | Fig. 6 Mechanical recycling general process of (a) PET bottles and (b) PE bags. Figure has been reproduced with permission from ref. 78. Copyright 2022, Royal Society of Chemistry. | |
Chemical recycling is a method that affects the molecular structure of materials using chemical reactions under certain conditions of temperature, pressure and catalysts to transform plastics into their original monomers and some other products such as hydrogen, oxygen, carbon monoxide, carbon dioxide, and methane.81 This method is not only able to recycle out-of-date plastic without a classified process but also provides a strategy to convert plastic waste into high-quality original monomers. Some typical methods of this technique include chemolysis, pyrolysis, gasification, bioconversion, and catalysis.78,79,81 However, current plastic recycling methods also face the problem of greenhouse gas emissions, which contribute to global warming and air pollution in the recycling area. Therefore, various emerging new methods have been developed to improve and overcome the limitations of traditional chemical methods. Fig. 7a shows traditional chemical recycling with some limitations related to environmental issues, and Fig. 7b shows recent advanced catalyst technologies under mild conditions, including enzymatic catalysts, photocatalysts, electrocatalysts, and low-temperature catalysts.82 Recently, catalysts have emerged as promising candidates for advanced technology that can not only stand independently but can also incorporate other traditional technologies, such as pyrolysis. Various catalyst methods are shown in Fig. 8a, and they can transfer the high-values output chemical products.83 Catalyst strategies are rising as one of the important technologies because they are green, efficient, and low-cost, and they provide many benefits, such as increased process efficiency and selectivity and reduced harsh reaction conditions. Moreover, catalyst processes help improve the thermal decomposition process, thereby minimizing the negative environmental impacts and creating sustainable fuels and chemicals from plastics. For example, Fig. 8b shows a schematic of the electroreforming of PET bottles into terephthalate and carbonate using palladium (Pd) modified nickel (Ni) foam anode and a nickel foam cathode.84 Fig. 8c illustrates the MoS2/g-C3N4 photocatalyst that efficiently upcycles poly(ethylene terephthalate) (PET) into valuable organic chemicals.85 Fig. 8d shows how CO2 works as a metal-free catalyst (Fig. 8e) to produce usable organic materials through alcoholysis in a chemical recycling process for common waste plastics of polyester and polycarbonate.86 Fig. 8e shows that the oxygen atom in CO2 serves as a Lewis base, activating alcohols, whereas the carbon atom in CO2 works as a Lewis acid, activating carbonyl groups in polyesters.86
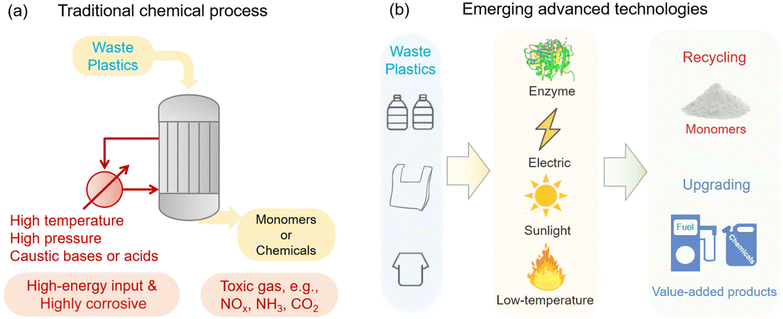 |
| | Fig. 7 Necessary transformation from (a) traditional chemical recycling of plastic waste into (b) emerging advanced technologies. Figure has been reproduced with permission from ref. 82. Copyright 2023, Royal Society of Chemistry. | |
 |
| | Fig. 8 (a) Concept of using catalytic technology to recover resources from plastic waste.83 (b) Diagram showing how PET is electroreformed into high-value compounds and H2 fuel.84 (c) Schematic of PET waste upcycling using the MoS2/g-C3N4 photocatalyst.85 (d) Depolymerization and recycling of waste polyesters and polycarbonates accelerated by CO2 and (e) MeOH and polyester are activated by CO2 as a Lewis acid–base pair.86 Figure (a) has been reproduced with permission from ref. 83, copyright 2023 Royal Society of Chemistry. (b) Represents with permission from ref. 84, copyright 2021, Royal Society of Chemistry. Figure (c) has been reproduced with permission from ref. 85, copyright 2023 American Chemical Society. | |
3. Preparation of graphene from plastic waste
Plastic pollution is a global problem for the plastic industry. Many studies have demonstrated the pollution and penetration of microplastics into soil, water, and biological bodies, including humans. However, plastic is also an important manufacturing and supporting industry with great potential because plastic products are used in all areas of life. In this context, the transition from a traditional economic model to a circular economic model is an approach towards sustainable development goals, which has been responded to and implemented by governments of many countries. The circular economy focuses on the reuse of raw materials, reducing raw material consumption, improving production efficiency, and minimizing environmental impacts. Therefore, plastic recycling has become a prerequisite for every country. In that trend, the idea of converting plastic into carbon materials has received great attention and focused research because of its feasibility and great applications if applied on an industrial scale. This idea, if put into industrial production, will solve a huge amount of plastic waste in the context of burying and burning plastic waste, which poses too many risks of poisoning water sources, soil, and organisms.
Among the various members of the carbon family that can be synthesized from plastic waste, graphene is the most interesting candidate because of its current large application scale. Graphene is a famous member of the carbon family with many outstanding advantages, including high stiffness, high mechanical strength, good electrical and thermal conductivity, and it is more flexible and stronger than steel.87–89 These properties make graphene an ideal material for many applications in fields such as electronics, composite materials, energy, and medicine.90–93
In general, plastic waste-derived graphene materials can be classified by two approaches: (i) top-down and (ii) bottom-up. Each approach has its advantages and disadvantages, with certain input conditions and different potential for industrial scale-up.
3.1 Top-down approach to preparing graphene material
The top-down trend of synthesizing graphene materials from plastic waste always includes two indispensable steps: cutting and grinding, which provide homogenous input materials for further steps. Some typical top-down methods can be listed as follows: pyrolysis, flash-joule heating (FJH), and electrocatalysis.
3.1.1 Pyrolysis method. The pyrolysis method involves several steps and normally incorporates catalyst chemicals to increase the efficiency of converting polymer materials into carbon form. Mechanical processing steps such as cleaning, chopping, and grinding are performed to create uniform input materials. The next steps could be to perform the pyrolysis process directly or blend with a catalyst to increase the conversion efficiency during the pyrolysis process or provide catalyst conditions during the pyrolysis process to create an output graphene product that meets the manufacturer's requirements. Some early researchers used plastic waste and mixed it directly with clay and montmorillonite minerals for the pyrolysis process at high temperatures to obtain residues. These residues were treated by soaking and washing in strong acids to remove the unconverted residual materials to obtain multilayer graphene.94–96 Recently, S. Pandey et al. reported an interesting strategy for preparing 3D graphene nanosheets for perovskite solar cell application by mixing plastic waste with pure nickel as a catalytic and degradation template.97 After two pyrolysis steps followed by washing and drying, graphene sheets were obtained as electrodes for the perovskite-structured solar cells. Numerous intriguing studies have enhanced the pyrolysis process for energy applications based on graphene obtained from plastic waste using various catalyst chemicals and creating innovative synthesis procedures. M. Karakoti and coworkers introduced their research on the preparation of graphene nanosheets using a ZnO catalyst for supercapacitor applications (Fig. 9).98
 |
| | Fig. 9 Process used to prepare graphene nanosheets from plastic waste. Figure has been reproduced with permission from ref. 98. Copyright 2022, Royal Society of Chemistry. | |
In this research, the shredded plastics were directly mixed with ZnO powder in a primary pyrolysis chamber via two steps: pyrolysis at 400 °C for 2 h and 920 °C for 3 h in an N2 atmosphere. The output graphene product showed good quality with a thin layer and uniform size, which was suitable for electrode fabrication in a symmetric supercapacitor using 1 M H3PO4 liquid electrolyte with a graphene nanosheet/H3PO4/graphene nanosheets sandwich structure. S. Dhali et al. introduced an interesting strategy for obtaining graphene nanosheets via two continuous pyrolysis steps (Fig. 10). Then, they prepared waste plastic graphene oxide (WpGO) by modified Hummers' method, and thermal annealing following the polyol process to synthesize WpNrGO-Pd–Ru (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) metal catalyst for fuel cell studies.99 Recently, K. K. Garg et al. reported two continuous pyrolysis steps at 400 °C and 850 °C under an N2 atmosphere, respectively, to produce graphene nanosheets by mixing mixed plastic with Al2O3 as a catalyst factor (Fig. 11).100 They discovered that the existence of edge defects caused the graphene nanosheets to thicken by 3–4 nm. Semiconducting characteristics were achieved in the produced graphene nanosheets due to the existence of edge defects inside the sheets.100
1) metal catalyst for fuel cell studies.99 Recently, K. K. Garg et al. reported two continuous pyrolysis steps at 400 °C and 850 °C under an N2 atmosphere, respectively, to produce graphene nanosheets by mixing mixed plastic with Al2O3 as a catalyst factor (Fig. 11).100 They discovered that the existence of edge defects caused the graphene nanosheets to thicken by 3–4 nm. Semiconducting characteristics were achieved in the produced graphene nanosheets due to the existence of edge defects inside the sheets.100
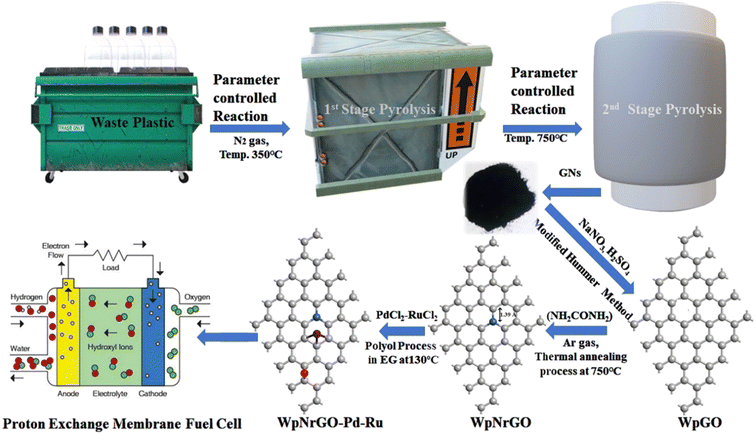 |
| | Fig. 10 Process to synthesize nitrogen-doped reduced graphene oxide from plastic waste and modify it to obtain nitrogen-doped reduced graphene oxide decorated core–shell nano-structured metal for proton exchange membrane fuel cells. Figure has been reproduced with permission from ref. 99. Copyright 2024, Royal Society of Chemistry. | |
 |
| | Fig. 11 Process diagram of the preparation of graphene nanosheets from plastic waste for thermoelectric applications. Figure has been reproduced with permission from ref. 100. Copyright 2022, Elsevier. | |
The process of pyrolysis involves the heat breakdown of many feedstocks without the presence of oxygen or air. The synergistic effects of various feedstocks provide pyrolysis with several advantages over solo pyrolysis. This includes the capacity to process mixed plastic waste, which can lower costs and improve the quality of pyrolysis products. Table 2 presents a summary of plastic waste-derived graphene materials using pyrolysis methods and applications.94–105
Table 2 Summary of various reports using pyrolysis methods to prepare the graphene material
| Plastic input materials |
Catalyst addition |
Pyrolysis conditions |
Graphene type and output applications |
Ref. |
| PP |
Organically modified montmorillonite |
Fast pyrolysis at 700 °C in 15 min under an inert atmosphere |
Graphene flakes |
94 |
| PP, PE and PS |
Nanoclay |
Slow pyrolysis at 400 °C and fast pyrolysis at 700 °C in N2 atmosphere |
Graphene nanosheets |
95 |
| Mixed plastics |
Bentonite nanoclay |
Slow pyrolysis at 400 °C for 50 min and fast pyrolysis at 800 °C for 80 min under inert atmosphere |
Graphene sheets as nanofillers in concrete (compressive strength of concrete mixture enhances by ∼42.86%, and tensile strength increases by ∼30% for 28 days) |
96 |
| PP, PE, and PET |
Pure nickel |
Slow pyrolysis at 400 °C for 80 min and fast pyrolysis at 850 °C for 85 min in an N2 atmosphere |
3D graphene nanosheets for perovskite solar cells (power conversion efficiency by ∼12.40%) |
97 |
| Mixed plastics |
ZnO |
First pyrolysis step at 400 °C for 2 h and 920 °C for 3 h in an N2 atmosphere |
Graphene nanosheets for supercapacitors (152 F g−1 at 1 A g−1) |
98 |
| Mixed plastics |
Nanoclay |
First pyrolysis step at 350 °C for 2 h and second pyrolysis at 750 °C for 3 h in an N2 atmosphere |
Nitrogen-doped reduced graphene oxide-decorated core–shell nano-structured Pd–Ru for fuel cells (∼43 m2 g−1 for the oxygen reduction reaction and half potential of 0.33 V) |
99 |
| Mixed plastics |
Al2O3 |
Two continuous carbonization steps at 400 and 850 °C under an N2 atmosphere |
Graphene nanosheets for thermoelectric applications (thermoelectric value ∼0.1 × 10−6 at 426 K) |
100 |
| PP |
Fe(NO3)2 and Co(NO3)2 |
First pyrolysis step at 450 °C for 20 min, second pyrolysis step at 800 °C for 1 h, and final step at 850 °C for 90 min in an N2 atmosphere |
Graphene sheets |
101 |
| Mixed plastics |
Graphene oxide |
First pyrolysis step at 600 °C for 3 h and second pyrolysis step at 2500 °C for 1 h in an Ar atmosphere |
Expanded graphite or graphene multiple sheets |
102 |
| PET |
FeCl3, Al(NO3)3·9H2O, and citric acid |
800 °C for 2 h in an N2 atmosphere |
Graphene sheets |
103 |
| Mixed plastics |
Bentonite nanoclay |
First pyrolysis step at 450 °C for 1 h and second pyrolysis step at 950 °C for 2 h in an N2 atmosphere |
Reduced graphene oxide (rGO) for NiCo2O4@WPrGO composite electrodes of supercapacitor (334 F g−1 at 0.5 A g−1) |
104 |
| LLDPE |
KCl and K2CO3 |
First pyrolysis step at 450 °C for 1 h, second pyrolysis step at 600 °C for 1 h, and final step at 950 °C for 1 h in an N2 atmosphere |
Graphene sheets for supercapacitors (1800 m2 g−1, 173 F g−1 at 0.25 A g−1) |
105 |
| Torrefied yellow poplar and HDPE |
HSZM-5 and Al-MCM-41 |
First step for calcination of catalyst materials at 550 °C in air for 3 h, second pyrolysis step at 600 °C |
Few layers of graphene-like char |
109 |
| Bio-oils and face masks |
Nickel foam-based catalyst |
First step of fast pyrolysis at 800 °C, second step of bio-oil collection and gas purification, following the third step of CVD |
Graphene film deposits on nickel foam (light weight of 16.46 mg cm−3 and less oxygen impurity of 3.57 at%) |
110 |
| PS |
|
Microwave-assisted pyrolysis at 390 °C |
Graphene quantum dots for ink |
111 |
| Waste tire (styrene butadiene) |
KOH |
Microwave-assisted pyrolysis at 900 °C |
Porous graphene (964.27 m2 g−1 with apparent density of 0.68 g cm−3 at 27 °C) |
112 |
The pyrolysis method is continuously improved to enhance the quality of output products, increase conversion efficiency, and, more importantly, reduce costs on an industrial production scale. Currently, some materials or methods have been integrated into the pyrolysis method to improve the quality of the output carbon materials, such as the co-pyrolysis of other waste materials together with plastic waste. A typical example is mixing biomass materials with plastic waste. The co-pyrolysis of plastic waste and biomass is a technical process in which plastic waste and biomass are simultaneously pyrolyzed to produce valuable solid, liquid and gaseous products.106–108 This process not only recycles plastic waste but also produces hydrogen and other organic compounds, thus optimizing energy production from both feedstocks.107 This method is improved using catalysts to increase the efficiency and product yield. Fig. 12 shows the general process of synthesizing highly graphitized carbon by incorporating plastic waste and biomass precursors.108 However, co-pyrolysis between plastic waste and biomass usually produces porous carbon materials, amorphous carbon and graphite, which makes it difficult to obtain high-quality graphene nanosheets.106–108 This could result from the combination of two input materials of plastic and biomass, which have significant structural, physical, and chemical differences. This has caused the pyrolysis process to convert the material to a carbon phase containing a 3D and amorphous structure belonging to biomass materials. Recently, there have been some reports combining some types of biomass and plastic wastes, such as torrefied yellow poplar and HDPE,109 face masks and heavy fractions of bio-oil110 to produce graphene materials and graphene thin films.
 |
| | Fig. 12 Schematic of the process used to synthesize highly graphitized carbon materials from plastic waste and biomass precursors. Figure has been reproduced with permission from ref. 108. Copyright 2022, American Chemical Society. | |
Microwave-assisted pyrolysis of plastic waste can prepare graphene, which provides a potential strategy for converting plastic waste into value-added products, including high-purity graphene. This method is promising for the recycling and efficient use of plastic waste. Microwave-assisted pyrolysis offers many benefits, including: (i) improved processing efficiency because microwaves can heat quickly and uniformly, which enhances the pyrolysis process efficiency, shortens the time, and saves energy; (ii) creates high-value products such as oil, gas, and graphene; (iii) microwave plastic recycling reduces plastic waste; (iv) this technology can produce graphene and other products with higher purity than traditional methods. D. Kumar et al. reported interesting research on microwave-assisted pyrolysis for the preparation of graphene quantum dots from Styrofoam (PS). The graphene quantum dots were obtained after a microwave furnace at 390 °C with a ramp rate of 10 °C min, which focused on quantum dot ink.111 W. Chen and co-workers presented microwave-assisted pyrolysis to produce porous graphene using KOH etching of waste tires.112 They found that the dielectric constant progressively increased with increasing temperature, peaking at around 700 °C and then leveling out at around 750 °C. The study also investigated the reflection loss and penetration depth of mixes with varying proportions. It was found that a waste tire–KOH mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 exhibits good dielectric properties to produce high-quality porous graphene. In general, in the pyrolysis-based synthesis of graphene, catalysts are essential because they affect the quality, shape, and structure of the output products. The thickness of the graphene sheet, surface area, defect density, and electrical conductivity are all affected by catalyst selection. It can be noted that catalysts play a key role in pyrolysis by lowering the activation energy needed for the rearrangement of carbon–carbon bonds, regulating the development kinetics of graphene layers, and preventing excessive graphitization, all of which result in regulated carbon deposition. Nanoclays, such as bentonite and montmorillonite, serve as catalysts in the pyrolysis of plastic waste, facilitating the transformation of plastics into valuable carbon materials like graphene. Their roles and effects include (i) catalytic activity for promoting the breakdown of polymer chains at lower temperatures and assisting in the formation of aromatic compounds, which are precursors for graphene structures; (ii) improvement of graphene properties; and (iii) optimal conditions to enhance graphene yield and quality.94,96,99 Moreover, metal oxides and metal salts can be used as chemical catalyst additions because of (i) carbon bond cleavage and arrangement; (ii) controlling carbon structure and defect density; and (iii) dehydrogenation.97,98,110 Furthermore, alkaline compounds, including KOH, NaOH, K2CO3, and KCl, can play pivotal roles as catalyst additions in the pyrolysis process of plastic waste, facilitating its transformation into graphene and other carbon-based nanomaterials. They provide some primary functional factors, such as enhanced surface area, lower activation energy, micropore formation, and synergistic effects in co-pyrolysis.105,112 Table 3 demonstrates the influence of specific catalysts on plastic-derived graphene properties.
2 exhibits good dielectric properties to produce high-quality porous graphene. In general, in the pyrolysis-based synthesis of graphene, catalysts are essential because they affect the quality, shape, and structure of the output products. The thickness of the graphene sheet, surface area, defect density, and electrical conductivity are all affected by catalyst selection. It can be noted that catalysts play a key role in pyrolysis by lowering the activation energy needed for the rearrangement of carbon–carbon bonds, regulating the development kinetics of graphene layers, and preventing excessive graphitization, all of which result in regulated carbon deposition. Nanoclays, such as bentonite and montmorillonite, serve as catalysts in the pyrolysis of plastic waste, facilitating the transformation of plastics into valuable carbon materials like graphene. Their roles and effects include (i) catalytic activity for promoting the breakdown of polymer chains at lower temperatures and assisting in the formation of aromatic compounds, which are precursors for graphene structures; (ii) improvement of graphene properties; and (iii) optimal conditions to enhance graphene yield and quality.94,96,99 Moreover, metal oxides and metal salts can be used as chemical catalyst additions because of (i) carbon bond cleavage and arrangement; (ii) controlling carbon structure and defect density; and (iii) dehydrogenation.97,98,110 Furthermore, alkaline compounds, including KOH, NaOH, K2CO3, and KCl, can play pivotal roles as catalyst additions in the pyrolysis process of plastic waste, facilitating its transformation into graphene and other carbon-based nanomaterials. They provide some primary functional factors, such as enhanced surface area, lower activation energy, micropore formation, and synergistic effects in co-pyrolysis.105,112 Table 3 demonstrates the influence of specific catalysts on plastic-derived graphene properties.
Table 3 Effect of specific catalyst addition in the pyrolysis method on graphene properties
| Catalyst |
Role in pyrolysis |
Impact on graphene quality |
Ref. |
| Nanoclays |
Enhanced degradation, aromatization |
- Lead to the formation of graphene with fewer defects and higher crystallinity |
94 and 95 |
| - The nanoclays are properly processed and dispersed within the plastic matrix can enhance its catalytic efficiency |
96 and 99 |
| NiO, ZnO, Al2O3 |
Carbon diffusion, template and defect control |
- Control of graphene structure and defect density |
97, 98 and 110 |
| - Controlling graphene flake size and layer thickness, and surface functionalization |
| Fe(NO3)2, Co(NO3)2, FeCl3, Al(NO3)3·9H2O |
Support and surface area enhancement, catalytic activity for carbon bond |
- Increasing surface area, controlling graphene thickness |
101 and 103 |
| - Controlling graphene flake size and layer thickness, and surface functionalization |
| Alkaline compounds (KOH, KCl and K2CO3) |
Enhancing surface area, lower activation energy, micropore formation, and synergistic effects in co-pyrolysis |
- Creating a porous structure with a large surface area |
105 and 112 |
| - Synergistic effect that enhances the yield and quality of the resulting graphene sheets |
Pyrolysis is a promising technology, but it generates various waste gases that must be effectively treated to minimize environmental impact. Gaseous emissions primarily consist of volatile organic compounds such as carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), and other hydrocarbons. Additionally, the presence of hazardous substances such as dioxins, furans, and other toxic byproducts necessitates the implementation of advanced gas treatment systems to ensure regulatory compliance and environmental safety. One of the most effective approaches for treating waste gas from plastic pyrolysis is thermal oxidation, where high temperatures are used to decompose harmful organic compounds into less harmful gases like CO2 and water vapor. Catalytic oxidation can further enhance this process using catalysts to lower the required temperature, thereby reducing energy consumption. Another key strategy is the use of the syngas generated during pyrolysis as an energy source. Gases produced during pyrolysis, which contain hydrogen, methane, and other combustible gases, can be cleaned through desulfurization and tar removal processes before being used in combustion engines or gas turbines to generate power. This not only mitigates emissions but also improves the overall energy efficiency of the process, making graphene production more sustainable. Thus, emergency strategies are available for implementing an integrated waste gas treatment system that ensures compliance with environmental regulations while maximizing the economic and ecological benefits of graphene production.
The pyrolysis method for converting plastic materials into graphene is considered to have great potential for industrial-scale applications in the near future. Currently, industrial chains mostly convert plastic into output products such as pyrolysis oil, syngas, char residue, and wax. With the target product being graphene, the industrial production chain needs to be improved with suitable boundary conditions, especially chemical catalysts to convert plastic into graphene.
3.1.2 Flash Joule heating method. Flash Joule Heating (FJH) is a technique that heats plastic materials to very high temperatures (above 3000 K) in an extremely short period, achieving a conversion efficiency of over 90% and transforming plastic waste into graphene with high purity and uniformity.113 FJH rapidly converts precursor materials, such as plastic waste, into graphene by rearranging the carbon atoms using a high applied voltage current, which generates tremendous heat and causes the material to flash at high temperatures above 3000 K in milliseconds.Fig. 13a shows a typical FJH system proposed by K. M. Wyss et al. The plastic materials are gently compressed inside quartz or ceramic tubes by two electrodes made of graphite or conductive metals.114 To aid in outgassing, the system can be operated at atmospheric pressure or in a moderate vacuum (∼10 mm Hg). In less than 100 milliseconds, a high-voltage electric discharge from a capacitor bank transforms the transfer materials into turbostratic flash graphene by raising the carbon source temperature to almost 3000 K. Fig. 13b illustrates a typical current discharge profile of the FJH procedure for converting plastic waste into flash graphene. Fig. 13c–h shows the good morphology and structure of flash graphene with Raman peaks and good TEM lattice spacing of two-dimensional graphene sheets.114 FJH uses the idea of fast heating a precursor material, like graphite, coal, or plastic waste, to high temperatures by applying an electric current. This technique breaks down the material into turbostratic flash graphene, a kind of graphene in disordered form and non-stacked layers that differ from typical graphene.115–117 The FJH technique is notable for its efficiency in turning waste materials into high-value graphene in a matter of seconds. This is a significant advance over traditional methods, which are sometimes hampered by slower production rates, expensive supplies, and high energy usage. However, it is necessary to optimize the system to be able to handle a large amount of input precursor per “flash”. Table 4 shows the properties of the FJH method for the preparation of flash graphene. FJH is a promising and highly feasible approach for producing high-quality graphene materials from plastic waste. However, this method still has some limitations that need to be overcome, such as high energy consumption and high-priced equipment. With the synchronous development of science and technology, the FJH method is expected to soon be transferred from the laboratory scale to the industrial scale in the next decade.
 |
| | Fig. 13 (a) Diagram for designing a dual capability FJH process at low and high current discharge, (b) current plot of the FJH process to transfer ELV-WP into ELV-WP-FG, and the structure and morphology of the ELV-WP-FG product: (c) and (d) Raman spectra, (e) XRD pattern, (f) XPS binding energy profiles, and (g and h) high-resolution TEM images with a scale bar of 10 nm. Figure has been reproduced with permission from ref. 114 Copyright 2022, Springer. | |
Table 4 Summary of various reports using flash Joule Heating methods to prepare flash graphene
| Plastic input materials |
Catalyst addition |
FJH conditions |
Graphene type and output applications |
Ref. |
| Mixed plastics (plastic bags) |
No |
Quartz tubes, AC 120 V, 60 Hz for ∼8 s in a vacuum desiccator (∼10 mmHg) |
Turbostratic flash graphene (average sheet size ∼16 nm, average interlayer spacing ∼3.45 Å) |
113 |
| Mixed plastics |
No |
Quartz tubes, AC from 1 to 25 A, over 15–20 s, DC 200 A at 104 mF capacitors charged to 150 V, discharged in 500 milliseconds |
Turbostratic flash graphene for polyurethane foams (average sheet size of 13.8 ± 7.1 nm, average interlayer spacing of 0.358 nm) |
114 |
| Mixed plastics |
No |
Quartz tubes, apply voltage of 50 V, 70 V, 90 V, and 160 V that each lasted 450 millisecond in total 1800 millisecond until a final resistance of 1 Ω resulted |
Turbostratic flash graphene for concrete (average sheet sizes from 20 nm to multiple microns, average interlayer spacing of 0.349 nm) |
115 |
| Rubber tire |
No |
Quartz tubes, AC charged to 150 V, discharged in 500 milliseconds |
Turbostratic flash graphene added to Portland cement (average sheet sizes ∼33 nm, average interlayer spacing ∼3.45 Å) |
116 |
| Blend of 5% carbon black: HDPE |
No |
Quartz tubes, AC charged to 120 V, discharged in 500 milliseconds |
Turbostratic flash graphene |
117 |
3.1.3 Other methods. Another simple and efficient method for producing graphene-based materials is carbonization. Carbonization does not require as many consecutive steps as pyrolysis or special systems like FHJ. In general, pyrolysis and carbonization are methods of converting plastic materials, in this case polymers, into carbon through heating steps at high temperatures in an inert or anaerobic atmosphere. Pyrolysis is the process by which biomaterials are decomposed into various chemical products, whereas carbonization is the process by which organic materials are mainly converted into carbon.To produce graphene material, many research groups have recycled plastic waste using high-temperature furnace systems under certain circumstances. J. Ma et al. reported a study on the synthesis of graphene-like porous carbon sheets through the pressurized carbonization of mixed plastic wastes (PP, PVC, PE, PS, PET) with the activation of magnesium oxide (Fig. 14).118 The study showed that the conversion from plastic to porous carbon sheets can be carried out at 500 °C and that the reaction time can affect the properties of the final product. The maximum BET surface area was 713 m2 g−1 at a MgO/plastic weight ratio of 4, and the maximum pore volume was 5.27 cm3 g−1 at a MgO/plastic weight ratio of 6. This study showed that at a high temperature of 500 °C using the MgO catalyst, the polymer could be converted to a porous carbon with a two-dimensional structure. PET plastic bottles are among the most prioritized types of plastic waste for recycling because of the huge amount discharged every day. S. Ko and coworkers introduced two high-temperature carbonization steps to convert PET bottled water to graphite.119 Fig. 15a shows a schematic of the preparation of graphite and graphene using PET bottles. First, the carbonization of PET flakes occurred in a tube furnace at 900 °C under N2 atmosphere for 1 h to obtain a carbonaceous material. This carbonaceous material was ground, sieved, mixed with boron powder and transferred into a box-type furnace for heating to 2400 °C for 1 h under flowing He gas. They found that the best ratio between carbon and boron powder (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) provided the highest graphite output material. However, this strategy has the major limitation of high cost due to the use of a large amount of boron powder. Using a source of medical waste plastic, K. T. Kumar et al. presented a carbonization method at extremely high temperatures for waste blister packs without chemical activation to transfer graphene-like turbostratic carbon for lithium-ion battery applications.120 In their study, the waste blister packs were washed in HCl and dissolved in THF solvent, followed by casting on a glass Petri dish to obtain slurry casting films. These films were carbonized at a high temperature of 1400 °C without activation to reach graphene-like turbostratic carbon with high crystallinity and quality (Fig. 15b).120 Table 5 lists the various reports on the use of carbonization methods to prepare graphene using diverse strategies.
5) provided the highest graphite output material. However, this strategy has the major limitation of high cost due to the use of a large amount of boron powder. Using a source of medical waste plastic, K. T. Kumar et al. presented a carbonization method at extremely high temperatures for waste blister packs without chemical activation to transfer graphene-like turbostratic carbon for lithium-ion battery applications.120 In their study, the waste blister packs were washed in HCl and dissolved in THF solvent, followed by casting on a glass Petri dish to obtain slurry casting films. These films were carbonized at a high temperature of 1400 °C without activation to reach graphene-like turbostratic carbon with high crystallinity and quality (Fig. 15b).120 Table 5 lists the various reports on the use of carbonization methods to prepare graphene using diverse strategies.
 |
| | Fig. 14 (a) Schematic of the preparation of porous carbon sheets, and morphology determined from (b–d) TEM images and (e) SEM images. Figure has been reproduced with permission from ref. 118. Copyright 2018, Royal Society of Chemistry. | |
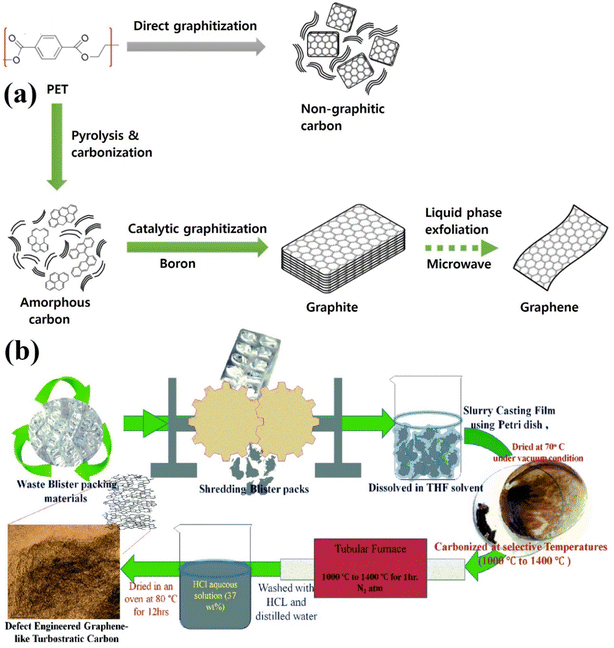 |
| | Fig. 15 (a) Diagram for turning PET plastic into graphite and graphene.119 (b) Illustration of the turbostratic carbon produced via a carbonization process from blister packs.120 Figure (a) has been reproduced with permission from ref. 119, copyright 2020, Elsevier. Figure (b) has been reproduced from ref. 120. Copyright 2022, Royal Society of Chemistry. | |
Table 5 Summary of various reports using carbonization methods for graphene preparation
| Plastic input materials |
Catalyst addition |
Carbonization conditions |
Graphene type and output applications |
Ref. |
| Mixed plastics |
MgO |
Autoclave, heating until 500 °C at a heating rate of 20 °C min−1 for 1 h |
Graphene-like porous carbon sheets (713 m2 g−1) |
118 |
| PET |
Boron |
First step at 900 °C under an N2 atmosphere for 1 h, following second step at 2400 °C for 1 h under flowing He gas |
Graphite and graphene (average lateral size ∼410 nm) |
119 |
| Blister packs |
No |
Dissolving in THF and drying, followed by 1400 °C for 1 h under N2 atmosphere |
Defect-engineered graphene-like turbostratic carbon for lithium-ion battery anode (∼11.4 m2 g−1, capacity of 594 mA h g−1 at 0.1C after 200 cycles) |
120 |
| PS |
MgO |
700 °C for 1 h under an Ag atmosphere |
Hierarchical porous carbon nanosheets for supercapacitors (2650 m2 g−1, specific capacitance of 323 F g−1 at 0.5 A g−1 in a 6 M KOH) |
121 |
| PET |
MgO/Co(C5H7O2)3 |
700 °C for 1 h under an Ag atmosphere |
3D porous carbon Nanosheets-MnO2 composites (561 m2 g−1 with pore volumes ∼ 2.4 cm3 g−1, 210.5 F g−1 at 0.5 A g−1) |
122 |
| PET |
No |
700 °C for 1 h under an anaerobic atmosphere |
Graphene sheets for dye adsorption (721.7 m2 g−1) |
123 |
3.2 Bottom-up approach to preparing graphene material
3.2.1 Chemical vapor deposition method. Chemical Vapor Deposition (CVD) is the most common bottom-up method for graphene preparation because of its scalability, good quality graphene, and relatively low cost. This method allows control over the domain size, thickness, and scale of graphene during the preparation process. This method is preferred when it is necessary to grow graphene thin films on substrates such as Cu, Si, and Ni. For instance, S. Sharma et al. reported an ambient pressure CVD process to grow a graphene layer on Cu foil using fruit protective foam plastic waste (Fig. 16).124 Fig. 16a shows two continuous furnaces that were set up at 500 °C and 1020 °C for plastic waste and Cu foil, respectively. The first furnace was used to heat plastic waste, and the second furnace was used for annealing to grow graphene sheets on the Cu foil. To minimize lattice misfits and dislocations and increase the sizes of Cu grains, annealing is a crucial step in limiting the number of nucleation sites. Fig. 16c shows the substrate heating, annealing, growth time, and cooling rate throughout the growth process. Fig. 16d shows the SEM images of thin crystalline graphene, which has a high crystalline structure, as revealed by the Raman spectra shown in Fig. 16e. L. Cui and coworkers presented research work on solid-state CVD utilizing different plastic waste as input sources along with Ar and H2 to prepare a graphene film on Ni foil, which can be transferred to a free-standing graphene film after etching Ni foil in ferric chloride/hydrochloride (Fig. 17).125 Table 6 summarizes some recent studies on the use of the CVD method to synthesize graphene films from plastic waste as input material.124–128
 |
| | Fig. 16 (a) Schematic of CVD for the preparation of graphene sheets on Cu foil; (b) fruit protective foam plastic waste; (c) the graphene development process's heating, annealing, growth time, and cooling rate; (d) TEM images of individual graphene crystals after growing for 90 minutes; and (e) Raman spectra of graphene films. Figure has been reproduced from ref. 124. Copyright 2014, Elsevier. | |
 |
| | Fig. 17 (a) Various plastic types for experimental, (b) solid-state CVD system, (c) image of one large graphene foil 17 × 10 cm2. (d and e) SEM images of the graphene foil edge, and (f) cross-sectional TEM images with clear lattice spacing. Figure has been reproduced with permission from ref. 125. Copyright 2017, Royal Society of Chemistry. | |
Table 6 Summary of various reports using bottom-up methods for graphene preparation
| Plastic input materials |
Catalyst addition |
Process conditions |
Graphene type and output applications |
Ref. |
| Fruit protective plastic waste |
No |
CVD using two continuous furnaces set up at 500 °C and 1020 °C under H2 and Ar atmosphere |
Graphene film growth on Cu foil |
124 |
| Mixed plastic waste |
No |
CVD using quartz tube furnace at 1050 °C for 120 min in an H2/Ar atmosphere |
Graphene film growth on Ni foil |
125 |
| Fruit protective plastic waste |
No |
CVD using two continuous furnaces set up at 500 °C and 1020 °C under H2 and Ar atmosphere |
Graphene film growth on Cu sheets |
126 |
| PS from petri dish |
No |
CVD using a quartz tube furnace, heating until 1050 °C for 15 min under an Ar atmosphere at 500 cm3 STP min−1 and H2 flow at 100 cm3 STP min−1 |
Graphene film growth on Cu sheets |
127 |
| PET |
No |
CVD using tube furnace, heating 1000 °C for 10 min with a cooling rate of 6.5 °C s−1 |
Monolayer graphene growth on Cu–Si substrate |
128 |
| PE |
No |
Atmospheric pressure microwave plasma (APMP) using a quartz tube furnace |
Graphene nanosheets |
129 |
3.2.2 Other methods. Although graphene can be produced in huge quantities as a powder using top-down methods, bottom-up approaches offer their benefits, most notably the capacity to create graphene thin films on various substrates for particular applications. Recently, M. A. Zafar and M. V. Jacob reported that an emerging technique known as atmospheric pressure microwave plasma (APMP) offers a significant advantage in graphene production.129 They used retired PE bottles as input plastic waste (Fig. 18a) in the APMP system (Fig. 18b), where a 2.45 GHz, 500 W microwave power supply was applied in 1 minute to make a plasma area under argon gas flow conditions. There are two phases to the production process in the plasma zone. The microplastics are first effectively broken down under plasma energy, which releases component gases such as carbon dioxide (CO2), hydrogen (H2), ethylene (C2H4), ethane (C2H6), methane (CH4), and carbon monoxide (CO). Hydrocarbons, especially methane, then go through further processing in the system, where they undergo plasma dissociation and become graphene.129 Table S1† shows the information of some typically large companies that provide the processes to transfer waste plastic and rubber tires to carbon-related products, especially graphene materials and related composites.
 |
| | Fig. 18 (a) Illustration showing dropper bottles sliced into tiny plastic particles ranging in size from 1 to 3 mm. (b) A graphical representation of the APMP technique used to create graphene from PE microplastics. Figure has been reproduced ref. 129. Copyright 2024, Wiley. | |
Table 7 illustrates various typical methods from top-down to bottom-up for the synthesis of graphene material from plastic waste resources. Each method has advantages and limitations that require improvement for large-scale operation. The pyrolysis method is more scalable and cost-effective but requires an inert atmosphere and produces lower-quality graphene. Flash Joule Heating provides a high graphene yield (80–90%) and good quality but requires higher energy demands, whereas carbonization is the most energy-efficient but yields lower quality, contains porous carbon and may need pre-treatment. For a bottom-up strategy, CVD produces monolayer graphene but is costly and energy-intensive, whereas microwave plasma offers higher efficiency (∼70–85%) and lower energy use but requires specialized plasma reactors with high costs. Thus, CVD is suitable for growing monolayer graphene, whereas microwave plasma is better for bulk applications like energy storage.
Table 7 Summary of various top-down and bottom-up methods for fabricating plastic waste-derived graphene material
| |
Methods |
Temperature (°C) |
Conversion efficiency (%) |
Advantages |
Disadvantages |
| Top-down |
Pyrolysis |
600–1000 |
60–80 |
- Acceptable cost and scalable |
- Requires inert gas condition |
| - Can process mixed plastic waste |
- Require chemical as catalyst addition |
| - Produces graphene with tunable properties |
- Lower graphene quality |
| - May produce byproducts (carbon black, gases) |
| Flash joule heating |
2700–3000 |
80–90 |
- Ultra-fast conversion (milliseconds) |
- High energy consumption |
| - No catalyst addition |
- Requires high-voltage pulsed current |
| - Produces high-purity graphene |
- High price equipment |
| - Works with contaminated plastic waste |
- Can be applied on an industrial scale but the output price is still high |
| Carbonization |
400–800 |
50–70 |
- Low energy consumption |
- Lower yield of graphene (∼50–70%) |
| - Can use biomass/plastic blends |
- Usually require pre-treatment |
| - Produces porous carbon structures |
- Graphene quality varies based on plastic type |
| Bottom-up |
Chemical vapor deposition (CVD) |
800–1200 |
30–50 |
- Produces high-quality monolayer graphene |
- Low conversion efficiency |
| - Requires metal catalysts (Cu, Ni) and hydrocarbon feedstock |
| - Precise control over graphene thickness |
- Expensive and energy-intensive |
| - Require strict aeration conditions |
| Microwave plasma |
500–1000 |
70–85 |
- Higher conversion efficiency |
- Requires specialized plasma reactors |
| - Lower temperature and energy consumption |
- Graphene quality varies based on microwave power and catalyst addition |
| - Can process various plastic waste types |
- High setup equipment cost |
4. Effects of plastic on the economy and future perspectives for plastic waste-derived carbon materials
4.1 Plastic recycling and the circular economy
The circular economy is an inevitable economic development model in the process of implementing the goal of green growth and sustainable development to achieve three main goals: (i) reducing the exploitation of natural resources; (ii) overcoming environmental pollution; (iii) harmoniously combining economic growth with environmental protection.130–132 In this inevitable development trend, plastic waste recycling is considered one of the important factors that satisfy all 3 criteria above: (i) Using plastic waste as input material for industrial production contributes to reducing dependence on coal, (ii) recycling waste contributes to reducing environmental pollution, and (iii) recycling and using waste as a resource to combine economic growth and environmental protection. Thus, the key to decreasing the detrimental impacts of plastic waste on the environment is a circular strategy, which includes reducing, reusing, and recycling (3R) plastic waste, as well as sorting and cleaning procedures to reduce waste. This strategy encourages sustainable behaviors, reduces the demand on natural resources, and contributes to the development of an economically and environmentally sustainable system for the management of plastic waste.74,133 The value-chain stages from plastic manufacture to plastic waste are shown in Fig. 19, along with the primary obstacles related to each stage.133 Contamination during the design and manufacturing stages makes it difficult to develop and create goods and packaging using recycled materials because it lowers the final product or package quality. Contamination impacts recycling chances throughout the end-of-life phase, resulting in technical and financial barriers to recycling. This suggests that in order to make sure that the procedures in one phase do not impede circularity in another, cooperation and research that addresses the relationships between the phases are necessary.130,133,134
 |
| | Fig. 19 From the manufacture of plastic to the disposal of plastic waste, every stage of the value chain represents tremendous challenges. Figure has been reproduced with permission from ref. 133. Copyright 2022, Elsevier. | |
The idea of using plastic waste to produce graphene presents a sustainable, long-term solution that addresses the reduction of fossil fuel consumption and plastic pollution while promoting a circular economy. By converting non-recyclable plastics into high-value graphene, this approach reduces reliance on natural carbon-based graphene production, lowers carbon emissions, and limits the demand for virgin plastic manufacturing. Additionally, it provides an economically viable recycling pathway, preventing plastic waste from accumulating in landfills and oceans. However, challenges such as high energy requirements for the synthesis process, scalability, and feedstock variability must be addressed through optimized and improved techniques and industrial-scale processing with standardized production methods. Currently, with continued advancements in technology, policy support, and industry investment, the idea of transferring plastic waste to graphene could be more practical and feasible, turning the idea from laboratory scale into industrial production.
4.2 Future perspectives on plastic waste-derived carbon materials
To date, no comprehensive method can use input plastics from a mixture of different types to produce a homogeneous graphene material. This is understandable because the chemical and physical properties of polymers have different characteristics. Therefore, more in-depth research is needed to focus on solving some existing problems, such as (i) a method that can simultaneously process many different types of plastics on the same line; (ii) reducing gas emissions during the conversion from plastic to carbon; and (iii) having a unified mechanism among countries on regulations for the treatment and recycling of plastic waste.
Fig. 20 illustrates current methods for plastic waste recycling, including various approaches and synthesis processes, and some new strategies that have been focused on recently.135 However, these methods still face some limitations related to cost and technology, as well as the quality of the output materials after recycling. Therefore, some improvements should be focused on development such as (i) optimizing the synchronous equipment system to reduce the amount of energy consumed during the recycling process and prioritizing the use of renewable energy such as solar power and wind as energy sources for the machinery system; (ii) developing catalysts to help increase conversion efficiency; (iii) using more biopolymers that can be safely decomposed after use.
 |
| | Fig. 20 An analysis of the main traditional and new approaches for the reuse and recycling of plastic waste. Figure has been reproduced with permission from ref. 135. Copyright 2022, Elsevier. | |
Graphene materials will be synthesized using new strategies that use plastic waste as input material, with the goal of processing the maximum amount of plastic waste to help balance environmental problems. Moreover, current methods will be improved with the aim of recycling more plastic waste on an industrial scale. In the future, environmentally sustainable methods will be tested to synthesize graphene materials from plastic waste, such as electrocatalysts and plasma-assisted conversion.
Graphene has revolutionized energy storage and conversion technologies due to its high electrical conductivity, large surface area, mechanical strength and chemical durability. For electrochemical battery applications, graphene enhances energy capacity and lifetime by improving charge transfer, cycle stability, and energy density, whereas for supercapacitor studies, its high surface area and good electron mobility enable fast charge/discharge rates, making it ideal for high-power applications.136–138 For energy conversion applications, especially in perovskite and organic photovoltaics, graphene provides an excellent strategy for fabricate transparent electrodes and charge transport layers, leading to improved efficiency and flexibility.139,140 A promising sustainable approach is the use of plastic waste-derived graphene, which not only reduces environmental pollution but also provides a low-cost alternative for energy applications. By refining conversion top-down and bottom-up techniques, such as pyrolysis, flash-joule heating, CVD, and microwave plasma, plastic-derived graphene can achieve high purity and performance, making it a viable material for next-generation energy storage and conversion systems. However, the application of graphene materials derived from industrial plastic waste sources needs to be further studied in relation to technological processes, environmental impacts and correlation with the plastic economy.
5. Conclusion
Recycling plastic waste is an excellent and effective fuel-saving waste treatment method. It will save more energy than producing new plastic materials to reduce activities such as mining, processing, and transportation. Reusing recycled plastic waste is an environmentally friendly method that helps reduce the consumption of natural resources and saves energy. Therefore, it will reduce a large amount of gas emissions every year. Currently, the industrial scale of amazing techniques for plastic waste-derived graphene faces policy barriers such as a lack of standardized regulations, weak recycling infrastructure, and limited government incentives, which slow investment and industry. Moreover, technological gaps, including high energy consumption, scalability issues, and inconsistent graphene quality, further hinder the commercialization of these materials. To overcome these challenges, clear regulatory frameworks and improved waste collection systems are needed, along with strong policy support, technological innovation, and market incentives; plastic-derived graphene could become a sustainable and economically viable material.
Recycling plastic waste into useful products based on carbonaceous materials represents a step forward towards a circular economy. The production and potential applications of plastic waste-derived graphene-based materials are reviewed in this report. Through the processes of top-down and bottom-up methods, plastic waste can be converted into various carbon compounds, especially graphene.
Data availability
No primary research results, software or code has been included and no new data were generated or analysed as part of this review.
Conflicts of interest
The authors declare that they have no known competing financial interests.
Acknowledgements
The authors wish to acknowledge the support provided by the Institute of Chemistry, Vietnam Academy of Science and Technology, Hanoi 10000, Vietnam.
References
- Y. Chen, A. K. Awasthi, F. Wei, Q. Tan and J. Li, Single-use plastics: Production, usage, disposal, and adverse impacts, Sci. Total Environ., 2021, 752, 141772, DOI:10.1016/j.scitotenv.2020.141772.
- J. G. Rosenboom, R. Langer and G. Traverso, Bioplastics for a circular economy, Nat. Rev. Mater., 2022, 7, 117–137, DOI:10.1038/s41578-021-00407-8.
- H. Ritchie, V. Samborska, and M. Roser, Data Page: Global plastics production, in Plastic Pollution, 2023 Search PubMed.
- S. S. V. Vuppaladadiyam, et al., Waste to energy: Trending key challenges and current technologies in waste plastic management, Sci. Total Environ., 2024, 913, 169436, DOI:10.1016/j.scitotenv.2023.169436.
- M. Bergmann, et al., Plastic pollution in the Arctic, Nat. Rev. Earth Environ., 2022, 3, 323–337, DOI:10.1038/s43017-022-00279-8.
- L. Huang, et al., Research progress on microplastics pollution in polar oceans, Polar Sci., 2023, 36, 100946, DOI:10.1016/j.polar.2023.100946.
- H. A. Leslie, M. J. M. van Velzen, S. H. Brandsma, A. D. Vethaak, J. J. Garcia-Vallejo and M. H. Lamoree, Discovery and quantification of plastic particle pollution in human blood, Environ. Int., 2022, 163, 107199, DOI:10.1016/j.envint.2022.107199.
- A. Sun and W. X. Wang, Human Exposure to Microplastics and Its Associated Health Risks, Environ. Health, 2023, 1(3), 139–149, DOI:10.1021/envhealth.3c00053.
- Y. Li, L. Tao, Q. Wang, F. Wang, G. Li and M. Song, Potential Health Impact of Microplastics: A Review of Environmental Distribution, Human Exposure, and Toxic Effects, Environ. Health, 2023, 1(4), 249–257, DOI:10.1021/envhealth.3c00052.
- M. Dokl, et al., Global projections of plastic use, end-of-life fate and potential changes in consumption, reduction, recycling and replacement with bioplastics to 2050, Sustain. Prod. Consum., 2024, 51, 498–518, DOI:10.1016/j.spc.2024.09.025.
- P. Roy, A. K. Mohanty and M. Misra, Microplastics in ecosystems: their implications and mitigation pathways, Environ. Sci.: Adv., 2022, 1(1), 9–29, 10.1039/d1va00012h.
- I. E. Napper and R. C. Thompson, Plastic Debris in the Marine Environment: History and Future Challenges, Global Challenges, 2020, 4, 1900081, DOI:10.1002/gch2.201900081.
- N. Evode, S. A. Qamar, M. Bilal, D. Barceló and H. M. N. Iqbal, Plastic waste and its management strategies for environmental sustainability, Case Stud. Chem. Environ. Eng., 2021, 4, 100142, DOI:10.1016/j.cscee.2021.100142.
- R. Kumar, et al., Impacts of plastic pollution on ecosystem services, sustainable development goals, and need to focus on circular economy and policy interventions, Sustainability, 2021, 13(17), 9963, DOI:10.3390/su13179963.
- G. Pathak, et al., Plastic pollution and the open burning of plastic wastes, Glob. Environ. Change, 2023, 80, 102648, DOI:10.1016/j.gloenvcha.2023.102648.
- S. Sangkham, et al., Evidence of microplastics in groundwater: A growing risk for human health, Groundw. Sustain. Dev., 2023, 23, 100981, DOI:10.1016/j.gsd.2023.100981.
- S. Viaroli, M. Lancia and V. Re, Microplastics contamination of groundwater: Current evidence and future perspectives. A review, Sci. Total Environ., 2022, 824, 153851, DOI:10.1016/j.scitotenv.2022.153851.
- S. Noventa, et al., Paradigms to assess the human health risks of nano- and microplastics, Microplast. Nanoplast., 2021, 1, 9, DOI:10.1186/s43591-021-00011-1.
- T. Atugoda, et al., Interactions between microplastics, pharmaceuticals and personal care products: Implications for vector transport, Environ. Int., 2021, 149, 106367, DOI:10.1016/j.envint.2020.106367.
- G. E. De-la-Torre, Microplastics: an emerging threat to food security and human health, Food Sci. Technol., 2020, 57, 1601–1608, DOI:10.1007/s13197-019-04138-1.
- S. Senarath and D. Kaushalya, Management of microplastics from sources to humans, in Microplastics in the Ecosphere: Air, Water, Soil,and Food, 2023, DOI:10.1002/9781119879534.ch24.
- Z. Yuan, R. Nag and E. Cummins, Human health concerns regarding microplastics in the aquatic environment - From marine to food systems, Sci. Total Environ., 2022, 823, 153730, DOI:10.1016/j.scitotenv.2022.153730.
- H. K. Ageel, S. Harrad and M. A. E. Abdallah, Occurrence, human exposure, and risk of microplastics in the indoor environment, Environ. Sci.: Processes Impacts, 2022, 24, 17–31, 10.1039/d1em00301a.
- P. G. C. Nayanathara Thathsarani Pilapitiya and A. S. Ratnayake, The world of plastic waste: A review, Cleaner Mater., 2024, 11, 100220, DOI:10.1016/j.clema.2024.100220.
- T. A. Saleh, Chapter 3 - Polymer science and polymerization methods toward hybrid materials, in Polymer Hybrid Materials and Nanocomposites, ed. T. A. Saleh, William Andrew Publishing, 2021, pp. 59–103, DOI:10.1016/B978-0-12-813294-4.00004-2.
- P. G. Ponnusamy and S. Mani, Material and Environmental Properties of Natural Polymers and Their Composites for Packaging Applications—A Review, Polymers, 2022, 14(19), 4033, DOI:10.3390/polym14194033.
- R. Geyer, Chapter 2 - Production, use, and fate of synthetic polymers, in Plastic Waste and Recycling, ed. T. M. Letcher, Academic Press, 2020, pp. 13–32, DOI:10.1016/B978-0-12-817880-5.00002-5.
- C. Cucci, G. Bartolozzi, V. Marchiafava, M. Picollo and E. Richardson, Study of semi-synthetic plastic objects of historic interest using non-invasive total reflectance FT-IR, Microchem. J., 2016, 124, 889–897, DOI:10.1016/j.microc.2015.06.010.
- A. J. Martín, C. Mondelli, S. D. Jaydev and J. Pérez-Ramírez, Catalytic processing of plastic waste on the rise, Chem, 2021, 7, 1487–1533, DOI:10.1016/j.chempr.2020.12.006.
- L. Faroni-Perez, Overcoming Plastic Pollution: Challenges Faced by Brazilian Policies and Perspectives for Stakeholder Engagement and Global Governance Opportunities, J. Sci. Policy Gov., 2023, 22(2) DOI:10.38126/jspg220204.
- T. Muringayil Joseph, et al., Polyethylene terephthalate (PET) recycling: A review, Case Stud. Chem. Environ. Eng., 2024, 9, 100673, DOI:10.1016/j.cscee.2024.100673.
- V. Soni, et al., Upcycling of polyethylene terephthalate (PET) plastic wastes into carbon-based nanomaterials: Current status and future perspectives, Eur. Polym. J., 2024, 215, 113249, DOI:10.1016/j.eurpolymj.2024.113249.
- F. Suvari and H. Gurvardar, Revitalizing high-density polyethylene (HDPE) waste: from environmental collection to high-strength hybrid yarns, Polym. Bull., 2024, 81(15), 14011–14029, DOI:10.1007/s00289-024-05367-x.
- K. Pinsuwan, P. Opaprakasit, A. Petchsuk, L. Dubas and M. Opaprakasit, Chemical recycling of high-density polyethylene (HDPE) wastes by oxidative degradation to dicarboxylic acids and their use as value-added curing agents for acrylate-based materials, Polym. Degrad. Stab., 2023, 210, 110306, DOI:10.1016/j.polymdegradstab.2023.110306.
- L. Lu, W. Li, Y. Cheng and M. Liu, Chemical recycling technologies for PVC waste and PVC-containing plastic waste: A review, Waste Manage., 2023, 166, 245–258, DOI:10.1016/j.wasman.2023.05.012.
- A. Turner and M. Filella, Polyvinyl chloride in consumer and environmental plastics, with a particular focus on metal-based additives, Environ. Sci.: Processes Impacts, 2021, 23, 1376–1384, 10.1039/d1em00213a.
- S. Sangwan, R. Bhattacharyya and D. Banerjee, Plastic compounds and liver diseases: Whether bisphenol A is the only culprit, Liver Int., 2024, 44, 1093–1105, DOI:10.1111/liv.15879.
- X. Wang, et al., Human health risk assessment of bisphenol A (BPA) through meat products, Environ. Res., 2022, 213, 113734, DOI:10.1016/j.envres.2022.113734.
- H. H. Senousy, H. M. Khairy, H. S. El-Sayed, E. R. Sallam, M. A. El-Sheikh and M. E. Elshobary, Interactive adverse effects of low-density polyethylene microplastics on marine microalga Chaetoceros calcitrans, Chemosphere, 2023, 311, 137182, DOI:10.1016/j.chemosphere.2022.137182.
- J. Boone, F. Lox and S. Pottie, Deficiencies of polypropylene in its use as a food-packaging material — a review, Packag. Technol. Sci., 1993, 6(5), 277–281, DOI:10.1002/pts.2770060508.
- J. L. Hu et al., Analysis of microplastics released from plastic take-out food containers based on thermal properties and morphology study, Food Addit. Contam.,: Part A, 2023, 40, 2, 305–318, DOI:10.1080/19440049.2022.2157894.
- I. B. Veroneze, L. A. Onoue and S. A. Cruz, Thermal Stability and Crystallization Behavior of Contaminated Recycled Polypropylene for Food Contact, J. Polym. Environ., 2022, 30(8), 3474–3482, DOI:10.1007/s10924-022-02447-9.
- F. J. Díaz-Galiano, M. J. Gómez-Ramos, I. Beraza, M. Murcia-Morales and A. R. Fernández-Alba, Cooking food in microwavable plastic containers: in situ formation of a new chemical substance and increased migration of polypropylene polymers, Food Chem., 2023, 417, 135852, DOI:10.1016/j.foodchem.2023.135852.
- C. Xu, et al., Safety assessment of polypropylene self-heating food container: The release of microplastics and volatile organic compounds, Food Packag. Shelf Life, 2024, 44, 101307, DOI:10.1016/j.fpsl.2024.101307.
- H. Lv, F. Huang and F. Zhang, Upcycling Waste Plastics with a C-C Backbone by Heterogeneous Catalysis, Langmuir, 2024, 40(10), 5077–5089, DOI:10.1021/acs.langmuir.3c03866.
- H. Gausepohl and N. Nießner, Polystyrene and Styrene Copolymers, in Encyclopedia of Materials: Science and Technology, ed. K. H. J. Buschow, R. W. Cahn, M. C. Flemings, B. Ilschner, E. J. Kramer, S. Mahajan, and P. Veyssière, Elsevier, Oxford, 2001, pp. 7735–7741, DOI:10.1016/B0-08-043152-6/01389-9.
- J. Wang, J. Lee, E. E. Kwon and S. Jeong, Quantitative analysis of polystyrene microplastic and styrene monomer released from plastic food containers, Heliyon, 2023, 9, e15787, DOI:10.1016/j.heliyon.2023.e15787.
- Y. Ma, et al., Exposure to different surface-modified polystyrene nanoparticles caused anxiety, depression, and social deficit in mice via damaging mitochondria in neurons, Sci. Total Environ., 2024, 919, 170739, DOI:10.1016/j.scitotenv.2024.170739.
- E. J. Werder, et al., Environmental styrene exposure and neurologic symptoms in U.S. Gulf coast residents, Environ. Int., 2018, 121, 480–490, DOI:10.1016/j.envint.2018.09.025.
- M. Gohil and G. Joshi, Chapter 18 - Perspective of polycarbonate composites and blends properties, applications, and future development: A review, in Green Sustainable Process for Chemical and Environmental Engineering and Science, ed. T. Altalhi and Inamuddin, Elsevier, 2022, pp. 393–424, DOI:10.1016/B978-0-323-99643-3.00012-7.
- U. Ali, K. J. B. A. Karim and N. A. Buang, A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA), Polym. Rev., 2015, 55, 678–705, DOI:10.1080/15583724.2015.1031377.
- G. I. Edo, et al., An updated review on the modifications, recycling, polymerization, and applications of polymethyl methacrylate (PMMA), J. Mater. Sci., 2024, 59(44), 20496–20539, DOI:10.1007/s10853-024-10402-3.
- T. A. Swetha, et al., A comprehensive review on polylactic acid (PLA) – Synthesis, processing and application in food packaging, Int. J. Biol. Macromol., 2025, 234, 123715, DOI:10.1016/j.ijbiomac.2023.123715.
- M. Cvek, U. C. Paul, J. Zia, G. Mancini, V. Sedlarik and A. Athanassiou, Biodegradable Films of PLA/PPC and Curcumin as Packaging Materials and Smart Indicators of Food Spoilage, ACS Appl. Mater. Interfaces, 2022, 14(12), 14654–14667, DOI:10.1021/acsami.2c02181.
- P. Saini, M. Arora and M. N. V. R. Kumar, Poly(lactic acid) blends in biomedical applications, Adv. Drug Delivery Rev., 2016, 107, 47–59, DOI:10.1016/j.addr.2016.06.014.
- M. Connolly, et al., Novel polylactic acid (PLA)-organoclay nanocomposite bio-packaging for the cosmetic industry; migration studies and in vitro assessment of the dermal toxicity of migration extracts, Polym. Degrad. Stab., 2019, 168, 108938, DOI:10.1016/j.polymdegradstab.2019.108938.
- B. Tyler, D. Gullotti, A. Mangraviti, T. Utsuki and H. Brem, Polylactic acid (PLA) controlled delivery carriers for biomedical applications, Adv. Drug Delivery Rev., 2016, 107, 163–175, DOI:10.1016/j.addr.2016.06.018.
- V. Lassalle and M. L. Ferreira, PLA nano- and microparticles for drug delivery: An overview of the methods of preparation, Macromol. Biosci., 2007, 7, 767–783, DOI:10.1002/mabi.200700022.
- M. G. Sahini, Polylactic acid (PLA)-based materials: a review on the synthesis
and drug delivery applications, Emergent Mater., 2023, 6, 1461–1479, DOI:10.1007/s42247-023-00551-7.
- A. A. Owadh, I. W. Parsons, J. N. Hay and R. N. Haward, Properties of nylon-1 polymers and copolymers in the solid state, Polymer, 1978, 19(4), 386–392, DOI:10.1016/0032-3861(78)90242-2.
- E. Braun and B. C. Levin, Nylons: A review of the literature on products of combustion and toxicity, Fire Mater., 1987, 11(2), 71–88, DOI:10.1002/fam.810110204.
- H. Yang, J. Zhao, M. Yan, S. Pispas and G. Zhang, Nylon 3 synthesized by ring opening polymerization with a metal-free catalyst, Polym. Chem., 2011, 2(12), 2888–2892, 10.1039/c1py00334h.
- N. C. Kim, J. H. Kim, J. H. Kim, S. W. Nam, B. S. Jeon and Y. J. Kim, Preparation of Nylon 4 microspheres via heterogeneous polymerization of 2-pyrrolidone in a paraffin oil continuous phase, J. Ind. Eng. Chem., 2015, 28, 236–240, DOI:10.1016/j.jiec.2015.02.020.
- M. Varghese and M. W. Grinstaff, Beyond nylon 6: polyamides via ring opening polymerization of designer lactam monomers for biomedical applications, Chem. Soc. Rev., 2022, 51, 8258–8275, 10.1039/d1cs00930c.
- A. McNeeley and Y. A. Liu, Assessment of Nylon-6 Depolymerization for Circular Economy: Kinetic Modeling, Purification, Sustainable Process Design, and Industrial Practice, Ind. Eng. Chem. Res., 2024, 63(40), 16953–16989, DOI:10.1021/acs.iecr.4c01975.
- J. D. Moore, Acrylonitrile-butadiene-styrene (ABS) - a review, Composites, 1973, 4(3), 118–130, DOI:10.1016/0010-4361(73)90585-5.
- J. V. Rutkowski and B. C. Levin, Acrylonitrile–butadiene–styrene copolymers (ABS): Pyrolysis and combustion products and their toxicity—a review of the literature, Fire Mater., 1986, 10(3–4), 93–105, DOI:10.1002/fam.810100303.
- J. R. Wagner, E. M. Mount, and H. F. Giles, 22 - Processing Recommendations for Various Resin Systems, in Extrusion, ed. J. R. Wagner, E. M. Mount, and H. F. Giles, William Andrew Publishing, Oxford, 2nd edn, 2014, pp. 255–275, DOI:10.1016/B978-1-4377-3481-2.00022-3.
- S. Olivera, H. B. Muralidhara, K. Venkatesh, K. Gopalakrishna and C. S. Vivek, Plating on acrylonitrile–butadiene–styrene (ABS) plastic: a review, J. Mater. Sci., 2016, 51, 3657–3674, DOI:10.1007/s10853-015-9668-7.
- S. Nur’aini, et al., Waste acrylonitrile butadiene styrene (ABS) incorporated with polyvinylpyrrolidone (PVP) for potential water filtration membrane, RSC Adv., 2022, 12(52), 33751–33760, 10.1039/d2ra05969j.
- V. Mishra, C. K. Ror, S. Negi, S. Kar and L. N. Borah, 3D printing with recycled ABS resin: Effect of blending and printing temperature, Mater. Chem. Phys., 2023, 309, 128317, DOI:10.1016/j.matchemphys.2023.128317.
- B. Thavornyutikarn, C. Aumnate, W. Kosorn, N. Nampichai and W. Janvikul, Acrylonitrile Butadiene Styrene/Thermoplastic Polyurethane Blends for Material Extrusion Three-Dimensional Printing: Effects of Blend Composition on Printability and Properties, ACS Omega, 2023, 8(47), 45013–45025, DOI:10.1021/acsomega.3c06595.
- M. Yan, W. Wei, Y. Huang, D. Gao and D. Wang, Grafting of maleic anhydride onto waste acrylonitrile butadiene styrene (ABS) and its application for compatibilization of ABS blends, J. Appl. Polym. Sci., 2025, 142(1), e56323, DOI:10.1002/app.56323.
- J. E. Lee, D. Lee, J. Lee and Y.-K. Park, Current methods for plastic waste recycling: Challenges and opportunities, Chemosphere, 2025, 370, 143978, DOI:10.1016/j.chemosphere.2024.143978.
- M. Shamsuyeva and H.-J. Endres, Plastics in the context of the circular economy and sustainable plastics recycling: Comprehensive review on research development, standardization and market, Compos., Part C: Open Access, 2021, 6, 100168, DOI:10.1016/j.jcomc.2021.100168.
- E. Naderi Kalali, S. Lotfian, M. Entezar Shabestari, S. Khayatzadeh, C. Zhao and H. Yazdani Nezhad, A critical review of the current progress of plastic waste recycling technology in structural materials, Curr. Opin. Green Sustainable Chem., 2023, 40, 100763, DOI:10.1016/j.cogsc.2023.100763.
- Z. O. G. Schyns and M. P. Shaver, Mechanical Recycling of Packaging Plastics: A Review, Macromol. Rapid Commun., 2021, 42, 2000415, DOI:10.1002/marc.202000415.
- H. Li, et al., Expanding plastics recycling technologies: chemical aspects, technology status and challenges, Green Chem., 2022, 24, 8899–9002, 10.1039/d2gc02588d.
- B. Jabłonska, P. Kiełbasa, M. Korenko and T. Drózdz, Physical and chemical properties of waste from pET bottles washing as a component of solid fuels, Energies, 2019, 12(11), 2197, DOI:10.3390/en12112197.
- J. M. Soto, G. Blázquez, M. Calero, L. Quesada, V. Godoy and M. Á. Martín-Lara, A real case study of mechanical recycling as an alternative for managing of polyethylene plastic film presented in mixed municipal solid waste, J. Clean. Prod., 2018, 203, 777–787, DOI:10.1016/j.jclepro.2018.08.302.
- H. Luo, H. Tyrrell, J. Bai, R. Ibrahim Muazu and X. Long, Fundamental, technical and environmental overviews of plastic chemical recycling, Green Chem., 2024, 26(23), 11444–11467, 10.1039/D4GC03127J.
- S. Zhang, M. Li, Z. Zuo and Z. Niu, Recent advances in plastic recycling and upgrading under mild conditions, Green Chem., 2023, 25, 6949–6970, 10.1039/d3gc01872e.
- Y. Chen, L. Bai, D. Peng, X. Wang, M. Wu and Z. Bian, Advancements in catalysis for plastic resource utilization, Environ. Sci.: Adv., 2023, 2, 1151–1166, 10.1039/d3va00158j.
- R. Shi, K. S. Liu, F. Liu, X. Yang, C. C. Hou and Y. Chen, Electrocatalytic reforming of waste plastics into high value-added chemicals and hydrogen fuel, Chem. Commun., 2021, 57(94), 12595–12598, 10.1039/d1cc05032j.
- H. Kang, et al., Concentration-Dependent Photocatalytic Upcycling of Poly(ethylene terephthalate) Plastic Waste, ACS Mater. Lett., 2023, 5(11), 3032–3041, DOI:10.1021/acsmaterialslett.3c01134.
- Q. Zhang, et al., CO2 catalyzed recycling of polyester and polycarbonate plastics, Green Chem., 2024, 26(24), 11976–11983, 10.1039/D4GC04782F.
- R. Mas-Ballesté, C. Gómez-Navarro, J. Gómez-Herrero and F. Zamora, 2D materials: to graphene and beyond, Nanoscale, 2011, 3(1), 20–30, 10.1039/C0NR00323A.
- J. Yang, M. Ma, L. Li, Y. Zhang, W. Huang and X. Dong, Graphene nanomesh: new versatile materials, Nanoscale, 2014, 6(22), 13301–13313, 10.1039/C4NR04584J.
- A. C. Ferrari, et al., Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems, Nanoscale, 2015, 7(11), 4598–4810, 10.1039/C4NR01600A.
- G. Zeller, et al., Demonstration of tritium adsorption on graphene, Nanoscale Adv., 2024, 6(11), 2838–2849, 10.1039/D3NA00904A.
- N. S. Singh, A. K. Mia and P. K. Giri, Role of oxygen functional groups and attachment of Au nanoparticles on graphene oxide sheets for improved photodetection performance, Nanoscale Adv., 2024, 6(8), 2136–2148, 10.1039/D3NA01120H.
- E. Mohebbi, et al., Towards graphene-based asymmetric diodes: a density functional tight-binding study, Nanoscale Adv., 2024, 6(5), 1548–1555, 10.1039/D3NA00603D.
- P. Zygouri, et al., Graphene oxide and oxidized carbon nanodiscs as biomedical scaffolds for the targeted delivery of quercetin to cancer cells, Nanoscale Adv., 2024, 6(11), 2860–2874, 10.1039/D3NA00966A.
- J. Gong, et al., Upcycling Waste Polypropylene into Graphene Flakes on Organically Modified Montmorillonite, Ind. Eng. Chem. Res., 2014, 53(11), 4173–4181, DOI:10.1021/ie4043246.
- S. Pandey, et al., Bulk synthesis of graphene nanosheets from plastic waste: An invincible method of solid waste management for better tomorrow, Waste Manage., 2019, 88, 48–55, DOI:10.1016/j.wasman.2019.03.023.
- G. Tatrari, et al., Waste plastic derived graphene sheets as nanofillers to enhance mechanical strength of concrete mixture: An inventive approach to deal with universal plastic waste, Clean Eng. Technol., 2021, 5, 100275, DOI:10.1016/j.clet.2021.100275.
- S. Pandey, et al., 3D graphene nanosheets from plastic waste for highly efficient HTM free perovskite solar cells, Nanoscale Adv., 2021, 3(16), 4726–4738, 10.1039/D1NA00183C.
- M. Karakoti, et al., A waste to energy approach for the effective conversion of solid waste plastics into graphene nanosheets using different catalysts for high performance supercapacitors: a comparative study, Mater. Adv., 2022, 3(4), 2146–2157, 10.1039/D1MA01136G.
- S. Dhali, et al., Waste plastic derived nitrogen-doped reduced graphene oxide decorated core–shell nano-structured metal catalyst (WpNrGO-Pd–Ru) for a proton exchange membrane fuel cell, Mater. Adv., 2024, 5(9), 3771–3782, 10.1039/D3MA01006F.
- K. K. Garg, S. Pandey, A. Kumar, A. Rana, N. G. Sahoo and R. K. Singh, Graphene nanosheets derived from waste plastic for cost-effective thermoelectric applications, Results Mater., 2022, 13, 100260, DOI:10.1016/j.rinma.2022.100260.
- A. A. Aboul-Enein, M. A. Azab, A. M. Haggar and A. E. Awadallah, Synthesis of high-quality graphene sheets via decomposition of non-condensable gases from pyrolysis of polypropylene waste using unsupported Fe, Co, and Fe–Co catalysts, J. Mater. Cycles Waste Manag., 2023, 25(1), 272–287, DOI:10.1007/s10163-022-01528-0.
- A. Gharpure, M. Kowalik, R. L. Vander Wal and A. C. T. van Duin, Upcycling Plastic Waste into Graphite Using Graphenic Additives for Energy Storage: Yield, Graphitic Quality, and Interaction Mechanisms via Experimentation and Molecular Dynamics, ACS Sustain. Chem. Eng., 2024, 12(11), 4565–4575, DOI:10.1021/acssuschemeng.3c07783.
- E. Salama, et al., Catalytic fabrication of graphene, carbon spheres, and carbon nanotubes from plastic waste, RSC Adv., 2024, 14(3), 1977–1983, 10.1039/D3RA07370J.
- D. Bhatt, et al., Upcycling waste plastic into 2D-carbon nanomaterials for high-performance supercapacitors by incorporating NiCo2O4: a sustainable approach to renewable energy, Mater. Adv., 2024, 5(15), 6255–6269, 10.1039/D4MA00469H.
- Y. Gao, N. T. Huynh, K.-J. Kim, C. Wang, V. H. Pham and C. Matranga, Upcycling linear low-density polyethylene waste to turbostratic graphene for high mass loading supercapacitors, Chem. Eng. J., 2024, 498, 155873, DOI:10.1016/j.cej.2024.155873.
- C. C. Seah, et al., Co-pyrolysis of biomass and plastic: Circularity of wastes and comprehensive review of synergistic mechanism, Results Eng., 2023, 17, 100989, DOI:10.1016/j.rineng.2023.100989.
- Z. Wang, K. G. Burra, T. Lei and A. K. Gupta, Co-pyrolysis of waste plastic and solid biomass for synergistic production of biofuels and chemicals-A review, Prog. Energy Combust. Sci., 2021, 84, 100899, DOI:10.1016/j.pecs.2020.100899.
- H. Weldekidan, A. K. Mohanty and M. Misra, Upcycling of Plastic Wastes and Biomass for Sustainable Graphitic Carbon Production: A Critical Review, ACS Environ. Au, 2022, 2(6), 510–522, DOI:10.1021/acsenvironau.2c00029.
- Y.-M. Kim, J. Jae, B.-S. Kim, Y. Hong, S.-C. Jung and Y.-K. Park, Catalytic co-pyrolysis of torrefied yellow poplar and high-density polyethylene using microporous HZSM-5 and mesoporous Al-MCM-41 catalysts, Energy Convers. Manage., 2017, 149, 966–973, DOI:10.1016/j.enconman.2017.04.033.
- Z. Luo, X. Zhu, J. Deng, K. Gong and X. Zhu, High-value utilization of mask and heavy fraction of bio-oil: From hazardous waste to biochar, bio-oil, and graphene films, J. Hazard. Mater., 2021, 420, 126570, DOI:10.1016/j.jhazmat.2021.126570.
- D. Kumar, B. Tom Mathew, S. Rani, A. Kumar, B. Nandan and R. K. Srivastava, Mechanism insights for formation of graphene quantum dots from Styrofoam waste and its application as security ink, Microchem. J., 2024, 196, 109681, DOI:10.1016/j.microc.2023.109681.
- W. Chen, et al., KOH etching catalyzed microwave pyrolysis of waste tires to
prepare porous graphene, Carbon Lett., 2024, 34(8), 2195–2209, DOI:10.1007/s42823-024-00804-3.
- W. A. Algozeeb, et al., Flash Graphene from Plastic Waste, ACS Nano, 2020, 14(11), 15595–15604, DOI:10.1021/acsnano.0c06328.
- K. M. Wyss, R. D. De Kleine, R. L. Couvreur, A. Kiziltas, D. F. Mielewski and J. M. Tour, Upcycling end-of-life vehicle waste plastic into flash graphene, Commun. Eng., 2022, 1(1), 3, DOI:10.1038/s44172-022-00006-7.
- K. M. Wyss, et al., Converting plastic waste pyrolysis ash into flash graphene, Carbon, 2021, 174, 430–438, DOI:10.1016/j.carbon.2020.12.063.
- P. A. Advincula, D. X. Luong, W. Chen, S. Raghuraman, R. Shahsavari and J. M. Tour, Flash graphene from rubber waste, Carbon, 2021, 178, 649–656, DOI:10.1016/j.carbon.2021.03.020.
- P. A. Advincula, et al., Waste plastic- and coke-derived flash graphene as lubricant additives, Carbon, 2023, 203, 876–885, DOI:10.1016/j.carbon.2022.12.035.
- J. Ma, J. Liu, J. Song and T. Tang, Pressurized carbonization of mixed plastics into porous carbon sheets on magnesium oxide, RSC Adv., 2018, 8(5), 2469–2476, 10.1039/C7RA12733B.
- S. Ko, Y. J. Kwon, J. U. Lee and Y.-P. Jeon, Preparation of synthetic graphite from waste PET plastic, J. Ind. Eng. Chem., 2020, 83, 449–458, DOI:10.1016/j.jiec.2019.12.018.
- K. Thileep Kumar, S. Raghu and A. M. Shanmugharaj, Transmogrifying waste blister packs into defect-engineered graphene-like turbostratic carbon: novel lithium-ion (Li-ion) battery anode with noteworthy electrochemical characteristics, Nanoscale, 2022, 14(11), 4312–4323, 10.1039/D1NR07183A.
- C. Ma, et al., Sustainable recycling of waste polystyrene into hierarchical porous carbon nanosheets with potential applications in supercapacitors, Nanotechnology, 2020, 31(3), 035402, DOI:10.1088/1361-6528/ab475f.
- X. Mu, et al., Controllable carbonization of plastic waste into three-dimensional porous carbon nanosheets by combined catalyst for high performance capacitor, Nanomaterials, 2020, 10(6), 1097, DOI:10.3390/nano10061097.
- N. A. El Essawy, S. M. Ali, H. A. Farag, A. H. Konsowa, M. Elnouby and H. A. Hamad, Green synthesis of graphene from recycled PET bottle wastes for use in the adsorption of dyes in aqueous solution, Ecotoxicol. Environ. Saf., 2017, 145, 57–68, DOI:10.1016/j.ecoenv.2017.07.014.
- S. Sharma, et al., Synthesis of graphene crystals from solid waste plastic by chemical vapor deposition, Carbon, 2014, 72, 66–73, DOI:10.1016/j.carbon.2014.01.051.
- L. Cui, X. Wang, N. Chen, B. Ji and L. Qu, Trash to treasure: converting plastic waste into a useful graphene foil, Nanoscale, 2017, 9(26), 9089–9094, 10.1039/C7NR03580B.
- N. A. M. Tahir, M. F. Bin Abdollah, N. Tamaldin, M. R. B. M. Zin and H. Amiruddin, Optimisation of graphene grown from solid waste using CVD method, Int. J. Adv. Des. Manuf. Technol., 2020, 106(1), 211–218, DOI:10.1007/s00170-019-04585-2.
- G. Ruan, Z. Sun, Z. Peng and J. M. Tour, Growth of Graphene from Food, Insects, and Waste, ACS Nano, 2011, 5(9), 7601–7607, DOI:10.1021/nn202625c.
- Y. You, et al., Growth of NiO nanorods, SiC nanowires and monolayer graphene via a CVD method, Green Chem., 2017, 19(23), 5599–5607, 10.1039/C7GC02523H.
- M. A. Zafar and M. V Jacob, Instant Upcycling of Microplastics into Graphene and Its Environmental Application, Small Sci., 2024, 4(10), 2400176, DOI:10.1002/smsc.202400176.
- I. O. Kunlere and K. U. Shah, A recycling technology selection framework for evaluating the effectiveness of plastic recycling technologies for circular economy advancement, Circular Economy, 2023, 2(4), 100066, DOI:10.1016/j.cec.2023.100066.
- S. Khadke, et al., Efficient plastic recycling
and remolding circular economy using the technology of trust–blockchain, Sustainability, 2021, 13(16), 9142, DOI:10.3390/su13169142.
- M. Shamsuyeva and H. J. Endres, Plastics in the context of the circular economy and sustainable plastics recycling: Comprehensive review on research development, standardization and market, Compos, Part C: Open Access, 2021, 6, 100168, DOI:10.1016/j.jcomc.2021.100168.
- M. R. Johansen, T. B. Christensen, T. M. Ramos and K. Syberg, A review of the plastic value chain from a circular economy perspective, J. Environ. Manage., 2022, 302, 113975, DOI:10.1016/j.jenvman.2021.113975.
- D. Hidalgo, J. M. Martín-Marroquín and F. Corona, A multi-waste management concept as a basis towards a circular economy model, Renew. Sustain. Energy Rev., 2019, 111, 481–489, DOI:10.1016/j.rser.2019.05.048.
- X. Zhao, et al., Plastic waste upcycling toward a circular economy, Chem. Eng. J., 2022, 428, 131928, DOI:10.1016/j.cej.2021.131928.
- W. Lv, Z. Li, Y. Deng, Q. H. Yang and F. Kang, Graphene-based materials for electrochemical energy storage devices: Opportunities and challenges, Energy Storage Mater., 2016, 2, 107–138, DOI:10.1016/j.ensm.2015.10.002.
- M. F. El-Kady, Y. Shao and R. B. Kaner, Graphene for batteries, supercapacitors and beyond, Nat. Rev. Mater., 2016, 1, 16033, DOI:10.1038/natrevmats.2016.33.
- X. Fu, Y. Liu, X. Wang, L. Kang and T. Qiu, Graphene-based advanced materials for energy storage and conversion systems: Progress, challenges, and commercial future, Appl. Energy, 2025, 386, 125566, DOI:10.1016/j.apenergy.2025.125566.
- K. Gong, et al., The roles of graphene and its derivatives in perovskite solar cells: A review, Mater. Des., 2021, 211, 110170, DOI:10.1016/j.matdes.2021.110170.
- L. M. Shaker, A. A. Abdulamie and A. A. Al-Amiery, Graphene-enabled advancements in solar cell technology, J. Alloys Compd., 2025, 1020, 179583, DOI:10.1016/j.jallcom.2025.179583.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*








![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) metal catalyst for fuel cell studies.99 Recently, K. K. Garg et al. reported two continuous pyrolysis steps at 400 °C and 850 °C under an N2 atmosphere, respectively, to produce graphene nanosheets by mixing mixed plastic with Al2O3 as a catalyst factor (Fig. 11).100 They discovered that the existence of edge defects caused the graphene nanosheets to thicken by 3–4 nm. Semiconducting characteristics were achieved in the produced graphene nanosheets due to the existence of edge defects inside the sheets.100
1) metal catalyst for fuel cell studies.99 Recently, K. K. Garg et al. reported two continuous pyrolysis steps at 400 °C and 850 °C under an N2 atmosphere, respectively, to produce graphene nanosheets by mixing mixed plastic with Al2O3 as a catalyst factor (Fig. 11).100 They discovered that the existence of edge defects caused the graphene nanosheets to thicken by 3–4 nm. Semiconducting characteristics were achieved in the produced graphene nanosheets due to the existence of edge defects inside the sheets.100


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 exhibits good dielectric properties to produce high-quality porous graphene. In general, in the pyrolysis-based synthesis of graphene, catalysts are essential because they affect the quality, shape, and structure of the output products. The thickness of the graphene sheet, surface area, defect density, and electrical conductivity are all affected by catalyst selection. It can be noted that catalysts play a key role in pyrolysis by lowering the activation energy needed for the rearrangement of carbon–carbon bonds, regulating the development kinetics of graphene layers, and preventing excessive graphitization, all of which result in regulated carbon deposition. Nanoclays, such as bentonite and montmorillonite, serve as catalysts in the pyrolysis of plastic waste, facilitating the transformation of plastics into valuable carbon materials like graphene. Their roles and effects include (i) catalytic activity for promoting the breakdown of polymer chains at lower temperatures and assisting in the formation of aromatic compounds, which are precursors for graphene structures; (ii) improvement of graphene properties; and (iii) optimal conditions to enhance graphene yield and quality.94,96,99 Moreover, metal oxides and metal salts can be used as chemical catalyst additions because of (i) carbon bond cleavage and arrangement; (ii) controlling carbon structure and defect density; and (iii) dehydrogenation.97,98,110 Furthermore, alkaline compounds, including KOH, NaOH, K2CO3, and KCl, can play pivotal roles as catalyst additions in the pyrolysis process of plastic waste, facilitating its transformation into graphene and other carbon-based nanomaterials. They provide some primary functional factors, such as enhanced surface area, lower activation energy, micropore formation, and synergistic effects in co-pyrolysis.105,112 Table 3 demonstrates the influence of specific catalysts on plastic-derived graphene properties.
2 exhibits good dielectric properties to produce high-quality porous graphene. In general, in the pyrolysis-based synthesis of graphene, catalysts are essential because they affect the quality, shape, and structure of the output products. The thickness of the graphene sheet, surface area, defect density, and electrical conductivity are all affected by catalyst selection. It can be noted that catalysts play a key role in pyrolysis by lowering the activation energy needed for the rearrangement of carbon–carbon bonds, regulating the development kinetics of graphene layers, and preventing excessive graphitization, all of which result in regulated carbon deposition. Nanoclays, such as bentonite and montmorillonite, serve as catalysts in the pyrolysis of plastic waste, facilitating the transformation of plastics into valuable carbon materials like graphene. Their roles and effects include (i) catalytic activity for promoting the breakdown of polymer chains at lower temperatures and assisting in the formation of aromatic compounds, which are precursors for graphene structures; (ii) improvement of graphene properties; and (iii) optimal conditions to enhance graphene yield and quality.94,96,99 Moreover, metal oxides and metal salts can be used as chemical catalyst additions because of (i) carbon bond cleavage and arrangement; (ii) controlling carbon structure and defect density; and (iii) dehydrogenation.97,98,110 Furthermore, alkaline compounds, including KOH, NaOH, K2CO3, and KCl, can play pivotal roles as catalyst additions in the pyrolysis process of plastic waste, facilitating its transformation into graphene and other carbon-based nanomaterials. They provide some primary functional factors, such as enhanced surface area, lower activation energy, micropore formation, and synergistic effects in co-pyrolysis.105,112 Table 3 demonstrates the influence of specific catalysts on plastic-derived graphene properties.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) provided the highest graphite output material. However, this strategy has the major limitation of high cost due to the use of a large amount of boron powder. Using a source of medical waste plastic, K. T. Kumar et al. presented a carbonization method at extremely high temperatures for waste blister packs without chemical activation to transfer graphene-like turbostratic carbon for lithium-ion battery applications.120 In their study, the waste blister packs were washed in HCl and dissolved in THF solvent, followed by casting on a glass Petri dish to obtain slurry casting films. These films were carbonized at a high temperature of 1400 °C without activation to reach graphene-like turbostratic carbon with high crystallinity and quality (Fig. 15b).120 Table 5 lists the various reports on the use of carbonization methods to prepare graphene using diverse strategies.
5) provided the highest graphite output material. However, this strategy has the major limitation of high cost due to the use of a large amount of boron powder. Using a source of medical waste plastic, K. T. Kumar et al. presented a carbonization method at extremely high temperatures for waste blister packs without chemical activation to transfer graphene-like turbostratic carbon for lithium-ion battery applications.120 In their study, the waste blister packs were washed in HCl and dissolved in THF solvent, followed by casting on a glass Petri dish to obtain slurry casting films. These films were carbonized at a high temperature of 1400 °C without activation to reach graphene-like turbostratic carbon with high crystallinity and quality (Fig. 15b).120 Table 5 lists the various reports on the use of carbonization methods to prepare graphene using diverse strategies.







