Open Access Article
Open Access ArticleSynthesis of polyurethane/vinyl polymer hybrids with unexpected mechanical properties using a macro chain transfer agent†
Ryota Uchida,
Seima Kondo and
Akinori Takasu *
*
Division of Soft Materials, Department of Engineering, Nagoya Institute of Technology, Gokiso-cho, Showa-ku, Nagoya 466-8555, Japan. E-mail: takasu.akinori@nitech.ac.jp
First published on 16th June 2025
Abstract
Polyurethane is one of the most essential polymer materials due to its wide range of applications in the forms of elastomer and foam. We here report the synthesis of customized polyurethane by hybridization with vinyl polymer using a polyurethane-based macroinitiator and successive radical polymerization. First, we performed step polymerization of a trithiocarbonate-containing diol, bis{4-[ethyl-(2-hydroxyethyl)carbamoyl]benzyl}trithiocarbonate, and isophorone diisocyanate to afford the polyurethane-based macroinitiator (PU-TTC: Mn = 27.0 × 103; Mn/Mw = 1.71) for reversible addition–fragmentation chain-transfer (RAFT) radical polymerization of vinyl monomers. We demonstrated polymerization of styrene and methyl methacrylate mediated by PU-TTC as the macro chain transfer agent to prepare a hybrid consisting of polyurethane and poly(styrene) or poly(methyl methacrylate). Compared to the methacrylate homopolymer, differential scanning calorimetry of the polyurethane hybrid showed improved thermal properties and tensile strength tests revealed unusual elongation behavior.
1. Introduction
The high performance of polymer materials has contributed to many applications not only in academia, but also in industrial fields. Among such materials, polyurethanes have fascinated polymer chemists because of their unique elastic properties and high thermal and chemical stabilities. Syntheses of commercially available polyurethanes are usually performed by step polymerization of bisphenol A and several diisocyanates including isophorone diisocyanate (IPDI).1–5 But recent urgent concerns about environmental pollution have promoted the exploration of the use of alternative chemicals instead of bisphenol A. However, a promising alternative synthetic approach that retains the conventional performance of polyurethane has not been reported.One of the evolving ideas is the hybridization of polyurethane and vinyl polymers.6,7 As the miscibility of a mixture of polyurethanes and vinyl polymers is extremely low,8 a reversible addition–fragmentation chain-transfer (RAFT) approach to well-defined telechelic vinyl polymers with hydroxyl terminals as polymeric diol-type building blocks (macromonomer) for polyurethanes was developed by Sudo et al.9 An alternative synthetic strategy of direct incorporation of vinyl monomer into a polyurethane backbone is worth considering because the reactivity of a macromonomer needs to be generally low for the step polymerization to obtain a desirable molecular weight.10 Our survey showed that the RAFT approach to synthesize multiblock vinyl polymers had been reported,11,12 but there is only one report dealing with polyurethane-based macroinitiators.10 In this report, we demonstrate RAFT radical polymerization using a polyurethane-base macroinitiator, prepared via step polymerization of bis{4-[ethyl-(2-hydroxyethyl)carbamoyl]benzyl}trithiocarbonate (TTCOH) and IPDI. Using this macroinitiator, poly(styrene) [poly(St)] and poly(methyl methacrylate) [poly(MMA)] segments are incorporated into the polyurethane backbone. We also investigated the thermal and mechanical properties of the resulting hybrid. We found an unexpected result that poly(MMA) incorporated into the macroinitiator appeared to be more ductile, compared with poly(MMA) prepared using a conventional RAFT agent.
2. Experimental section
2.1. Materials
Styrene (St), methyl methacrylate (MMA), tris(2-phenylpyridinato)iridium(III) [Ir(ppy)3](purified by sublimation), isophorone diisocyanate (IPDI), 1,5,7-triazabicyclo[4,4,0]dec-5-ene (TBD) were purchased from Tokyo Chemical Industry Co., Ltd (Tokyo Japan). Bis{4-[ethyl-(2-hydroxyethyl)carbamoyl]benzyl}trithiocarbonate (TTCOH), dibutyltin(IV) dilaurate (DBTDL), N,N-dimethylformamide (DMF) (deoxidized) were purchased from Wako Pure Chemical Industries, Ltd (Osaka Japan). Acetone, tetrahydrofuran (THF), hexane, and methanol were purchased from Nacalai Tesque Co., Ltd (Kyoto, Japan). MMA was distilled under reduced pressure under a nitrogen atmosphere. St was purified by passing through a basic alumina column.2.2. Synthesis of polyurethane containing the trithiocarbonate moiety (PU-TTC)
All operations were carried out in a glove box, and the flasks were flushed with dry nitrogen. TTCOH (395.8 mg, 0.76 mmol), IPDI (168.9 mg, 0.76 mmol), and DMF (1.00 mL) were premixed at room temperature, then DMF solution of DBTDL was added and heated to 60 °C for a predetermined time. The reaction mixture was quenched with methanol and the synthesized polymers were purified using THF and acetone/water (1/2 v/v) as good and poor solvents, respectively. The products were dried at 50 °C under reduced pressure.2.3. RAFT polymerization of MMA or St with PU-TTC as macro chain transfer agents
All operations were carried out in a glove box, and the flask were flushed with dry nitrogen. PU-TTC (18.6 mg, 0.025 mmol trithiocarbonate unit), MMA (750.9 mg, 7.5 mmol) or St (781.1 mg, 7.5 mmol) were dissolved in DMF (1.02 mL) in a flask and Ir(ppy)3 was added at 15 ppm (mol/mol) relative to the monomer. The polymerization was performed under blue-LED irradiation (456.1 nm, 15.04 lm W−1, D160 series 24V 10 mm LED dot type, JW-system Co. Ltd) at room temperature. After a predetermined time, polymerization was quenched by stopping the blue-LED irradiation. The synthesized polymers were purified by reprecipitation using THF as good solvent and methanol [for poly(MMA)] or hexane [for poly(St)] as poor solvents. The products were dried at 50 °C under reduced pressure.For the polymerization without blue-LED irradiation, AIBN was dissolved in a flask at a ratio of trithiocarbonate unit/AIBN = 1/0.2 (mol/mol) and magnetically stirred at 70 °C for a predetermined time.
2.4. Synthesis of MMA homopolymer
As a control sample, MMA homopolymer was synthesized by RAFT polymerization. All operations were carried out in a glove box, and the flask flushed with dry nitrogen. TTCOH (13.0 mg, 0.025 mmol), MMA (1251.5 mg, 12.5 mmol) were dissolved in deoxidized DMF (4.90 mL) in a flask and AIBN was added at a ratio of TTCOH/AIBN = 1/0.2 (mol/mol) and magnetically stirred at 70 °C. After 24 h, the synthesized polymers were purified using THF as a good solvent and methanol as a poor solvent. The product was dried at 50 °C under reduced pressure.2.5. Photodegradation of polyurethane–poly(MMA)
Reprecipitated polyurethane–poly(MMA) (10 mg), DMF (0.5 mL) and hydroquinone (1.0 mg) were mixed in a flask. The sample was exposed under a UV lamp (365 nm, 41 mW cm−2, MAX-303, Asahi spectra) at room temperature for 14 hours. The solution was withdrawn for SEC measurements without reprecipitation.2.6. Characterization
1H NMR (400 MHz) spectra were acquired using a Bruker Analytik DPX400 at 27 °C. FT-IR spectra (KBr disc method) were acquired using a FT/IR-6600 spectrometer (JASCO). The number average molecular weight (Mn) and polydispersity (Mw/Mn) values of the products were assessed using a size exclusion chromatography (SEC) system, which included a PU-4185 semi-micro pump (JASCO) that was connected to an RI-4035 differential refractometer (JASCO) and a CO-2065 Plus Intelligent column oven (JASCO) equipped with a TSK-gel SuperMultipore HZ-M columns (TOSOH) was used. The measurement conditions were THF as eluent, 40 °C, flow rate of 0.35 mL min−1 and sample concentration of 1 mg mL−1. Poly(methyl methacrylate) standards were used for calibration. Differential scanning calorimetry (DSC) experiments were performed using a DSC7020 (HITACHI) system. The first heating was carried out from 30 °C to 200 °C. The temperature was then lowered to −40 °C. The second heating was carried out from −40 °C to 200 °C. The temperature change rate was set at 10 °C min−1. All measurements were performed under nitrogen gas flow. The tensile tests were performed using an AGS-500NX (SHIMADZU) apparatus. In carrying out the tests, dogbone-shaped samples with length of 13 mm and width of 4 mm were prepared using a cutting die. The tests were carried out at room temperature at an elongation rate of 10 mm min−1. Scanning electron microscopy (SEM) was performed using a JSM-7800F (JEOL) machine. Specimens coated with platinum palladium at a thickness of 5 nm were attached to carbon tape. The acceleration voltage was set at 5 kV.3. Results and discussion
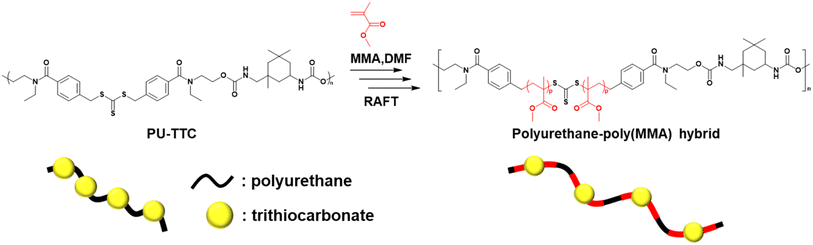
According to Scheme 1, step polymerization, using 1,5,7-triazabicyclo[4,4,0]dec-5-ene (TBD) and dibutyltin(IV) dilaurate (DBTDL) as catalysts, of commercially available TTCOH and IPDI was performed at 60 °C to give polyurethane containing trithiocarbonate (PU-TTC) moieties. In both cases, the expected step polymerizations proceeded to give viscous polymeric materials after the reaction. NMR measurements (Fig. S1–S3†) showed no presence of remaining monomer and fitted the expected spectrum of the PU-TTC product. The structure of the PU-TTC product was confirmed by IR (Fig. S4†). Characteristic peaks at 3318 cm−1 (NH stretching), 1716 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the urethane group), and 1535 cm−1 (NH vibration) were observed in the IR spectrum, suggesting the formation of urethane bonds, and also a peak around 1620 cm−1 attributed to C
O stretching of the urethane group), and 1535 cm−1 (NH vibration) were observed in the IR spectrum, suggesting the formation of urethane bonds, and also a peak around 1620 cm−1 attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the TTCOH unit was observed. These results confirmed the synthesis of our expected polyurethane containing a trithiocarbonate structure.
O stretching of the TTCOH unit was observed. These results confirmed the synthesis of our expected polyurethane containing a trithiocarbonate structure.
The details of the reaction results are summarized in Table 1, in which it seems that DBTDL is a superior catalyst (runs 2 and 3), similar to results seen in other polyurethanes syntheses.13–15 For these runs, the number average molecular weight (Mn) and polydispersity a (Mw/Mn), estimated by SEC measurements calibrated using poly(MMA) standards, Mn were 2.7 × 104 for both runs and Mw/Mns were 1.68 and 1.71, respectively for runs 2 and 3.
Subsequently, styrene monomer was incorporated into the PU-TTC backbone under blue-LED irradiation (Scheme 2 and run 1 in Table 2). The polyurethane-based macroinitiator acted at room temperature and monomer consumption was confirmed from 1H NMR, accompanied by a shift of the SEC results to higher molecular weights. After 48 h irradiation, the Mn (initially 27.0 × 103 for the parent macroinitiator) increased to 32.8 × 103, accompanied by a broadening of Mw/Mn (from 1.71 to 2.91) (run 1 in Table 2). The broadening of Mw/Mn indicated that the RAFT radical polymerization was not a controlled polymerization.
| Run | [M]0/[TTC]0 | [M]0 (mol L−1) | Radical trigger | Time (h) | Conv.b (%) | Mnc (kg mol−1) | Mw/Mnc | Tg (°C) |
|---|---|---|---|---|---|---|---|---|
| a Polymerization using PU-TTC (run 3 in Table 1) as chain transfer agent.b Calculated from 1H NMR in CDCl3.c Determined by SEC with poly(methyl methacrylate) standards.d Polymerization with Ir(ppy)3 as a redox catalyst by blue-LED irradiation.e Styrene was used as monomer.f Polymerization with AIBN as a radical trigger under 70 °C ([TTC linkage]/[AIBN] = 1/0.2). | ||||||||
| 1d,e | 300 | 4.0 | Redox-LED | 48 | 48 | 32.8 | 2.91 | 98 |
| 2d | 300 | 2.0 | Redox-LED | 24 | 82 | 32.8 | 1.73 | 116 |
| 3f | 100 | 2.0 | AIBN | 24 | 80 | 79.8 | 2.20 | 118 |
| 4f | 300 | 2.0 | AIBN | 24 | 68 | 122.1 | 1.98 | 116 |
MMA was also polymerized using PU-TTC as the macroinitiator in a similar procedure to St. After monomer addition of MMA ([M]0: 2.0 M), blue-LED irradiation was performed at room temperature (run 2 in Table 2). The SEC trace characteristic of the macroinitiator (Mn = 27.0 × 103, Mw/Mn = 1.71) shifted to higher molecular weights (Mn = 32.8 × 103), but without broadening of the polydispersity index (Mw/Mn = 1.73). The results revealed that the RAFT radical polymerization indeed proceeded in a controlled manner.
On the other hand, polymerization of MMA with AIBN as an alternative radical trigger was carried out instead of using blue LED irradiation. Heating at 70 °C produced polymerization similar to that seen under blue LED irradiation but showed a more marked increase in Mn and a broader polydispersity (runs 3–4 in Table 2 and Fig. S5†). This may be due to the cleavage of the main chain of PU-TTC by photolysis of the trithiocarbonate unit in RAFT polymerization with blue-LED, as reported by several groups.16,17 Actually, the correlation between the monomer conversion and number average molecular weight in RAFT polymerization by blue LED irradiation via PU-TTC was investigated (Fig. S6 and Table S1†). Although, the monomer conversion showed a linear increase with time, a decreasing trend in molecular weight was observed during the course of polymerization. This suggested that photolysis of trithiocarbonate occurred simultaneously with propagation reaction. Photolysis of trithiocarbonate unit is considered to have caused the main chain being cleaved and the formation of degradation products such as thioetethers, which don't show RAFT polymerization activity, resulting in the polymerization being terminated while keeping low molecular weight and remaining the monomer. Low molecular weight tailing is also observed in the SEC trace after polymerization of MMA with AIBN, which means that the initiation of MMA was not regulated due to the bulkiness of the macro CTA, PU-TTC. Main chain scission was carried out by UV irradiation18–20 in the presence of hydroquinone in order to check that the polymerization proceeded in a radical fashion. As shown in Fig. S7,† after UV irradiation the SEC trace of polyurethane–poly(MMA) hybrid shifted to the low molecular weight region, indicating that the polymerization actually occurred in a radical manner. As shown in Schemes 1 and 2, it is expected that chain initiation is proposed here through C–S bond cleavage. Compared with 1H NMR spectrum of parent PU-TTC (Fig. S8†), we could new 1H NMR signal assigned to methylene protons adjacent to trithiocarbonate moiety at 2.3 ppm as the evidence of new C–S bond formation during polymerization of MMA (Fig. S9†), which is different from the corresponding methylene (at 4.6 ppm) of the parent PU-TTC (Fig. S8†).
Finally, we checked the thermal and mechanical properties of the polyurethane–vinyl polymer hybrids by DSC and tensile strength (Fig. 1) measurements, respectively. To compare thermal properties, MMA homopolymer poly(MMA) was synthesized via RAFT polymerization using TTCOH as the RAFT agent. The poly(MMA) polydispersity and Mn were estimated by SEC to be 1.98 and 172.6 × 103, respectively. The DSC trace for the polyurethane–poly(MMA) hybrid (run 3 in Table 2) shows a heat capacity inflection point at 118.1 °C (Tg) (Fig. S10†). This is approximately 10 °C higher than that of the poly(MMA) (108.4 °C), and the Tg of the parent PU-TTC was observed at 4 °C (Fig. S11†), indicating improved thermal properties due to hybridization. The higher Tg value for the hybrid relative to that of the poly(MMA) is thought to be due to the formation of hydrogen bonds between the urethane groups (–NHCOO–) present in the main chain and the carbonyl groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of the MMA or amide groups (–NRCO–) derived TTCOH.
O) of the MMA or amide groups (–NRCO–) derived TTCOH.
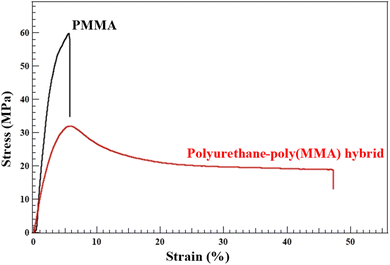 | ||
| Fig. 1 Stress–strain curves of polyurethane–poly(MMA) hybrid (run 3 in Table 2) and MMA homopolymer (room temperature, 10 mm min−1). | ||
Although PU-PMMA hybrid looks transparent (Fig. 2), quantitative analysis of the transparency evaluated the transmittance at 590 nm showed 7%, which is much lower than that (86%) of PMMA homopolymer (Table S2†). The tensile strength of the hybrids was characterized by uniaxial elongation tests conducted at room temperature. Fig. 1 shows a comparison of the stress–strain curves of polyurethane–poly(MMA) hybrids with those of the MMA homopolymer as a control sample. The latter showed typical brittle fracture behavior, whereas the polyurethane–poly(MMA) hybrid exhibited ductile fracture behavior with a distinct yield point. The introduction of intermolecular interactions such as hydrogen bonds was expected to improve tensile strength, but the results showed opposite behavior with a Young's modulus of 1.6 GPa for the PMMA homopolymer, compared to 1.0 GPa for poly(urethane-MMA). The Young's modulus was calculated from the initial slope. It is noteworthy that in the polyurethane–poly(MMA) hybrid, elongation caused whitening near the fracture zone, as noted by others in a rubber-poly(MMA) composite.21 Hayashi et al.22,23 have already reported that such whitening in amorphous glassy polymers, polyester-graft-poly(MMA), is due to cavitation. Such whitening was not observed for the poly(MMA) homopolymer. As the cavitation phenomenon is known to usually produce a large amount of voids,24 SEM observations of the samples after elongation were carried out (Fig. 2) to search for such voids. Many scattered voids (ca. 1 μm of diameter) were observed in the whitened region, whereas a uniform smooth surface was observed in other regions. Observations of the fracture surface were also performed, which revealed a dense porous surface. Furthermore, attention to the inside of the voids confirmed the formation of numerous fine fibrils. They were considered to have been formed by the elongation of voids, suggesting that the voids observed in this study were not generated during the forming process of the specimens, but were produced during the tensile test. The increased elongation at break may be due to more energy absorption caused by the formation of voids resulting from the preferential cleavage of physical crosslinking due to intermolecular hydrogen bonds in the urethane. The formation of hydrogen bonds was confirmed by IR measurements (Fig. S12†). Full range of the FT IR spectrum was also shown in Fig. S13.† A shoulder peak was observed in the peak derived from carbonyl stretching, which may be due to the overlapping absorptions of the free carbonyl (1727 cm−1) and hydrogen bonded carbonyl (1714 cm−1), and a peak (1635 cm−1) derived from hydrogen bonded esters was also observed,25 indicating that the intermolecular hydrogen bonding formation is likely responsible. In view of the above results, the observation of the whitening phenomenon may indicate an increase in the toughness of polymeric materials, as cavitation can prevent the formation of crazes before they reach an unstable state that can cause macroscopic cracks. As a control, the parent polyurethane, PU-TTC, showed the excellent ductility as shown in Fig. S14,† in which PU-TTC showed over 400%. We now concluded that the improved ductility of polyurethane–PMMA hybrid is ascribed to the urethane linkages dispersed in the PMMA matrix.
 | ||
| Fig. 2 (a) Fractured specimen of poly(urethane-MMA) (run 3 in Table 2). (b) SEM images of the transparent region and whitened region (0.1 mm from the edge) of the fracture piece. (c) SEM images of the fracture surface of the fracture piece. | ||
4. Conclusion
In this paper, we demonstrated polymerization of styrene and methyl methacrylate mediated by PU-TTC as the macro chain transfer agent to prepare a hybrid consisting of polyurethane and poly(styrene) or poly(methyl methacrylate). Compared to the methacrylate homopolymer, polyurethane–poly(MMA) hybrid showed improved thermal properties and ductility in the tensile strength measurement. These fundamental results should activated the recent researches aiming at frontier materials including flexible coatings, impact-resistant films, or elastomeric actuators.Data availability
The data supporting this article are available in the manuscript and the ESI.† Additional data can be obtained from the authors upon request.Author contributions
Monomer and polymer synthesis, structural characterization, property investigation and manuscript preparation were per formed by R. U.; data analyses and treatments were directed by A. T.; and guidance on manuscript writing, review and editing was provided by A. T.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
A. T. and R. U. are grateful to Ms Maho Suzuki and Dr Mikihiro Hayashi in their help and fruitful discussion in tensile strength measurement of the polyurethane–poly(MMA) hybrid. R. U. also acknowledge to Prof. Kenji Nagata for technical support in SEM measurements. A. T. is taking financial support of Grants-in-Aid for Scientific Research ‘KAKENHI’ (no. 24K01538).References
- O. Bayer, Das di-isocyanat-polyadditionsverfahren (polyurethane), Angew. Chem., 1947, 59, 16 CrossRef.
- B. K. Kim, J. C. Lee and K. H. Lee, Polyurethane Anionomer Dispersion from Ether-Type Polyols and Isophorone Diisocyanate, J. Macromol. Sci., Pure Appl. Chem., 1994, 31(9), 1241–1257 CrossRef.
- N. Khamma, D. Krisiri, P. Tachaprasertporn and N. Chantarasiri, Thermally stable polyurethane-ureas and copolyurethane-ureas containing zinc and nickel dihydroxysaltrien complexes, J. Appl. Polym. Sci., 2008, 108(1), 245–255 CrossRef CAS.
- C. L. Yu, J. H. Shan, Y. Chen, J. T. Shao and F. A. Zhang, Preparation and adsorption properties of rosin-based bisphenol A molecularly imprinted microspheres, Mater. Today Commun., 2020, 24, 101076 CrossRef CAS.
- S. Adeboye, A. Adebowale, B. Durodola, B. Olanipekun, K. Ajanaku, E. Akintayo, P. Basak, R. Narayan and T. Siyanbola, Synthesis and Property Evaluations of a Bio-Based Anticorrosive Urethane Composites Containing Bisphenol a as Extender, ChemistrySelect, 2024, 9(36), e202402216 CrossRef CAS.
- Y. Y. Wei, C. Z. Zong and F. F. Wang, Preparation of vinyl polymer/polyurethane hybrid latex particles and their application in nano-sized vinyl polymer particle/thermoplastic polyurethane blends, e-Polym., 2010, 99 Search PubMed.
- S. Goswami and J. K. Ranjan, Study of Mechanical and Thermomechanical Properties of Vinyl Ester/Polyurethane Interpenetrating Polymer Network Based Hybrid Composites, Fibers Polym., 2020, 21(5), 1096–1114 CrossRef CAS.
- A. Danait and D. D. Deshpande, Miscibility behaviour of blends of a thermoplastic polyester polyurethane with vinyl polymers, Macromol. Symp., 1999, 148, 489–497 CrossRef CAS.
- A. Sudo, T. Hamaguchi, N. Aoyagi and T. Endo, RAFT-approach to well-defined telechelic vinyl polymers with hydroxyl terminals as polymeric diol-type building blocks for polyurethanes, J. Polym. Sci., Part A: Polym. Chem., 2012, 51(2), 318–326 CrossRef.
- Y. Higaki, H. Otsuka, T. Endo and A. Takahara, Polyurethane macroinitiator for controlled monomer insertion of styrene, Macromolecules, 2003, 36(5), 1494–1499 CrossRef CAS.
- Q. Li, X. Hu and R. Bai, Synthesis of Photodegradable Polystyrene with Trithiocarbonate as Linkages, Macromol. Rapid Commun., 2015, 36(20), 1810–1815 CrossRef CAS PubMed.
- L. Zhang, Q. Wang, P. Lei, X. Wang, C. Wang and L. Cai, Multiblock poly(4-vinylpyridine) and its copolymer prepared with cyclic trithiocarbonate as a reversible addition–fragmentation transfer agent, J. Polym. Sci., Part A: Polym. Chem., 2007, 45(13), 2617–2623 CrossRef CAS.
- Y. S. Lu and L. Zhang, Effects of NCO/OH molar ratio on miscibility and properties of semiinterpenetrating polymer networks from polyurethane and benzyl konjac glucomannan, J. Appl. Polym. Sci., 2003, 88(5), 1304–1310 CrossRef CAS.
- M. A. Semsarzadeh and A. H. Navarchian, Effects of NCO/OH ratio and catalyst concentration on structure, thermal stability, and crosslink density of poly(urethane-isocyanurate), J. Appl. Polym. Sci., 2003, 90(4), 963–972 CrossRef CAS.
- A. Ohno, M. Hayashi and A. Takasu, Synthesis of sulfone-containing non-ionic polyurethanes for electrophoretic deposition coating, Polym. J., 2018, 50(10), 959–966 CrossRef CAS.
- R. N. Carmean, M. B. Sims, C. A. Figg, P. J. Hurst, J. P. Patterson and B. S. Sumerlin, Ultrahigh Molecular Weight Hydrophobic Acrylic and Styrenic Polymers through Organic-Phase Photoiniferter-Mediated Polymerization, ACS Macro Lett., 2020, 9(4), 613–618 CrossRef CAS PubMed.
- I. Kurowska, M. Odnoroh, O. Ivanchenko, M. Guerre and M. Destarac, Synthesis of high molar mass all-(meth)acrylic thermoplastic elastomers by photo-iniferter RAFT polymerisation, Polym. Chem., 2024, 15(47), 4888–4893 RSC.
- T. P. Davis, E. Rizzardo, L. Barner, C. Barner-Kowollik and J. F. Quinn, Reversible addition-fragmentation chain transfer polymerization initiated with ultraviolet and gamma radiation: A comparison, Abstr. Pap. Am. Chem. Soc., 2002, 224, U448 Search PubMed.
- J. F. Quinn, L. Barner, C. Barner-Kowollik, E. Rizzardo and T. P. Davis, Reversible addition-fragmentation chain transfer polymerization initiated with ultraviolet radiation, Macromolecules, 2002, 35(20), 7620–7627 CrossRef CAS.
- H. Wang, Q. B. Li, J. W. Dai, F. F. Du, H. T. Zheng and R. K. Bai, Real-Time and in Situ Investigation of "Living"/Controlled Photopolymerization in the Presence of a Trithiocarbonate, Macromolecules, 2013, 46(7), 2576–2582 CrossRef CAS.
- M. Razavi, D. Huang, S. Q. Liu, H. L. Guo and S. Q. Wang, Examining an Alternative Molecular Mechanism To Toughen Glassy Polymers, Macromolecules, 2020, 53(1), 323–333 CrossRef CAS.
- M. Hayashi, K. Shibata and S. Nobukawa, Advantage of graft architecture with a flexible main chain for implantation of ductile nature into brittle amorphous acrylic glass, Polymer, 2021, 236, 124316 CrossRef CAS.
- K. Yamawake, M. Hayashi and S. Nobukawa, Preparation of All Amorphous PMMA Resins Based on the Graft Architecture with a Flexible Main Chain for Simultaneous Enhancement of Thermal and Mechanical Toughness, Macromol. Chem. Phys., 2022, 223(24), 2200255 CrossRef CAS.
- X. A. Hou, S. Chen, J. J. Koh, J. H. Kong, Y. W. Zhang, J. C. C. Yeo, H. M. Chen and C. B. He, Entropy-Driven Ultratough Blends from Brittle Polymers, ACS Macro Lett., 2021, 10(4), 406–411 CrossRef CAS PubMed.
- Y. Hu, C.-K. Lin, M. F. Beristain and M. Soucek, Preparation and Investigation of a Urethane Methacrylate Containing a Cyclohexyl Terminal Group in Urethane/Acrylate Hybrid Latexes, Polymer, 2024, 307, 127300 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra01234a |
| This journal is © The Royal Society of Chemistry 2025 |