Open Access Article
Open Access ArticleHigh-precision optical thermometry using Pr3+-doped NaCaY(MoO4)3 luminophores: a multi-spectral and chromaticity-based approach to non-contact temperature sensing†
Zein El Abidine Aly Taleb,
Kamel Saidi and
Mohamed Dammak
and
Mohamed Dammak *
*
Laboratoire de Physique Appliquée, Groupe des Matériaux Luminescents, Faculté des Sciences de Sfax, Département de Physique, Université de Sfax, Sfax, BP 1171, Tunisia. E-mail: madidammak@yahoo.fr; Mohamed.dammak@fss.usf.tn
First published on 17th February 2025
Abstract
A series of NaCaY(MoO4)3 (NCYM) phosphors doped with Pr3+ ions was synthesized to develop advanced materials for optical temperature sensing. The structures, morphologies, and luminescent characteristics of these phosphors were thoroughly analyzed. X-ray diffraction (XRD) results confirm that all phosphors exhibit a tetragonal phase with a scheelite-type structure. Optical properties were characterized using UV-visible absorption and photoluminescence (PL) spectroscopy. Under 450 nm excitation, optimal luminescence intensity was achieved at a Pr3+ concentration of 30 mol%. Fluorescence intensity ratio (FIR) techniques, based on emissions from various excited states of Pr3+ (3P1 → 3H5 and 3P0 → 3H5; 3P1 → 3H5 and 3P0 → 3F2), were employed for thermometric characterization over the 298–498 K range. The results indicate excellent temperature detection performance, with maximum relative sensitivities of 0.69% per K and 1.2% per K at 298 K, respectively. Additionally, a study of temperature uncertainty (δT) demonstrated values below 0.06 K, with repeatability (R) exceeding 97%. The temperature-induced shift in chromaticity further improves the material's functionality, as the CIE coordinates change from (0.3806, 0.4278) at 298 K to (0.3772, 0.4229) at 498 K, demonstrating a stable transition towards yellow. These findings suggest that Pr3+-activated NCYM phosphors have significant potential for application in non-contact optical thermometry.
1. Introduction
Temperature is a fundamental physical parameter that holds a pivotal role across diverse domains, including scientific research, engineering, and industrial applications. In recent decades, luminescent materials have emerged as an innovative solution to the inherent limitations of traditional contact thermometers.1,2 These remote temperature measurement systems have been extensively explored and applied in numerous fields, offering distinct advantages. One key benefit of optical thermometry is its exceptional accuracy, which is crucial in precision-demanding applications such as semiconductor manufacturing, food safety monitoring, and medical diagnostics.3,4 Moreover, optical thermometers are particularly advantageous in extreme environments where contact-based methods are impractical, such as high-temperature or corrosive conditions.5 Recent technological advancements have further refined the precision of optical thermometry, making it an increasingly preferred option for a wide range of applications.6 Among the various approaches, the fluorescence intensity ratio (FIR) technique in phosphor materials has garnered significant attention. This method is extensively studied for its advantages, including non-contact measurement, rapid response times, and high spatial and temporal resolution, enabling effective temperature detection in challenging conditions or for rapidly moving objects.7,8 In FIR thermometry, two distinct emission peaks serve as reference signals. The efficacy of FIR-based thermometric materials is defined by three critical parameters: high absolute temperature sensitivity (Sa), high relative temperature sensitivity (Sr), and excellent signal discriminability.9,10 Rare-earth ions are highly suitable for temperature sensing due to their unique optical properties, which include sharp emission bands, narrow linewidths, long lifetimes, and high quantum efficiencies. These features make them highly responsive to temperature variations. Most studies in this field focus on thermally coupled level pairs (TCLs) of rare-earth ions, such as the 2H11/2 and 4S3/2 levels of Er3+, the 3F2,3 and 3H4 levels of Tm3+, and the 4F7/2 and 4F3/2 levels of Nd3+.11–15 In these systems, the populations of the upper and lower TCLs exhibit an inverse relationship as temperature increases, leading to variations in the fluorescence intensity ratio (FIR).The energy gap between TCLs significantly impacts thermometric performance. A narrow gap enhances absolute temperature sensitivity (Sa) but compromises relative sensitivity (Sr) and signal discriminability due to overlapping emission peaks. Conversely, a wider energy gap improves Sr and signal discriminability but reduces thermal coupling, leading to a lower Sa.16,17 Consequently, achieving simultaneous optimization of Sa, Sr, and signal discriminability remains a challenge in TCL-based optical thermometry. In addition, the selection of host materials is a critical factor in designing effective optical sensors, as it influences the overall performance and suitability of the thermometric system.
In this study, we propose a highly sensitive and precise method for monitoring elevated temperatures, up to approximately 498 K, utilizing a newly developed Pr3+-doped NCYM phosphor. Molybdates, represented by the general formula NaCaY(MoO4)3 (NCYM), stand out for several reasons. These materials exhibit broad, intense absorption bands in the near-UV region, mainly attributed to charge transfer (CT) transitions. Among the various molybdate compounds, NCYM was chosen for its stable physical and chemical properties, as well as its particularly low phonon energy, and can accommodate higher concentrations of rare earth ions.
This material is not only cost-effective but also straightforward to synthesize, exhibiting broad and intense absorption bands in the near-UV region, primarily attributed to charge transfer transitions (CTB). The molybdate host matrix serves as an excellent platform due to its stable chemical properties, low phonon energy, and the ability to accommodate high concentrations of rare-earth ions. These attributes, combined with the crystal structure and luminescent characteristics of Pr3+-activated NCYM phosphors, make them highly suitable for temperature sensing applications. To characterize its thermometric properties, we examined the thermal responses of Pr3+ emissions over the temperature range of 298–498 K, focusing on specific transitions: (3P1 → 3H5 and 3P0 → 3H5) and (3P1 → 3H4 and 3P0 → 3F2). The temperature sensor's performance was evaluated using three fluorescence intensity ratio (FIR)-based optical thermometry models, which demonstrated high sensitivity and consistent reliability in temperature measurement.
2. Experimental
2.1. Materials
In this study, all chemical reagents were obtained from Sigma-Aldrich and used without additional purification. NaCaY(MoO4)3:xPr3+ phosphor samples (x = 0.1, 0.2, 0.3, and 0.4) were synthesized using a citrate-based sol–gel method. The raw materials included sodium nitrate [(Na(NO3)3, 99.0%)], calcium nitrate tetrahydrate [(Ca(NO3)2·4H2O, 99.0%)], yttrium(III) nitrate hexahydrate [(Y(NO3)3·6H2O, 99.9%)], praseodymium(III) nitrate hexahydrate [(Pr(NO3)3·6H2O, 99.9%)], ammonium molybdate tetrahydrate [((NH4)6Mo7O24·4H2O, 99.9%)], and citric acid [C6H8O7, 99.0%].2.2. Synthesis procedure
At the outset of the synthesis process, stoichiometric amounts of sodium nitrate, calcium nitrate tetrahydrate, yttrium nitrate hexahydrate, praseodymium nitrate hexahydrate, and ammonium molybdate tetrahydrate were dissolved in 200 mL of deionized water. To this solution, 5 moles of citric acid were added as a chelating agent, ensuring a citric acid-to-metal ion ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. This process produced a total of 1.5 g of the final product. The solution was stirred at room temperature for one hour to ensure homogeneity. Upon heating, the initially transparent solution gradually turned blue, eventually forming a blue wet gel. The container was then left uncovered to facilitate liquid evaporation. The resultant blue gel was dried at 423 K for 12 hours in an oven, producing a xerogel—a stable, porous matrix. This xerogel was subsequently annealed at 673 K for 3 hours, yielding black particles. Finally, the sample underwent sintering in air at 973 K for 3 hours, resulting in pure-phase crystalline particles.
2. This process produced a total of 1.5 g of the final product. The solution was stirred at room temperature for one hour to ensure homogeneity. Upon heating, the initially transparent solution gradually turned blue, eventually forming a blue wet gel. The container was then left uncovered to facilitate liquid evaporation. The resultant blue gel was dried at 423 K for 12 hours in an oven, producing a xerogel—a stable, porous matrix. This xerogel was subsequently annealed at 673 K for 3 hours, yielding black particles. Finally, the sample underwent sintering in air at 973 K for 3 hours, resulting in pure-phase crystalline particles.
3. Characterizations techniques
The characterization of the synthesized samples involved multiple analytical techniques. X-ray powder diffraction (PXRD) patterns were recorded at room temperature using a Bruker D8-Advance diffractometer with CuKα1 radiation (λ = 1.5406 Å) 20°–80° 2θ degrees. The morphology of the samples was examined using a Zeiss Supra55VP field-emission scanning electron microscope (FEG-SEM), equipped with a Bruker XFlash 5030 detector for enhanced imaging resolution. UV-vis-NIR absorption spectra were acquired using a PerkinElmer Lambda 365 spectrometer. For photoluminescence (PL) characterization, excitation spectra were measured with a Horiba Fluoromax 4P spectrometer, utilizing a xenon lamp as the excitation source at room temperature. The absolute quantum (QY) yield was determined by the integrating sphere method on the HORIBA JOBIN YVON Fluoromax-4 spectrofluorometer. An Easy Life-Horiba instrument was employed to measure the emission lifetime. The light source was a 450 nm diode laser. The optical thermometry study was conducted in a custom-built nanometric-heating chamber, ensuring temperature control accuracy within ±0.5 K. Emission spectra were recorded under excitation by a 450 nm diode laser operating at a constant power of 30 mW. A Horiba Jobin Yvon iHR320 monochromator spectrometer, equipped with an 1800 lines per mm grating optimized for 500 nm, was used for spectral analysis. A Hamamatsu R928 photomultiplier tube detected luminescence across the blue, green, and red emission bands. Sample temperatures were systematically varied from room temperature to 498 K in 10 K increments.4. Results and discussion
4.1. X-ray diffraction analysis
X-ray diffraction (XRD) patterns were employed to examine the crystal structure and phase purity of the NSGM:xPr3+ microcrystals (where x = 0.1, 0.2, 0.3, and 0.4). Fig. 1(a) displays the XRD patterns of these samples in the 20–80° 2θ range. All observed diffraction peaks are indexed to a pure scheelite-type tetragonal structure with the space group I41/a, consistent with the Joint Committee on Powder Diffraction Standards (JCPDS) pattern no. 25-0828. The absence of impurity peaks confirms that the Pr3+ ions were successfully incorporated into the Y3+ sites, resulting in the intended pure molybdate structure. In order to confirm the crystal structure, the structural parameters of NCYM:xPr3+ were refined using the Rietveld method (Fig. S1(a–d)†). The diffraction peaks show good agreement between the observed and calculated patterns. The refined lattice parameters, cell volume, and structural details are provided in Table S1,† which further reinforce the structural integrity of the synthesized material. Fig. 1(b) illustrates the of NCYM doped with Pr3+ and the coordination of Na/Ca/Y/Pr and Mo coordination. Given the similar ionic radii of Y3+ (1.019 Å, C. N. = 8) and Pr3+ (1.126 Å, C. N. = 8),18,19 the Pr3+ ions are most likely to replace Y3+ ions and occupy the Y positions. The radius percentage difference (Dr) between the potential substituted ions and dopants is calculated using the following equation:20
 | (1) |
4.2. Morphology characterization
The scanning electron microscope (SEM) technique was utilized to explore the surface morphology and grain size distribution. The SEM images of NCYM:xPr3+ samples are shown in Fig. 2(a–d). It is evident that the irregular shapes result from the aggregation of grains and are on the micrometer scale, which is favorable for improving their photoluminescence (PL) properties.4.3. Optical characterization
The plots of [F(R∞)hν]2 versus photon energy (hν) for NCYM:xPr3+ (x = 0.1, 0.2, 0.3 and 0.4) are shown in Fig. S2(a–d),† the direct optical band gap values (Eg) can be obtained by using K–M function and Tauc formulae:23,24
 | (2) |
| [F(R∞)hν] = B(hv − Eg)n | (3) |
 | ||
| Fig. 4 (a) PL spectrum of NCYM:0.3Pr3+ excited by 450 nm; (b) the schematic of the energy levels of Pr3+ ions and the possible transitions when excited by 450 nm. | ||
The energy bandgap law states that if the difference between two energy levels is greater than five times the value of the highest energy phonon in the host medium, the probability of multiphonon relaxation will be negligible. The multiphonon non-radiative transition rates “WNR” can be written using the formula:28,29
| WNR = (1/t0)exp(−ap) | (4) |
The 602 nm emission associated with the 1D2 → 3H4 transition is weak in the studied molybdate, whereas the 3P0-related emissions at 490, 621, and 651 nm are dominant. Similar phenomena have been observed in other luminophores, such as PMN-PT:Pr3+ and La2MgTiO6:Pr3+.34,35 A simplified diagram of the Pr3+ energy levels is shown in the Fig. 4(b).
The quantum yield provides valuable insights into the optical performance of phosphors. The quantum yield (QY) of the CDs was determined using the integrating sphere method, which allows for an absolute measurement by directly quantifying both the emitted and absorbed photons. The general equation is as follows:
| QY = number of photons emitted/number of photons absorbed |
Fig. S4† presents the curve of the absolute quantum yield measured using an integrating sphere. A quantum yield of 75% was observed for the studied, demonstrating its exceptional optical efficiency. Furthermore, the quantum yield of our analyzed phosphor exceeds that of several other commercial phosphors, such as Ca2GdSbO6:Eu3+ (73%),36 Pr3+-doped CAS glasses (37%),37 GCs:Pr3+/Yb3+ (38.9%)38 and Bi4Si3O12:Eu3+ (14.5%).39
In order to demonstrate ideal light qualities in the host under study findings, various concentrations of Pr3+ ions were doped into the host material, and their emission properties were examined. PL emission spectra at room temperature for different concentrations of Pr3+ ions doped into NCYM are shown in Fig. 5(a) NCYM:xPr3+ (x = 0.1, 0.2, 0.3 and 0.4), which shows only the emission bands of note for Pr3+ ions, and their intensities are controlled by the doping concentration at 450 nm excitation.
The photoluminescence (PL) intensity is observed to increase to 0.3 and subsequently decrease, as depicted in Fig. 5(a), due to the process of concentration quenching. Typically, concentration quenching occurs through one of two mechanisms: exchange interaction or electric multipolar interaction. Exchange interaction occurs when the wave functions of acceptor and donor ions overlap within a defined critical distance (RC). This overlap reduces the intensity of emission peaks and increases nonradiative energy transfer between the ions. The critical distance between the two Pr3+ ions present in the NCYM lattice is calculated.40–42
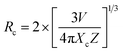 | (5) |
The formula for the critical distance for Pr3+ ions in NCYM arrays is estimated as Rc = 7.86 Å, demonstrating the presence of the electric multipole interaction.
The emission decay curves of lanthanide-activated phosphors were also analyzed, and the correlation between activator concentration, luminescence characteristics, and decay duration could strongly support concentration quenching during the energy migration process. Fig. 5(b) shows the logarithmic intensity decay curves of red emissions at 650 nm in NCYM:xPr3+ phosphors (x = 0.1, 0.2, 0.3, and 0.4) under 450 nm excitation. The observed emission at 650 nm corresponds to the 3P0 → 3F2 transition of Pr3+, confirming that the initial excited state for this luminescence is 3P0^3P_03P0. All the decay curves can be fitted by the following:43
 | (6) |
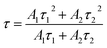 | (7) |
| Sample | A1 | τ1 (μs) | A2 | τ2 (μs) | τ (μs) |
|---|---|---|---|---|---|
| NCYM:0.1Pr3+ | 0.61 | 0.35 | 0.43 | 0.69 | 0.54 |
| NCYM:0.2Pr3+ | 0.75 | 0.45 | 0.27 | 0.81 | 0.59 |
| NCYM:0.3Pr3+ | 0.71 | 0.45 | 0.25 | 0.88 | 0.62 |
| NCYM:0.4Pr3+ | 0.63 | 0.28 | 0.41 | 0.64 | 0.50 |
4.4. Temperature sensing
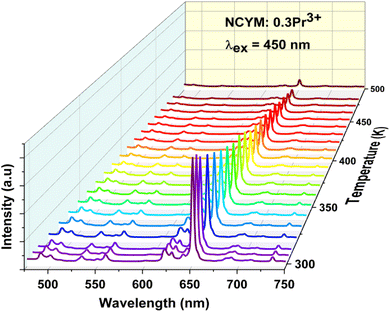
In order to determine the potential of the materials studied for optical temperature measurement, the high-temperature photoluminescence (PL) spectra of NCYM:0.3Pr3+ were recorded. Fig. 6 shows the PL spectra recorded from 298 K to 498 K under excitation at 450 nm. Thanks to the presence of different Pr3+ emission bands, especially those of the 3P0 → 3H4, 3P1 → 3H5, 3P0 → 3H5 and 3P0 → 3F2 transitions, the microcrystal obtained can act as optical temperature monitors, based on the light intensity ratio of these bands. The total intensity of luminescence decreases progressively with increasing temperature. Therefore, two fluorescence intensity ratio (FIR) patterns are used as temperature detection signals due to the marked differences in temperature dependence between the three emissions (3P1 → 3H5 and 3P0 → 3H5 (FIR1)), and (3P1 → 3H4 and 3P0 → 3F2 (FIR2)).The integrated intensities of the three emissions in Fig. 7(a and b) show that the intensities of FIR1 and FIR2 increase monotonically with increasing temperature. The FIR of the two models can be deduced and expressed as follows:44,45
 | (8) |
 | ||
| Fig. 7 (a and b) Variations of FIR as a function of temperature for NCYM:0.3Pr3+ between (a) (3P1–3H5/3P0–3H5), and (b) (3P1–3H5/3P0–3F2). | ||
In order to quantitatively determine the sensitivity of the two modes for optical thermometry, it is very important to analyze the relative and absolute sensitivity of the sensor, which is the ratio of the variation of the FIR with temperature and can be defined as follows:46,47
 | (9) |
 | (10) |
The values of Sr and Sa, calculated by eqn (9) and (10), are shown in Fig. 8(a and b). The maximum Sr values for the models reach 0.69% per K and 1.2% per K at 298 K for FIR1 and FIR2, respectively. Similarly, the maximum Sa values reach 0.0054 per K (at 298 K) and 0.00085 per K (at 298 K) for FIR1 and FIR2, respectively. The NCYM:Pr3+ phosphors had three FIR models monitored. When one of the FIR patterns is in error, other patterns can be simultaneously monitored to correct the inaccuracy. In this way, the self-calibrating optical thermometer can be made by simultaneously monitoring two FIR patterns. To give the reader an idea of the current state of the art, a summary of the various luminescent thermometers based on the FIR technique is given in Table 2. It can be concluded that, compared with other works, our material offers superior performance.
 | ||
| Fig. 8 (a and b) Sa and Sr values corresponding to (a) FIR1 (3P1–3H5/3P0–3H5), and (b) FIR2 (3P1–3H5/3P0–3F2). | ||
| Materials | Sr max (% per K) | T (K) | T-range (K) | Transitions | λ (nm) | Ref. |
|---|---|---|---|---|---|---|
| CaZnOS:Er3+ | 0.33 | 603.15 | 303–603 | 2H11/2/4S3/2 | 520/539 | 48 |
| PKAZLF glass:Er3+ | 0.79 | 630 | 298–773 | 2H11/2/4S3/2 | 530/550 | 49 |
| α-NaYF4:Nd3+ | 0.12 | 273 | 273–423 | 4F3/2–4I9/2 (Stark) | 863/870 | 50 |
| LaF3:Nd3+ | 0.26 | 296 | 296–345 | 4F3/2–4I9/2 (Stark) | 865/885 | 51 |
| β-NaYF4:Yb3+, Ho3+ | 0.9 | 300 | 300–500 | 5F5–5I8/2S2, 5F4–5I8 | 545/650 | 52 |
| SrF2:Yb3+, Er3+ | 1.20 | 298 | 298–383 | 2H11/2/4S3/2 | 525/545 | 53 |
| β-NaLuF4:Yb3+, Er3+, Ho3+ | 0.74 | 298 | 298–503 | 2H11/2/4S3/2 | 527/547 | 54 |
| GdVO4:Er3+/Yb3+ | 0.7 | 300 | 300–453 | 2H11/2/4S3/2 | 530/550 | 55 |
| LaMgTiO6:Pr3+ | 1.28 | 350 | 77–500 | 1D2, 3P0–3H4 | 608/499 | 35 |
| PMN-PT-PYN:Er3+ | 0.3 | — | 113–413 | 2H11/2/4S3/2 | 525/539 | 56 |
| PMN-PT:Pr3+ | 0.70 | — | 223–313 | 3P1–3H5/3P0–3H5 | 534/500 | 34 |
| PMN-PT:Pr3+ | 0.06 | — | 223–313 | 3P1–3H5/3P0–3F2 | 534/650 | 34 |
| NCYM:Pr3+ | 0.69 | 298 | 298–498 | 3P1–3H5/3P0–3H5 | 533/558 | This work |
| NCYM:Pr3+ | 1.2 | 298 | 298–498 | 3P1–3H5/3P0–3F2 | 533/651 | This work |
Temperature resolution, also known as uncertainty (δT), is another key parameter that determines the smallest temperature change that can be detected by an optical thermometer and describes the possible error in temperature reading. The temperature uncertainty (δT) using luminescent thermometry can be calculated using the equation as follows:57,58
 | (11) |
In this study, the standard deviation of the fluorescence intensity ratio (δFIR) is defined as the variability of FIR values obtained from repeated measurements at a constant temperature. The FIR itself represents the average value derived from these measurements. To establish the detection limit, 40 independent measurements were performed at room temperature under controlled conditions. The data are summarized in the histograms displayed in Fig. S5(a and b).† The uncertainty in FIR (δFIR) was computed based on the statistical spread of the data for the NCYM:0.3Pr3+ sample, yielding values of 0.016 and 0.003 for FIR1 and FIR2, respectively. Using eqn (11), the corresponding temperature uncertainties (δT) were calculated and found to be below 0.06 K. These results highlight the high precision and reliability of the FIR-based models across the investigated temperature range.
This analysis underscores the exceptional temperature resolution of the NCYM phosphor. Specifically, the NCYM:0.3Pr3+ sample exhibits high thermal sensitivity coupled with minimal temperature measurement errors, making it a promising candidate for optical thermometry using the FIR method. The δT values for FIR1 and FIR2 are shown in Fig. 9(a and b), further demonstrating the robustness of this material in temperature sensing applications.
 | ||
| Fig. 9 (a and b) Temperature resolution values δT, corresponding to (a) FIR2 (3P1–3H5/3P0–3H5) and (b) FIR3 (3P1–3H5/3P0–3F2) for NCYM:0.3Pr3+. | ||
The repeatability (R) is another important way, to verify the accuracy of the temperature sensing techniques used. The thermometric parameters determined (FIR values) were measured repeatedly by moving the sample from a low temperature to a high temperature, as shown in Fig. 10(a and b). The calculation used to determine repeatability (R) was as follows:59
 | (12) |
4.5. Temperature-dependent color shifts and their implications for thermal sensing
The chromaticity diagram, along with the Commission Internationale de l'Éclairage (CIE) coordinates, offers a precise method for evaluating the emission color characteristics of luminescent materials. In this study, the impact of temperature on the emission color of NCYM:0.3Pr3+ was systematically analyzed through CIE chromaticity coordinates, measured under 450 nm excitation across a temperature range from 298 K to 498 K. As depicted in Fig. 11, the CIE coordinates of the sample shift from (x = 0.3806, y = 0.4278) at 298 K to (x = 0.3772, y = 0.4229) at 498 K, indicating a clear and stable transition in emission color toward the yellow region as the temperature increases. The change in the x and y values reflects the continuous and stable shift in emission towards yellow, which occurs as a result of the thermal activation of different electronic states in the Pr3+ ions. | ||
| Fig. 11 CIE (x,y) coordinate diagram of the emission color at different temperature for NCYM:0.3Pr3+. | ||
This color shift provides critical insights into the temperature-dependent behavior of the material, suggesting that NCYM:0.3Pr3+ could be employed as a visual thermal sensor. The color change is stable and consistent over the studied temperature range, which is a crucial characteristic for practical thermal safety applications. Unlike traditional temperature sensors, which rely on electronic measurements, the color shift observed here offers an optical, non-contact alternative for temperature monitoring, making it suitable for environments where conventional sensors may be impractical.
The observed temperature-induced color shift is attributed to the thermal population of different electronic states in the Pr3+ ion, specifically the 3P0 and 3P1 states, which are responsible for the observed visible emissions. The correlation between temperature and emission color could thus be exploited for real-time monitoring of temperature fluctuations. This feature enhances the material's potential as a thermal safety indicator, where rapid visual detection of temperature variations could serve as an early warning system in sensitive applications, such as in electronics, automotive, or aerospace industries.
Additionally, the stable shift in CIE coordinates towards yellow over the entire temperature range suggests that NCYM:0.3Pr3+ can be a versatile material for applications requiring continuous monitoring or dynamic color changes with temperature. The gradual shift across the chromaticity diagram could be utilized in color-based thermal imaging systems, offering a straightforward method for visualizing temperature distributions. The precise CIE coordinates at room temperature (298 K) of (x = 0.3806, y = 0.4278) and at 498 K of (x = 0.3772, y = 0.4229) confirm that the material's emission color transitions in a controlled, repeatable manner, making it a reliable choice for real-time thermal imaging applications.
5. Conclusion
In summary, Pr3+-doped NCYM luminophores, synthesized using the citrate-based sol–gel method, demonstrate significant potential for advanced optical applications. The scheelite-type tetragonal structure with space group I41/a was confirmed by XRD and Rietveld refinement analysis. SEM analysis revealed an average particle size of approximately 3 μm. Absorption measurements revealed the molybdate host's characteristic absorption band in the visible region. The optical band gap (3.36–3.84 eV) showed minimal impact from Pr3+ doping on the host's electronic structure. Photoluminescence spectra under 450 nm excitation exhibited strong cyan, green, and red emissions, with optimal luminescence achieved at a 0.3 mol% doping concentration. These microparticles displayed excellent potential as optical, non-contact temperature sensors, with temperature-dependent emission intensity ratios yielding high sensitivity (0.69, and 1.2% per K for 533/558, and 533/651 nm ratios, respectively). Luminescence thermometry demonstrated impressive precision, with temperature uncertainty (δT) below 0.06 K and repeatability over 97%. Additionally, the temperature-induced chromaticity shift further enhances the material's functionality, as the CIE coordinates shifted from (0.3806, 0.4278) at 298 K to (0.3772, 0.4229) at 498 K, indicating a stable transition toward yellow. This highlights NCYM:0.3Pr3+ as a promising candidate for practical thermal indicators and sensors in high-precision, non-contact temperature measurements. Overall, the superior accuracy and reliability of Pr3+-doped NCYM as an optical thermometer position it for transformative applications in precision temperature monitoring, thermal management, and safety systems. This work lays the groundwork for future advancements in optical thermometry.Data availability
All data underlying the results are available as part of the article and no additional source data are required.Conflicts of interest
There are no conflicts to declare.References
- X. Wang, O. S. Wolfbeis and R. J. Meier, Luminescent probes and sensors for temperature, Chem. Soc. Rev., 2013, 42, 7834–7869 RSC.
- M. D. Dramićanin, Sensing temperature via downshifting emissions of lanthanide-doped metal oxides and salts. A review, Methods Appl. Fluoresc., 2016, 4, 042001 CrossRef PubMed.
- J. Zhong, et al., A review on nanostructured glass ceramics for promising application in optical thermometry, J. Alloys Compd., 2018, 763, 34–48 CrossRef CAS.
- K. Saidi, C. Hernández-Álvarez, M. Runowski, M. Dammak and I. R. Martín, Ultralow pressure sensing and luminescence thermometry based on the emissions of Er3+/Yb3+ codoped Y2Mo4O15 phosphors, Dalton Trans., 2023, 52, 14904–14916 RSC.
- M. Quintanilla, M. Henriksen-Lacey, C. Renero-Lecuna and L. M. Liz-Marzán, Challenges for optical nanothermometry in biological environments, Chem. Soc. Rev., 2022, 51, 4223–4242 RSC.
- K. Saidi, I. Kachou, K. Soler-Carracedo, M. Dammak and I. R. Martín, Ba2YV3O11 Er3+/Yb3+ Nanostructures for Temperature Sensing in the Presence of Bismuth Ions, ACS Appl. Nano Mater., 2023, 6, 17681–17690 CrossRef CAS.
- Z. Wang, et al., Nd3+ -sensitized NaLuF4 luminescent nanoparticles for multimodal imaging and temperature sensing under 808 nm excitation, Nanoscale, 2015, 7, 17861–17870 RSC.
- S. Zheng, et al., Lanthanide-doped NaGdF4 core–shell nanoparticles for non-contact self-referencing temperature sensors, Nanoscale, 2014, 6, 5675–5679 RSC.
- W. Xu, et al., Optical temperature sensing through the upconversion luminescence from Ho3+/Yb3+ codoped CaWO4, Sens. Actuators, B, 2013, 188, 1096–1100 CrossRef CAS.
- D. Chen, et al., Dual-activator luminescence of RE/TM:Y3 Al5 O12 (RE = Eu3+ , Tb3+ , Dy3+ ; TM = Mn4+ , Cr3+) phosphors for self-referencing optical thermometry, J. Mater. Chem. C, 2016, 4, 9044–9051 RSC.
- Z. E. A. A. Taleb, K. Saidi and M. Dammak, Dual-mode optical ratiometric thermometry using Pr3+-doped NaSrGd(MoO4)3 phosphors with tunable sensitivity, Dalton Trans., 2023, 52, 18069–18081 RSC.
- K. Saidi and M. Dammak, Upconversion luminescence and optical temperature sensing characteristics of Er3+/Yb3+ codoped Na3Gd(PO4)2 phosphors, J. Solid State Chem., 2021, 300, 122214 CrossRef CAS.
- M. Yangui, K. Saidi, C. Hernández-Álvarez, M. Dammak and I. R. Martín, Ratiometric luminescent thermometry with excellent sensitivity in the visible and near-infrared (NIR-II/III) based on thermally coupled and non-thermally coupled energy levels of Er3+ and Ho3+ ions, J. Alloys Compd., 2025, 1010, 177958 CrossRef CAS.
- C. Hernández-Álvarez, et al., Multifunctional optical sensing platform of temperature, pressure (vacuum) and laser power density:NaYF4:Gd3+, Yb3+, Er3+ nanomaterial as luminescent thermometer, manometer and power meter, J. Mater. Chem. C, 2023, 11, 10221–10229 RSC.
- I. Kachou, K. Saidi, C. Hernández-Álvarez, M. Dammak and I. R. Martín, Enhancing thermometric precision: modulating the temperature of maximum sensitivity via erbium dopant addition in Ba2GdV3O11:Tm3+/Yb3+ nano phosphors, Mater. Adv., 2024, 5, 8280–8293 RSC.
- W. Xu, X. Gao, L. Zheng, Z. Zhang and W. Cao, An optical temperature sensor based on the upconversion luminescence from Tm3+/Yb3+ codoped oxyfluoride glass ceramic, Sens. Actuators, B, 2012, 173, 250–253 CrossRef CAS.
- D. Chen, et al., Bulk glass ceramics containing Yb3+/Er3+: β-NaGdF4 nanocrystals: Phase-separation-controlled crystallization, optical spectroscopy and upconverted temperature sensing behavior, J. Alloys Compd., 2015, 638, 21–28 CrossRef CAS.
- R. A. Talewar, V. M. Gaikwad, P. K. Tawalare and S. V. Moharil, Opt Laser. Technol., 2019, 115, 215–221 CrossRef CAS.
- L. Li, P. Yang, W. Xia, Y. Wang, F. Ling, Z. Cao, S. Jiang, G. Xiang, X. Zhou and Y. Wang, Ceram. Int., 2021, 47, 769–775 CrossRef CAS.
- Y. Bahrouni, I. Kachou, K. Saidi, T. Kallel, M. Dammak, I. Mediavilla and J. Jiménez, Mater. Adv., 2025 Search PubMed , advance (in press).
- L. Li, P. Yang, W. Xia, Y. Wang, F. Ling, Z. Cao, S. Jiang, G. Xiang, X. Zhou and Y. Wang, Ceram. Int., 2021, 47, 769–775 CrossRef CAS.
- J. Wang, N. Chen, J. Li, Q. Feng, R. Lei, H. Wang and S. Xu, J. Lumin., 2021, 238, 118240 CrossRef CAS.
- M. Enneffati, M. Rasheed, B. Louati, K. Guidara and R. Barillé, Opt. Quantum Electron., 2019, 51, 299 CrossRef.
- K. Baishya, J. S. Ray, P. Dutta, P. P. Das and S. K. Das, Appl. Phys. A, 2018, 124, 704 CrossRef.
- Y. Gao, F. Huang, H. Lin, J. Xu and Y. Wang, Sens. Actuators, B, 2017, 243, 137–143 CrossRef CAS.
- M. Fhoula, K. Saidi, C. Hernández-Álvarez, K. Soler-Carracedo, M. Dammak and I. R. Martín, J. Alloys Compd., 2024, 979, 173537 CrossRef CAS.
- K. Saidi, M. Yangui, C. Hernández-Álvarez, M. Dammak, I. Rafael Martín Benenzuela and M. Runowski, ACS Appl. Mater. Interfaces, 2024, 16, 19137–19149 CrossRef CAS PubMed.
- M. J. Weber, Multiphonon Relaxation of Rare-Earth Ions in Yttrium Orthoaluminate, Phys. Rev. B, 1973, 8, 54–64 CrossRef CAS.
- S. A. Payne and C. Bibeau, Picosecond nonradiative processes in neodymium-doped crystals and glasses, J. Lumin., 1998, 79, 143–159 CrossRef CAS.
- M. V. V. Kumar, K. R. Gopal, R. R. Reddy, G. V. L. Reddy, N. S. Hussain and B. C. Jamalaiah, J. Non-Cryst. Solids, 2013, 364, 20–27 CrossRef CAS.
- V. Naresh and B. S. Ham, J. Alloys Compd., 2016, 664, 321–330 CrossRef CAS.
- H. Dornauf and J. Heber, J. Lumin., 1980, 22, 1–16 CrossRef CAS.
- C. De Mello Donegá, A. Meijerink and G. Blasse, J. Phys. Chem. Solids, 1995, 56, 673–685 CrossRef.
- Y. Qin, et al., Fluorescence intensity ratio (FIR) analysis of the temperature sensing properties in transparent ferroelectric PMN-PT:Pr3+ ceramic, Ceram. Int., 2021, 47, 24092–24097 CrossRef CAS.
- R. Shi, L. Lin, P. Dorenbos and H. Liang, Development of a potential optical thermometric material through photoluminescence of Pr3+ in La2MgTiO6, J. Mater. Chem. C, 2017, 5, 10737–10745 RSC.
- Z. Zhang, L. Sun, B. Devakumar, J. Liang, S. Wang, Q. Sun, S. J. Dhoble and X. Huang, J. Lumin., 2020, 221, 117105 CrossRef CAS.
- C. Y. Morassuti, et al., Spectroscopic investigation and interest of Pr3+-doped calcium aluminosilicate glass, J. Lumin., 2019, 210, 376–382 CrossRef CAS.
- D. Ding, J. Gao, S. Zhang and L. Duo, The photoluminescence properties of Pr3+-Yb3+ co-doped gallo-germanate glasses and glass ceramics as energy converter, J. Lumin., 2020, 226, 117512 CrossRef CAS.
- J. Y. Park, S. J. Park and H. K. Yang, Investigation of red-emitting Bi4Si3O12:Eu3+ phosphor under the deep UV irradiation as a novel material for white light and color tunable emission, Optik, 2018, 166, 69–76 CrossRef CAS.
- K. Saidi, W. Chaabani and M. Dammak, Highly sensitive optical temperature sensing based on pump-power-dependent upconversion luminescence in LiZnPO4 :Yb3+–Er3+/Ho3+ phosphors, RSC Adv., 2021, 11, 30926–30936 RSC.
- I. Kachou, K. Saidi, R. Salhi and M. Dammak, Synthesis and optical spectroscopy of Na3 Y(VO4)2:Eu3+ phosphors for thermometry and display applications, RSC Adv., 2022, 12, 7529–7539 RSC.
- Z. E. A. A. Taleb, K. Saidi and M. Dammak, The dual-model up/down-conversion green luminescence of NaSrGd(MoO4)3:Er3+ and its application for temperature sensing, RSC Adv., 2024, 14, 8366–8377 RSC.
- J. Wang, N. Chen, J. Li, Q. Feng, R. Lei, H. Wang and S. Xu, J. Lumin., 2021, 238, 118240 CrossRef CAS.
- J. Xing, F. Shang and G. Chen, Ceram. Int., 2021, 47, 8330–8337 CrossRef CAS.
- L. Li, P. Yang, W. Xia, Y. Wang, F. Ling, Z. Cao, S. Jiang, G. Xiang, X. Zhou and Y. Wang, Ceram. Int., 2021, 47, 769–775 CrossRef CAS.
- Z. E. A. A. Taleb, K. Saidi, M. Dammak, D. Przybylska and T. Grzyb, Ultrasensitive optical thermometry using Tb3+ doped NaSrGd(MoO4)3 based on single band ratiometric luminescence, Dalton Trans., 2023, 52, 4954–4963 RSC.
- X. Zhou, et al., Tunable emission color of Li2SrSiO4:Tb3+ due to cross-relaxation process and optical thermometry investigation, J. Am. Ceram. Soc., 2018, 101, 3076–3085 CrossRef CAS.
- H. Zhang, D. Peng, W. Wang, L. Dong and C. Pan, Mechanically Induced Light Emission and Infrared-Laser-Induced Upconversion in the Er-Doped CaZnOS Multifunctional Piezoelectric Semiconductor for Optical Pressure and Temperature Sensing, J. Phys. Chem. C, 2015, 119, 28136–28142 CrossRef CAS.
- N. Vijaya, et al., Optical characterization of Er3+-doped zinc fluorophosphate glasses for optical temperature sensors, Sens. Actuators, B, 2013, 186, 156–164 CrossRef CAS.
- D. Wawrzynczyk, A. Bednarkiewicz, M. Nyk, W. Strek and M. Samoc, Neodymium (III) doped fluoride nanoparticles as non-contact optical temperature sensors, Nanoscale, 2012, 4, 6959–6961 RSC.
- L. Marciniak, A. Pilch, S. Arabasz, D. Jin and A. Bednarkiewicz, Heterogeneously Nd3+ doped single nanoparticles for NIR-induced heat conversion, luminescence, and thermometry, Nanoscale, 2017, 9, 8288–8297 RSC.
- H. Li, Y. Zhang, L. Shao, Z. Htwe and P. Yuan, Luminescence probe for temperature sensor based on fluorescence intensity ratio, Opt. Mater. Express, 2017, 7, 1077–1083 CrossRef CAS.
- S. Balabhadra, M. L. Debasu, C. D. S. Brites, R. A. S. Ferreira and L. D. Carlos, Upconverting Nanoparticles Working As Primary Thermometers In Different Media, J. Phys. Chem. C, 2017, 121, 13962–13968 CrossRef CAS.
- N. Niu, et al., Tunable multicolor and bright white emission of one-dimensional NaLuF4:Yb3+, Ln3+ (Ln = Er, Tm, Ho, Er/Tm, Tm/Ho) microstructures, J. Mater. Chem., 2012, 22, 10889 RSC.
- J. Postlethwait, et al., Correction: Vertebrate genome evolution and the zebrafish gene map, Nat. Genet., 1998, 19, 303 CrossRef.
- J. Zhu, et al., Improving the up/down-conversion luminescence via cationic substitution and dual-functional temperature sensing properties of Er3+ doped double perovskites, Chem. Eng. J., 2023, 471, 144550 CrossRef CAS.
- M. Rajendran and S. Vaidyanathan, High performance red/deep-red emitting phosphors for white LEDs, New J. Chem., 2020, 44, 5354–5365 RSC.
- M. S. Pudovkin, O. A. Morozov, V. V. Pavlov, S. L. Korableva, E. V. Lukinova, Y. N. Osin, V. G. Evtugyn, R. A. Safiullin and V. V. Semashko, Physical Background for Luminescence Thermometry Sensors Based on Pr3+:LaF3 Crystalline Particles, J. Nanomater., 2017, 2017(1), 3108586 Search PubMed.
- A. Ćirić, et al., Comparison of three ratiometric temperature readings from the Er3+ upconversion emission, Nanomaterials, 2020, 10, 627 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00093a |
| This journal is © The Royal Society of Chemistry 2025 |