 Open Access Article
Open Access ArticleHighly efficient hydrogenation of furfural to furfuryl alcohol over Cu–Al2O3–ZnO catalyst†
Junqi Zhang,
Yongwang Li,
Zhiwei Zhang,
Zheng Wang,
Jiaxing Zhang,
Shuai Liu,
Yang Qin,
Bingxin Zhu,
Tongxue Zhang,
Hongyu Wang,
Fumin Wang * and
Xubin Zhang
* and
Xubin Zhang *
*
School of Chemical Engineering and Technology, Tianjin University, Tianjin 300350, China. E-mail: tjzxb@tju.edu.cn
First published on 10th February 2025
Abstract
The development of simple, efficient and economical catalysts for the hydrogenation of biomass to produce high value-added chemicals is of great significance in solving the energy crisis. In this work, a series of non-precious metal catalysts (Cu–Al2O3–ZnO) with different defect sites were prepared by etching Devarda's alloy. Under optimized mild reaction conditions, the furfural conversion and furfuryl alcohol selectivity are both greater than 99.0%, and the catalyst has good reusability. Characterisation and experiments were used to investigate the activate species for hydrogenation reaction. It can be proved that the low-valent Cu species in the Cu–Al2O3–ZnO catalysts play an important role as adsorption and dissociation sites for H2. Different etching degrees and sample reduction temperatures of the alloy can be used to adjust the content of acidic sites such as Al2O3 and CuO, which have appropriate adsorption properties for furfural. ZnO promotes the dispersion of the Cu species and enhances the accessibility of the active sites. The etching method achieves the interaction between species to further enhance the stability and activity of the catalyst. The catalytic performance of the catalyst is very competitive and this study provides a new method for the efficient hydrogenation of furfural to furfuryl alcohol.
1. Introduction
In the context of fossil fuel depletion and global warming, the extraction of high value-added products from a wide range of biomass platform chemicals has gradually become a viable alternative to petroleum products in order to solve a range of environmental problems and the energy crisis, and to reduce over-reliance on fossil fuels.1–3 Furfural (FAL) derived from lignocellulosic biomass, has attracted increasing attention in recent years as a valuable platform chemical that can be used as an intermediate to provide a variety of products for the energy, chemical and pharmaceutical industries,4,5 furthermore, in the hydrogenation reaction, due to the presence of various unsaturated groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in the FAL molecule, a wide range of products can be produced, such as furfuryl alcohol (FOL), tetrahydrofurfuryl alcohol, 2-methylfuran, 2-methyltetrahydrofuran, 1,2-pentanediol and 1,5-pentanediol.6–10
O) in the FAL molecule, a wide range of products can be produced, such as furfuryl alcohol (FOL), tetrahydrofurfuryl alcohol, 2-methylfuran, 2-methyltetrahydrofuran, 1,2-pentanediol and 1,5-pentanediol.6–10
Catalytic hydrogenation has been used as the main application for the conversion of FAL to various high-value chemicals. The majority of FAL worldwide (∼65%) are used in the production of FOL.11 FOL is considered a high-value intermediate that can be used as a raw material to manufacture products such as vitamin C and lysine,12,13 and has a wide range of applications in the fields of polymers, pharmaceuticals and cosmetics.12–14 Generally speaking, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond is active, and the FOL can further hydrogenate the C
C bond is active, and the FOL can further hydrogenate the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond to form tetrahydrofurfuryl alcohol.15,16 In addition, hydrolysis of the FOL moiety can produce other by-products, resulting in a variety of complex and competing reaction pathways.17 Since excessive hydrogenation or hydrolysis produces other by-products, which is the main problem with the hydrogenation of FAL to FOL, it is necessary to ensure high hydrogenation activity while preferentially hydrogenating C
C bond to form tetrahydrofurfuryl alcohol.15,16 In addition, hydrolysis of the FOL moiety can produce other by-products, resulting in a variety of complex and competing reaction pathways.17 Since excessive hydrogenation or hydrolysis produces other by-products, which is the main problem with the hydrogenation of FAL to FOL, it is necessary to ensure high hydrogenation activity while preferentially hydrogenating C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups and avoiding excessive hydrogenation.
O groups and avoiding excessive hydrogenation.
At present, various transition metal catalysts are often used for the selective hydrogenation of FAL to FOL. In general, precious metal catalysts tend to have higher catalytic activity than non-precious metal catalysts, and they are often used in combination with non-precious metal elements to catalyze hydrogenation reactions. For example, precious metal elements Pt, Ru and Pd-based catalysts18–21 exhibit high activity in the hydrogenation of FAL to FOL under relatively mild reaction conditions (30–100 °C, 0.8–2 MPa H2). However, factors such as the high cost of precious metal catalysts and the difficulty of recycling also severely limit their large-scale practical application. Compared to precious metal catalysts, Ni, Co and Cu-based non-precious metal catalysts have also achieved high FAL conversion rates and high FOL yields in the hydrogenation of FAL to FOL. For example, CuCo/MgO,11 Cu–ZnO–Al2O3,22 Cu2Ni1AlOy,23 CoAl,24 C@Ni3P25 have achieved more than 95% yields of FOL. However, some non-precious metal catalysts also have the disadvantages of complicated preparation process and low reactivity. Among the non-precious metal catalysts, the transition metal Cu initially adsorbs preferentially on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of FAL rather than the furan ring due to the overlap of the 3d band of Cu atoms with the antibonding orbitals of the furan ring, which is highly selective for FOL.26–30 However, the interaction between monometallic Cu-based catalysts and FAL is generally weak, the accessibility of the active center is severely restricted, and the reaction conditions for Cu-based catalysts have been relatively harsh in the past.31,32 Therefore, enhancing the interaction between Cu-based catalysts and FAL and improving catalytic activity are the main issues that need to be addressed in the hydrogenation of FAL to FOL.
O of FAL rather than the furan ring due to the overlap of the 3d band of Cu atoms with the antibonding orbitals of the furan ring, which is highly selective for FOL.26–30 However, the interaction between monometallic Cu-based catalysts and FAL is generally weak, the accessibility of the active center is severely restricted, and the reaction conditions for Cu-based catalysts have been relatively harsh in the past.31,32 Therefore, enhancing the interaction between Cu-based catalysts and FAL and improving catalytic activity are the main issues that need to be addressed in the hydrogenation of FAL to FOL.
Inspired by the widespread application and high activity of skeletal Cu-based catalysts derived from etched alloys in hydrogenation reactions,33–36 as well as the versatility of RANEY® nickel37,38 and etched alloy39–41 catalysts in hydrogenation processes, we explored the potential of Devarda's alloy (CuAlZn) as an ideal catalyst for the selective hydrogenation of FAL to FOL. In our study, skeletal Cu-based catalysts were prepared by etching Al and Zn from the Devarda's alloy using alkaline solutions of varying concentrations. These catalysts were subsequently applied to the hydrogenation of FAL, demonstrating their effectiveness in achieving selective and efficient production of FOL. The analysis shows that during the etching process of the alloy, some Al(OH)3 would be formed and remains in the Cu skeleton. After high-temperature calcination and reduction, the unetched Al and the Al(OH)3 remaining in the skeleton are oxidized and dehydrated to form amorphous Al2O3, which provide acidic sites for the adsorption of FAL. Meanwhile, the highly dispersed low-valent Cu species in the catalyst provide sites for H2 adsorption and dissociation. The synergistic effect of the low-valent Cu species and the acidic sites enhance the activity of hydrogenation of FAL to FOL. The optimal catalytic performance of the catalysts depends on the ratio between the low-valent metal sites and the acidic sites. Meanwhile, the catalyst prepared by alloy etching method can make the Cu species more dispersed to enhance the accessibility of the active sites, and ZnO can further disperse the Cu species. The interactions among Al2O3, ZnO and Cu species in the samples can help to bring out the synergistic effect between the hydrogenation sites and the acidic sites and make the catalysts more structurally stable, with stronger catalytic activity, and the catalysts also have good reusability.
2. Experimental
2.1 Materials
FAL (>99.5%) and Devarda's alloy particles (M27196) were purchased from Morell (Shanghai) Chemical Technology Co., Ltd FOL (>99.0%), γ-Al2O3, Cu(NO3)2·3H2O (99.0%) and Zn(NO3)2·6H2O (99.0%) were purchased from Aladdin Reagent (Shanghai) Co., Ltd NaOH (AR) and ethanol (AR) were purchased from Tianjin Jiangtian Chemical Co., Ltd H2 gas (purity 99.99%) and hydrogen–argon gas mixture (10 vol% H2/Ar) were purchased from Tianjin Liufang Industrial Gas Co., Ltd.2.2 Catalyst synthesis
The desired catalyst was synthesised using the etching method. In a typical synthesis, 2.0 g of Devarda's alloy (CuAlZn) particles were placed in 15.0 g of distilled water and stirred, named Sample A. Then, 15.0 g of 10 wt% NaOH solution was prepared, named Solution B. During stirring at a constant temperature of 25 °C, Solution B was added dropwise to Solution A to etch the alloy, and stirring lasted 11 h while the temperature was maintained at 25 °C. The precipitate was washed and centrifuged to the solution pH of about 7. The sample was dried in a vacuum oven at 60 °C overnight to obtain a powder sample. The dried sample was heated in a muffle furnace at 4 °C min−1 up to 400 °C, held for 3 h. After cooling, the sample was transferred to a tube furnace and heated at 3.8 °C min−1 up to 200 °C (reduction temperature), held for 3 h (10 vol% H2/Ar), to obtain the CAZ1.5/2.0-200 catalyst (CAZx/y-M: C stands for Cu, A stands for Al, Z stands for Zn, x/y stands for the mass ratio of NaOH to CuAlZn alloy, and M stands for the reduction temperature). The same process was used to prepare CAZ1.0/2.0-200, CAZ1.5/2.0-0, CAZ1.5/2.0-100, CAZ1.5/2.0-200, CAZ1.5/2.0-300, CAZ2.0/2.0-200 and CAZ2.5/2.0-200 catalysts with different reduction temperatures and etching concentrations, and were compared in terms of their performance.Cu/Al2O3 was synthesized by the traditional impregnation method, in which Cu (35 wt% of γ-Al2O3) was loaded on γ-Al2O3. A 35% Cu/Al2O3 catalyst was prepared by dissolving a certain amount of Cu(NO3)2·3H2O in deionised water, then slowly dropping the Cu solution into a solution sample containing the γ-Al2O3 support while stirring, leaving it to stand for 12 h, drying in an oven at 120 °C for 12 h. The dried sample was heated in a muffle furnace at 4 °C min−1 up to 400 °C, held for 3 h. After cooling, the sample was transferred to a tube furnace and heated at 3.8 °C min−1 up to 200 °C, held for 3 h (10 vol% H2/Ar) to obtain the 35%Cu/Al2O3 catalyst. The process for preparing ZnO/Cu/Al2O3 was the same as that for preparing Cu/Al2O3, except that a certain amount of Cu(NO3)2·3H2O and Zn(NO3)2·6H2O (1.33 wt% Zn of γ-Al2O3) were dissolved together in deionised water to produce the final catalyst 1.33% ZnO/35%Cu/Al2O3. The performance of these catalysts was compared.
2.3 Catalytic test conditions
The reaction was carried out in a 200 mL autoclave with a mechanical stirring speed of 600 rpm. Firstly, the ethanol solvent (60.0 g), FAL (1.20 g) and a certain amount of catalyst powder were added to the autoclave. Before each run, the vessel was sealed and the air in the vessel was replaced with 1.0 MPa N2 four times. Then H2 was introduced at the corresponding pressure, and the reactor was heated to a certain temperature with vigorous stirring. The reaction time was started when the temperature reached the predetermined value. After the reaction was completed, the reactor was allowed to cool down by itself. In the catalyst cycle stability test, the reaction solution was centrifuged after each reaction to recover the catalyst. The recovered catalyst was thoroughly washed with ethanol three times in sequence, and then the catalyst was dried in a vacuum oven at 60 °C for 12 h for the next reaction. Before the reaction, it was heated in a muffle furnace at 4 °C min−1 up to 400 °C, held for 3 h. After cooling, the catalyst was transferred to a tube furnace and heated at 3.8 °C min−1 up to 200 °C, held for 3 h (10 vol% H2/Ar). During the recovery process, about 10–15% of the catalyst was lost by weight, and the loss was compensated for by supplementing the recovery experiment with fresh catalyst (before each reaction, the quantities of catalyst, reactants, and solvent were standardised).The product filtrate was analysed using a Beifen Ruili GC-3420A equipped with a FID detector and a capillary column (KB-Wax, 30 m × 0.32 mm × 0.5 μm, Kromat Corporation). The estimated experimental error was less than ±5% in all activity tests and the FAL conversion and selectivity and the product yield were calculated using the following eqn (1), (2) and (3).
 | (1) |
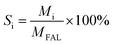 | (2) |
| Yi = CFAL × Si | (3) |
2.4 Catalyst characterisation
The specific surface area was measured by using SSA-7000 (BET) low-temperature N2 adsorption/desorption device. All samples were degassed at 150 °C for 4 h, and then adsorption and desorption were measured at −196 °C. X-ray diffraction (XRD) measurement was carried out on a Bruker D8 ADVANCE X-ray using Cu-Kα for crystallinity in the 2θ range of 10°–80° at a scanning speed of 8° min−1 to measure the catalyst precursors with different degrees of etching and the calcined reduced catalysts, and the energy dispersive spectroscopy was analysed by field emission scanning electron microscope (Apreo S LoVac-EDS). The sample was measured by field emission transmission electron microscopy (TEM) JEMF200. The surface functional groups of the samples were determined by Fourier Transform Infrared Spectroscopy (FT-IR) on a Nicolet-380 instrument with a DTGS detector. X-ray photoelectron spectroscopy (XPS) measurements were performed using K-Alpha+ from Thermo Fisher Scientific. The analysis was calibrated using the C 1s standard binding energy (284.8 eV). The catalyst with different etching concentrations at the same reduction temperature was measured by inductively coupled plasma optical emission spectrometry (ICP-OES, Agilent 725 ES & Agilent 5110). Ammonia Temperature-Programmed Desorption (NH3-TPD) test was measured on a TP-5080-B instrument. The sample was pretreated at 150 °C in a helium atmosphere for 1 h. After cooling to 50 °C, a 10% NH3/He mixture was injected to saturation, and then the He gas flow was switched to purge for 1 h to remove the weak physical adsorption NH3 on the surface. Finally, the temperature was increased in a He atmosphere at 10 °C min−1 to 700 °C, and the removed gas was detected using a TCD. The Hydrogen Temperature-Programmed Desorption (H2-TPD) test was measured on a TP-5080-B instrument. The sample was pretreated in a helium atmosphere at 150 °C for 1 h. After cooling to 50 °C, it was saturated with a 10% H2/He mixture, and then purged with He gas for 1 h to remove the weak physical adsorption H2 on the surface. Finally, the temperature was increased to 700 °C in a He atmosphere at 10 °C min−1 to 700 °C, and the removed gas was detected using a TCD. Pyridine-Infrared (Py-IR) test was measured on a Bruker Tensor 27 at 150 °C, 200 °C and 350 °C. The sample was evacuated at 350 °C for 1 h to remove impurity gases, then cooled naturally to 25 °C. Subsequently, the sample was vaporized with pyridine for 30 min, evacuated to 150 °C/200 °C/350 °C for 30 min. The spectra were taken at 25 °C.3. Results and discussion
A series of Cu-based skeleton catalysts CAZ0.5/2.0-200, CAZ1.0/2.0-200, CAZ1.5/2.0-200, CAZ2.0/2.0-200, and CAZ2.5/2.0-200 with different degrees of etching were successfully synthesized to explore the effect of different component content ratios on the performance of the catalyst. The influence of the reduction temperature was explored by using the CAZ1.5/2.0-100/200/300 catalyst (optimum etch level catalysts), and the cyclic reusability of the catalyst was explored by using CAZ1.5/2.0-200 as the experimental object.3.1 Characterisation of CAZx/2.0-200
| Entry | Catalysts | SBET (m2 g−1) | Actual atom ratio (Cu/Al/Zn)d | FAL conversione (%) | FOL selectivitye (%) | FOL yield (%) |
|---|---|---|---|---|---|---|
| a FAL (1.20 g), catalyst (0.24 g), ethanol (60.0 g), H2 (1.5 MPa), 80 °C, 5 h.b FAL (1.20 g), catalyst (0.24 g), ethanol (60.0 g), N2 (1.5 MPa), 80 °C, 5 h.c FAL (1.20 g), catalyst (0.24 g), ethanol (60.0 g), H2 (1.5 MPa), 85 °C, 2 h.d Actual bulk atom ratio of Cu/Al/Zn was measured by ICP-OES.e Experimental error <±5%. | ||||||
| 1 | CAZ0.5/2.0-200a | 168.21 | 54.85/34.82/4.48 | 64.22 | 99.71 | 64.03 |
| 2 | CAZ1.0/2.0-200a | 121.13 | 59.70/24.65/3.96 | 68.75 | 99.28 | 68.26 |
| 3 | CAZ1.5/2.0-200a | 39.69 | 74.35/16.63/3.96 | 98.57 | 99.69 | 98.26 |
| 4 | CAZ2.0/2.0-200a | 16.47 | 81.61/12.98/3.12 | 58.22 | 99.55 | 57.96 |
| 5 | CAZ2.5/2.0-200a | 9.17 | 84.08/12.25/1.97 | 45.35 | 99.27 | 45.92 |
| 6 | CAZ1.5/2.0-200b | 39.69 | 74.35/16.63/3.96 | 0.45 | 0 | 0 |
| 7 | CAZ1.5/2.0-200 (R)c | — | 72.95/15.13/4.02 | 72.25 | 96.15 | 69.47 |
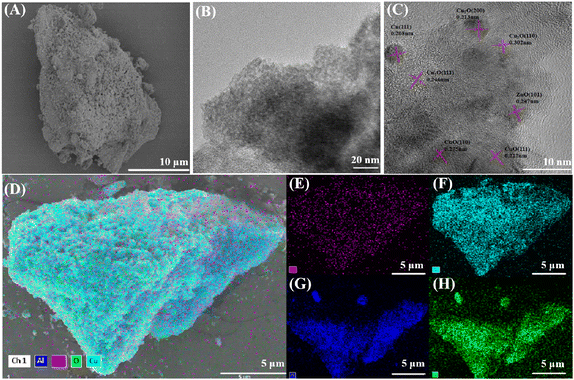 | ||
| Fig. 2 (A) SEM image; (B) TEM image; (C) HRTEM image; (D–H) EDS elemental mappings of the CAZ1.5/2.0-200 catalyst. | ||
TEM was used to further determine the structure of the Cu-based skeleton catalyst (Fig. 2B). The HRTEM image (Fig. 2C) of CAZ1.5/2.0-200 shows a lattice stripe spacing of 0.208 nm, which belongs to Cu (111).46 Meanwhile, the catalyst also consists of several components with different lattice spacings, and compared with the XRD standard card, it can be determined that 0.213 nm, 0.302 nm and 0.246 nm correspond to the (200), (110), and (111) crystal planes of Cu2O, respectively, and that 0.175 nm and 0.212 nm correspond to the (110) and (111) crystal planes of CuO, respectively, indicating that the presence of Cu species with different valence states on the surface of the catalyst. Combined with the SEM analysis, this further confirms the highly dispersed nature of the Cu species. Meanwhile, 0.247 nm corresponds to the (101) crystal plane of ZnO, confirming the presence of ZnO particles.
It is difficult to distinguish between Cu0 and Cu+ based only on the binding energy of Cu 2p,48 because the kinetic energy difference between XPS distinguishing Cu0 and Cu+ is small, which may lead to overlapping results and large analytical errors. Therefore, we used XAES spectroscopy to distinguish between Cu0 and Cu+ species (Fig. 3B), and the corresponding kinetic energies of Cu0, Cu2+ and Cu+ are observed to be 568.6 eV, 573.1 eV, and 576.4 eV, respectively. These results suggest that Cu exists in all three valence states at the catalyst surface.49 In the Al 2p XPS spectrum of each CAZx/2.0-200 (Fig. 3C), a signal peak at 74.8 eV belongs to Al3+ in the CAZx/2.0-200 sample,50 confirming that Al exists in the form of an oxide. The valence electron state of Zn can be accurately determined by using the XAES spectroscopy. As shown in Fig. 3D, the Zn 2p XAES spectra of each CAZx/2.0-200 sample shows a signal peak belonging to the ZnO species at 989.1 eV, which indicates that Zn exists mainly in the form of oxides on the catalyst surface.
As illustrated in Table S3 and Fig. S3,† the Cu atomic contents in XPS analysis of the CAZ1.0/2.0-200, CAZ1.5/2.0-200, and CAZ2.0/2.0-200 catalysts are 3.92%, 12.19%, and 14.58%, respectively, which are much lower than the corresponding ICP contents (Table 1): 59.70%, 74.35% and 81.61%, respectively, and the Al content is significantly higher, indicating that most of the Cu is stored in the bulk rather than on the surface, the Al content on the surface of the sample is higher than in the bulk, which may be attributed to the fact that the Al in the samples is partially forms into Al(OH)3 and covers on the surface of the samples by the etching process, which is consistent with the results of the XRD and SEM analysis.
 | ||
| Fig. 4 (A) NH3-TPD of the catalyst CAZ1.0/2.0-200, CAZ1.5/2.0-200 and CAZ2.0/2.0-200, (B) Py-IR of the catalyst CAZ1.5/2.0-200 at different test temperatures. | ||
The presence of Lewis acid sites on CAZ1.5/2.0-200 were confirmed by Py-IR at different adsorption temperatures. As shown in Fig. 4B, the total acid content in the catalyst is measured at 150 °C. When the temperature reaches 200 °C, some weak acids decompose, resulting in a subsequent decrease in the acid content. Most of the medium and weak acids decompose at 350 °C. The bands at 1450 cm−1 and 1610 cm−1 correspond to the adsorption of pyridine at the Lewis acid sites. The Al2O3 can be used as a Lewis acidic center to adsorb the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the FAL,53 and the CuO can also provide Lewis acidic enhancement to interact with the FAL.54 The weak peak at 1486 cm−1 is a combination of Lewis acid sites and Brønsted acid sites. The small amount of Brønsted acid sites may come from Al2O3·xH2O55 with different degrees of dehydration and CuOx.54 These acidic sites promote the adsorption of FAL on the catalyst. The adsorption of pyridine mainly occurs at Lewis acid sites. The total amount of acid in the catalyst CAZ1.5/2.0-200 is shown in Table S5,† which indicates that the catalyst is rich in medium-strength acid sites, and that these acids are mainly present in the form of Lewis acids.
O in the FAL,53 and the CuO can also provide Lewis acidic enhancement to interact with the FAL.54 The weak peak at 1486 cm−1 is a combination of Lewis acid sites and Brønsted acid sites. The small amount of Brønsted acid sites may come from Al2O3·xH2O55 with different degrees of dehydration and CuOx.54 These acidic sites promote the adsorption of FAL on the catalyst. The adsorption of pyridine mainly occurs at Lewis acid sites. The total amount of acid in the catalyst CAZ1.5/2.0-200 is shown in Table S5,† which indicates that the catalyst is rich in medium-strength acid sites, and that these acids are mainly present in the form of Lewis acids.
The position of the characteristic absorption peak (Fig. 5B) of the sample shifts after calcination and reduction, moving from the typical bayerite 1023 cm−1 band to the boehmite 1076 cm−1 band, which may be contributed to that after calcination reduction, bayerite is dehydrated and converted to boehmite. Meanwhile, there is a broader typical absorption peak at 3450 cm−1, which may be due to pseudoboehmite.58 However, it can still be seen that the in situ generated Al2O3·xH2O has a distinct characteristic peak at 738 cm−1 and the band at 738 cm−1 may be attributed to Al–OH stretching and bending vibrations of boehmite.59 The broad characteristic peak at around 589 cm−1 may also be due to the conversion of bayerite to boehmite.59
FT-IR characterisation analysis shows that Al2O3·xH2O continues to dehydrate in the samples after calcination reduction treatment, verifying the presence of Al2O3 in the CAZx/2.0-200 samples, which, combined with XRD analysis, is predominantly present in an amorphous form and supports the origin of the acidic sites in the NH3-TPD and Py-IR.
3.2 Properties of CAZ1.5/2.0-M under different reduction conditions
This study used FAL selective hydrogenation technology to evaluate the catalytic activity of CAZx/2.0-M catalysts. The activity evaluation was carried out under reaction conditions of 1.5 MPa H2 pressure, 80 °C, and a reaction time of 5 h. We tested the catalytic performance of CAZ1.5/2.0-0, CAZ1.5/2.0-100, CAZ1.5/2.0-200, and CAZ1.5/2.0-300 catalyst powders for FAL selective hydrogenation. As shown in Table 2, the reduced catalyst exhibits significantly higher activity than the unreduced catalyst, which is most likely attributed to the presence of low-valent Cu species (Cu0 and Cu+), particularly metallic Cu0,60,61 serving as essential active sites for hydrogen adsorption and dissociation. Reduction treatment converts oxidised Cu species (CuO) into low-valent species, which play a crucial role in facilitating hydrogenation reactions by providing active sites for H2 dissociation and the subsequent interaction with the substrate.62,63 For catalysts prepared under reducing conditions, this process results in a higher proportion of low-valent Cu species compared to catalysts that are only calcined. As a result, the reduced catalyst exhibits superior catalytic activity due to its enhanced ability to activate hydrogen and promote the reaction.| Catalysts | Actual atom ratio (Cu0/Cu2+/Cu+)b | (Cu0 + Cu+)/(Cu0 + Cu+ + Cu2+)b (%) | Conversionc (%) | Selectivityc (%) | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: FAL (1.20 g), catalyst (0.24 g), ethanol (60.0 g), 5 h, H2 (1.5 MPa), 80 °C.b Actual bulk atom ratio of Cu0/Cu2+/Cu+ and (Cu0 + Cu+)/(Cu0 + Cu+ + Cu2+) were measured by Cu XAES.c Experimental error <± 5%. | |||||
| CAZ1.5/2.0-100a | 73.48/17.94/8.58 | 82.06 | 28.90 | 98.95 | 28.60 |
| CAZ1.5/2.0-200a | 76.93/12.27/10.80 | 87.73 | 98.57 | 99.69 | 98.26 |
| CAZ1.5/2.0-300a | 84.99/10.96/4.05 | 89.04 | 76.05 | 98.88 | 75.20 |
| CAZ1.5/2.0-0a | — | — | 19.66 | 98.75 | 19.41 |
The influence of different reduction temperatures on the catalytic activity of this reaction is obvious. As the reduction temperature increases, the activity of the catalyst tends to increase and then decrease. In contrast, the catalytic activity of the CAZ1.5/2.0-200 sample is significantly improved, with a FAL conversion rate of 98.57% and FOL selectivity of 99.69%. Therefore, so the 200 °C is the optimal reduction temperature, and a series of catalytic characterisations were carried out to explain the changes in activity.
The results show that the reduction temperature does not change the crystalline phases of the Cu species, demonstrating the structural stability of the catalyst across different preparation conditions. However, we observed that reduction temperature influences the relative abundance of Cu species with different valence states, which could impact the availability and distribution of active sites crucial for catalytic performance. These findings underscore the significance of carefully controlling reduction parameters to optimise the catalyst's composition and activity.
 | ||
| Fig. 7 (A) NH3-TPD of the catalyst CAZ1.0/2.0-200, CAZ1.5/2.0-200 and CAZ2.0/2.0-200, (B) H2-TPD of the catalyst CAZ1.0/2.0-200, CAZ1.5/2.0-200 and CAZ2.0/2.0-200. | ||
Analysis of H2-TPD (Fig. 7B) shows that the main component is chemisorbed (50–200 °C) and dissociated (200–400 °C) hydrogen on the low-temperature peak (<400 °C).65 The amount of H2 adsorption capacity is shown in Table S6.† The CAZ1.5/2.0-300 sample has the strongest adsorption and dissociation capacity for H2, while the poor H2 adsorption capacity of the CAZ1.5/2.0-100 is not conducive to the hydrogenation reaction. The adsorption and dissociation capacities of the samples for H2 increase with the increase of reduction temperature, which may be due to the fact that more CuO is reduced to low-valent Cu with the increase of reduction temperature, and the low-valent Cu species can be used as the sites for adsorption and dissociation of H2 and thus promote the hydrogenation reaction.66,67
The relationship between catalyst activity and the distribution of active components was studied by establishing a structure–activity relationship based on the results of the activity data, NH3-TPD, H2-TPD and XPS analysis. The results show that different reduction temperatures control the distribution of Cu species in different valence states, which leads to different catalytic activities in the hydrogenation reaction. The reason for the higher conversion of CAZ1.5/2.0-300 than CAZ1.0/2.0-100 while ensuring high selectivity (greater than 98.5%) is that CAZ1.5/2.0-300 has the strongest ability to adsorb and dissociate H2, but the reason why the conversion rate of CAZ1.5/2.0-200 is significantly the highest is that the optimal ratio of acidic sites and hydrogenation sites has been adjusted, with an appropriate amount of acidic sites and hydrogenation sites. The acidic sites come from the moderate amount of Lewis acid provided by Al2O3 and CuO, while the hydrogenation sites come from the adsorption and dissociation of H2 by low-valent Cu. Together, they synergistically enhance the catalytic activity of FAL for hydrogenation to FOL.
3.3 Catalytic hydrogenation performance
| Catalysts | Conversionb (%) | Selectivityb (%) | Yield (%) |
|---|---|---|---|
| a Reaction conditions: FAL (1.20 g), catalyst (0.24 g), ethanol (60.0 g), 5 h, H2 (1.5 MPa), 80 °C.b Experimental error <± 5%. | |||
| CAZ1.5/2.0-200a | 98.57 | 99.69 | 98.26 |
| 25% Cu/Al2O3a | 6.07 | 88.14 | 5.35 |
| 35% Cu/Al2O3a | 18.37 | 93.25 | 17.13 |
| 45% Cu/Al2O3a | 14.96 | 97.74 | 14.62 |
| 1.33% ZnO/35% Cu/Al2O3a | 24.18 | 95.47 | 23.08 |
| Catalysts | Reaction conditions | FAL conversion (%) | FOL selectivity (%) | mFAL (g)/mCat (g) | Productivity (mol FOL g (cat)−1 h−1) |
|---|---|---|---|---|---|
| C@Ni3P25 | 140 °C, 1.4 MPa H2, 4 h | 97 | 96.3 | 5.0 | 0.01215 |
| Cu2Ni1AlOy23 | 120 °C, 1.6 MPa H2, 1.5 h | 98 | 99 | 5.0 | 0.03366 |
| Cu/MgO76 | 110 °C, 2 MPa H2, 80 min | 99.9 | 99.9 | 1.152 | 0.00897 |
| NiCoCuZnFe/C-800 (ref. 77) | 90 °C, 3 MPa H2, 9 h | 87.3 | 100 | 6 | 0.00606 |
| Fh-Al0.67 (ref. 78) | 150 °C, 1 MPa N2, 5 h | 100 | 100 | 1.92 | 0.00340 |
| Ru/ZP-A79 | 90 °C, 1.25 MPa H2, 7 h | 62 | 97 | 2.07 | 0.00185 |
| Ni–Mg–Al80 | 140 °C, 3 h | 98.5 | 98.98 | 2.304 | 0.00780 |
| Fe3O4@C81 | 200 °C, 2 MPa N2, 4 h | 93.6 | 98.9 | 3.84 | 0.00925 |
| 15Cu–15Ni/MCM-41 (ref. 82) | 160 °C, 4 h | 94.5 | 99 | 1.92 | 0.00467 |
| 2% Ni–5% Cu/SiO2 (ref. 83) | 100 °C, 2 MPa H2, 2 h | 94 | 64 | 0.290 | 0.00091 |
| CAZ1.5/2-200 (this work) | 85 °C, 1.5 MPa H2, 3 h | >99.0 | >99.0 | 5 | 0.01700 |
3.4 Characterisation of the recovered catalyst
In order to analyse the mechanism of catalyst deactivation, the recovered catalyst (after five reaction processes and calcination then reduction, named CAZ1.5/2.0-200 (R)) was further studied. XRD analysis of the recovered catalyst (Fig. 9A) shows that the intensities of the corresponding crystalline phase peaks of CuO, Cu2O and alloys decrease, and the relative intensity of the Cu crystalline phase increases, indicating that the recovered catalyst is more readily reducible to low-valent metals. Compared with fresh catalyst, ICP analysis of the recovered catalyst (Table 1) shows that Cu (CAZ1.5/2.0-200 (R): 72.95%; CAZ1.5/2.0-200: 74.35%) and Al (CAZ1.5/2.0-200 (R): 15.13%; CAZ1.5/2.0-200:16.63%) have slightly leach. Comparing the morphology (Fig. 9B) and mapping characterisation results (Fig. 9C–G) of fresh and recovered catalyst, it is relatively consistent. Therefore, the recovered catalyst can be reused well, but the slight decrease in activity can be attributed to the reduction of low-valent Cu species and the slight leaching of Cu and Al.4. Conclusions
In summary, this study utilised the etching method to synthesise a composite metal oxide catalyst for the selective hydrogenation of FAL to FOL. Among the catalysts tested, CAZ1.5/2-200 demonstrated the highest catalytic performance, achieving over 99.0% FAL conversion and 99.0% FOL selectivity under mild conditions of 1.5 MPa H2 at 85 °C for 3 h. This exceptional activity is primarily attributed to the synergistic effect between low-valent Cu species, which act as active sites for H2 adsorption and dissociation, and Lewis acid sites provided by Al2O3 and CuO, which enhance FAL adsorption. The interaction between ZnO and Cu modifies the electronic environment around the Cu species, facilitating their reduction to low-valent states and improving Cu dispersion. Compared to traditional impregnation methods, the alloy etching approach strengthens interfacial interactions between species, promoting a stronger synergistic effect between acidic and hydrogenation sites. This leads to a more stable catalyst structure with enhanced activity and reusability. Furthermore, the simple and cost-effective preparation process offers a promising strategy for the rational design of efficient hydrogenation catalysts for FAL to FOL conversion under mild reaction conditions.Data availability
Characterisation and evaluations are displayed in the manuscript, and other characterisation analysis figures, tables and the specific experimental data supporting this article have been included as part of the ESI.†Author contributions
Junqi Zhang: conceptualization, methodology, formal analysis, investigation, writing – original draft. Yongwang Li: conceptualization, methodology, supervision. Zhiwei Zhang: conceptualization, supervision, writing – review & editing. Zheng Wang: data curation, supervision. Jiaxing Zhang: conceptualization, methodology, supervision. Shuai Liu: validation, data curation, formal analysis. Yang Qin: validation, data curation. Bingxin Zhu: validation, data curation. Tongxue Zhang: validation, data curation. Hongyu Wang: validation, data curation. Fumin Wang: conceptualization, methodology, supervision. Xubin Zhang: validation, data curation, formal analysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22211540711, 22479109), the Natural Science Foundation of Shandong Province (Grant No. ZR2023ZD22), and the Major R&D Program of Shandong Province (Grant No. 2023CXGC010601).References
- W. Gao, S. Liang, R. Wang, Q. Jiang, Y. Zhang, Q. Zheng, B. Xie, C. Y. Toe, X. Zhu, J. Wang, L. Huang, Y. Gao, Z. Wang, C. Jo, Q. Wang, L. Wang, Y. Liu, B. Louis, J. Scott, A.-C. Roger, R. Amal, H. He and S.-E. Park, Industrial carbon dioxide capture and utilization: state of the art and future challenges, Chem. Soc. Rev., 2020, 49, 8584–8686 RSC.
- Y. Yang, Z. Ren, S. Zhou and M. Wei, Perspectives on multifunctional catalysts derived from layered double hydroxides toward upgrading reactions of biomass resources, ACS Catal., 2021, 11, 6440–6454 CrossRef CAS.
- L. Lei, Y. Wang, Z. Zhang, J. An and F. Wang, Transformations of biomass, its derivatives, and downstream chemicals over ceria catalysts, ACS Catal., 2020, 10, 8788–8814 CrossRef CAS.
- P. Zhou, Y. Chen, P. Luan, X. Zhang, Z. Yuan, S. X. Guo, Q. Gu, B. Johannessen, M. Mollah, A. L. Chaffee, D. R. Turner and J. Zhang, Selective electrochemical hydrogenation of furfural to 2-methylfuran over a single atom Cu catalyst under mild pH conditions, Green Chem., 2021, 23, 3028–3038 RSC.
- J. P. Lange, E. Van Der Heide, J. van Buijtenen and R. Price, Furfural-a promising platform for lignocellulosic biofuels, ChemSusChem, 2012, 5(1), 150–166 CrossRef CAS PubMed.
- P. Jia, X. C. Lan, X. D. Li and T. Wang, Highly selective hydrogenation of furfural to cyclopentanone over a NiFe bimetallic catalyst in a methanol/water solution with a solvent effect, ACS Sustain. Chem. Eng., 2019, 7, 15221–15229 CrossRef CAS.
- K. Yan, G. Wu, T. Lafleur and C. Jarvis, Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals, Renewable Sustainable Energy Rev., 2014, 38, 663–676 CrossRef CAS.
- V. Pace, P. Hoyos, L. Castoldi, P. Domínguez de María and A. R. Alcántara, 2-Methyltetrahydrofuran (2-MeTHF): a biomass-derived solvent with broad application in organic chemistry, ChemSusChem, 2012, 5(8), 1369–1379 CrossRef CAS.
- Z. Wang, X. Wang, C. Zhang, Y. Yang, L. Zhou, H. Cheng and F. Zhao, Selective hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-pentanediol over PrOx promoted Ni catalysts, Catal. Today, 2022, 402, 79–87 CrossRef CAS.
- X. Fu, X. Ren, J. Shen, Y. Jiang, Y. Wang, Y. Orooji, W. Xu and J. Liang, Synergistic catalytic hydrogenation of furfural to 1,2-pentanediol and 1,5-pentanediol with LDO derived from CuMgAl hydrotalcite, Mol. Catal., 2021, 499, 111298 CrossRef CAS.
- J. Zhang, Y. Liu, Z. Jia, S. Liu, L. Li, Q. Wu and C. Xu, Selective hydrogenation of furfural to furfuryl alcohol over copper-cobalt bimetallic catalyst, Chem. Eng. J., 2024, 490, 151677 CrossRef CAS.
- Y. Shi, T. Wu, Z. Wang, C. Liu, J. Bi and L. Wu, Photocatalytic precise hydrogenation of furfural over ultrathin Pt/NiMg-MOF-74 nanosheets: Synergistic effect of surface optimized NiII sites and Pt clusters, Appl. Surf. Sci., 2023, 616, 156553 CrossRef CAS.
- Y. Ren, Y. Yang, L. Chen, L. Wang, Y. Shi, P. Yin, W. Wang, M. Shao, X. Zhang and M. Wei, Synergetic effect of Cu0-Cu+ derived from layered double hydroxides toward catalytic transfer hydrogenation reaction, Appl. Catal. B-Environ., 2022, 314, 121515 CrossRef CAS.
- R. Mariscal, P. Maireles-Torres, M. Ojeda, I. Sadaba and M. L. Granados, Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels, Energy Environ. Sci., 2016, 9(4), 1144–1189 RSC.
- Z. Zhao, R. Bababrik, W. Xue, Y. Li, N. M. Briggs, D. T. Nguyen, U. Nguyen, S. P. Crossley, S. Wang, B. Wang and D. E. Resasco, Solvent-mediated charge separation drives alternative hydrogenation path of furanics in liquid water, Nat. Catal., 2019, 2(5), 431–436 CrossRef CAS.
- Y. Dai, X. Gao, X. Chu, C. Jiang, Y. Yao, Z. Guo, C. Zhou, C. Wang, H. Wang and Y. Yang, On the role of water in selective hydrogenation of cinnamaldehyde to cinnamyl alcohol on PtFe catalysts, J. Catal., 2018, 364, 192–203 CrossRef CAS.
- Q. Shen, Y. Li, F. Wang, X. Zhang, Z. W. Zhang, Z. L. Zhang, Y. Yang, C. Bing, X. Fan, J. Zhang and X. He, Controlled targeted conversion of furfural to 1,5-pentanediol or 2-methylfuran over Ni/CoAlOx catalyst, Mol. Catal., 2024, 556, 113919 CrossRef CAS.
- X. Li, P. Jia and T. Wang, Furfural: A promising platform compound for sustainable production of C4 and C5 chemicals, ACS Catal., 2016, 6(11), 7621–7640 CrossRef CAS.
- C. P. Jiménez-Gómez, J. A. Cecilia, D. Durán-Martín, R. Moreno-Tost, J. Santamaría-González, J. Mérida-Robles, R. Mariscal and P. Maireles-Torres, Gas- phase hydrogenation of furfural to furfuryl alcohol over Cu/ZnO catalysts, J. Catal., 2016, 336, 107–115 CrossRef.
- K. Fulajtárova, T. Soták, M. Hronec, I. Vávra, E. Dobročka and M. Omastová, Aqueous phase hydrogenation of furfural to furfuryl alcohol over Pd-Cu catalysts, Appl. Catal., A, 2015, 502, 78–85 CrossRef.
- M. J. Taylor, S. K. Beaumont, M. J. Islam, S. Tsatsos, C. A. M. Parlett, M. A. Issacs and G. Kyriakou, Atom efficient PtCu bimetallic catalysts and ultra dilute alloys for the selective hydrogenation of furfural, Appl. Catal., B, 2021, 284, 119737 CrossRef CAS.
- G. R. Bertolini, C. P. Jiménez-Gómez, J. A. Cecilia and P. Maireles-Torres, Gas-phase hydrogenation of furfural to furfuryl alcohol over Cu-ZnO-Al2O3 catalysts prepared from layered double hydroxides, Catalysts, 2020, 10(5), 486 CrossRef CAS.
- L. Luo, F. L. Yuan, F. Zaera and Y. J. Zhu, Catalytic hydrogenation of furfural to furfuryl alcohol on hydrotalcite-derived CuxNi3-xAlOy mixed-metal oxides, J. Catal., 2021, 404, 420–429 CrossRef CAS.
- M. Kalong, P. Hongmanorom, S. Ratchahat, W. Koo-amornpattana, K. Faungnawakij, S. Assabumrungrat, A. Srifa and S. Kawi, Hydrogen-free hydrogenation of furfural to furfuryl alcohol and 2-methylfuran over Ni and Co-promoted Cu/γ-Al2O3 catalysts, Fuel Process. Technol., 2021, 214, 106721 CrossRef CAS.
- Y. Tian, Y. Z. Wang, H. Y. Zhang, L. F. Xiao and W. Wu, Novel C@Ni3P nanoparticles for highly selective hydrogenation of furfural to furfuryl alcohol, Catal. Lett., 2022, 152(3), 883–894 CrossRef CAS.
- S. H. Liu, N. Govindarajan and K. Chan, Understanding activity trends in furfural hydrogenation on transition metal surfaces, ACS Catal., 2022, 12, 12902–12910 CrossRef CAS.
- M. J. Islam, M. G. Mesa, A. Osatiashtiani, J. C. Manayil, M. A. Isaacs, M. J. Taylor and S. T. G. Kyriakou, PdCu single atom alloys supported on alumina for the selective hydrogenation of furfural, Appl. Catal. B-Environ., 2021, 299, 120652 CrossRef CAS.
- M. J. Islam, M. G. Mesa, A. Osatiashtiani, M. J. Taylor, J. C. Manayil, C. M. Parlett and G. Kyriakou, The effect of metal precursor on copper phase dispersion and nanoparticle formation for the catalytic transformations of furfural, Appl. Catal. B-Environ., 2020, 273, 119062 CrossRef CAS.
- W. B. Gong, C. Chen, Y. Zhang, H. Zhou, H. Wang, H. Zhang, Y. Zhang, G. Z. Wang and H. J. Zhao, Efficient synthesis of furfuryl alcohol from H2-hydrogenation/transfer hydrogenation of furfural using sulfonate group modified Cu catalyst, ACS Sustain. Chem. Eng., 2017, 5, 2172–2180 CrossRef CAS.
- J. Lee, J. H. Seo, C. Nguyen-Huy, E. Yang, J. G. Lee, H. Lee, E. J. Jang, J. H. Kwak, J. H. Lee, H. Lee and K. An, Cu2O(100) surface as an active site for catalytic furfural hydrogenation, Appl. Catal. B-Environ., 2021, 282, 119576 CrossRef CAS.
- X. Q. Wang, Q. Chen, Y. J. Zhou, H. M. Li, J. W. Fu and M. Liu, Cu-based bimetallic catalysts for CO2 reduction reaction, Adv. Energy Mater., 2022, 1(3), 100023 Search PubMed.
- D. Sharma, J. Suriyaprakash, A. Dogra, S. Alijani, A. Villa and N. Gupta, Versatile carbon supported mono and bimetallic nanocomposites: synthesis, characterization and their potential application for furfural reduction, Mater. Today Chem., 2020, 17, 100319 CrossRef CAS.
- L. Ma and M. S. Wainwright, Development of skeletal copper–chromia catalysts: I. Structure and activity promotion of chromia on skeletal copper catalysts for methanol synthesis, Appl. Catal., A, 1999, 187(1), 89–98 CrossRef CAS.
- X. Liu, D. Sun, Y. Ji, S. Zu, Y. Pei, S. Yan, M. Qiao, X. Zhang and B. Zong, Effect of NaOH concentration on rapidly quenched Cu-Al alloy-derived Cu catalyst for CO2 hydrogenation to CH3OH, Catalysts, 2024, 14(6), 391 CrossRef CAS.
- R. Yao, Y. Li, X. Zhang, Y. Zhao, Y. Wang, X. Lang, Q. Jiang, H. Tan and Y. Li, Self-supported nanoporous CuNiAl alloy as highly efficient electrocatalyst for nitrobenzene hydrogenation to aniline, Chem. Eng. J., 2023, 471, 144487 CrossRef CAS.
- L. Geng, Z. Lin, Z. Li, S. An, X. Zhang, Z. Liu, D. S. Zhang, Y. Z. Zhang, S. Gao and H. Han, Facile synthesis of holey lamellar CuO via ultrasonic chemical etching toward highly efficient hydrogenation of 4-nitrophenol under mild conditions, J. Solid State Chem., 2020, 292(1), 121698 CrossRef CAS.
- H. Hu, F. Xie, Y. Pei, M. Qiao, S. Yan, H. He, K. Fan, H. Li, B. Zong and X. Zhang, Skeletal Ni catalysts prepared from Ni-Al alloys rapidly quenched at different rates: Texture, structure and catalytic performance in chemoselective hydrogenation of 2-ethylanthraquinone, J. Catal., 2006, 237(1), 143–151 CrossRef CAS.
- Z. Sun, Z. H. Zhang, T. Q. Yuan, X. Ren and Z. Rong, Raney Ni as a versatile catalyst for biomass conversion, ACS Catal., 2021, 11, 10508–10536 CrossRef CAS.
- Y. Jiang, Q. Chang, L. Guan, B. Teng, C. Xu, Y. Zhang, X. Li, Q. Sheng, Y. Yao, S. Lu and Y. Qin, Efficient C = O bond hydrogenation of cinnamaldehyde over Pt-Cu alloy frame: Insight into the morphology and Pt species state, Chem. Eng. J., 2023, 477, 146854 CrossRef CAS.
- T. Zheng, Y. Zhu, R. Ma, L. Bai, H. Yu, S. Zhao, Y. He, Y. Liu and D. Li, Construction of Enriched Metal Vacancies to Enhance Catalytic Behavior for Selective Hydrogenation Over Au-Cu Nanoalloys, Ind. Eng. Chem. Res., 2024, 63, 2717–2725 CrossRef CAS.
- Y. B. Huang, J. L. Zhang, X. Zhang, X. Luan, H. Z. Chen, B. Hu, L. Zhao, Y. L. Wu and Q. Lu, Catalytic depolymerization of lignin via transfer hydrogenation strategy over skeletal CuZnAl catalyst, Fuel Process. Technol., 2022, 237, 107448 CrossRef CAS.
- B. S. Vasile, G. Dobra, S. Iliev, L. Cotet, I. A. Neacsu, V. A. Surdu, A. I. Nicoara, A. Boiangiu and L. Filipescu, Thermally activated Al (OH)3 part II-effect of different thermal treatments, Ceram., 2021, 4(4), 564–575 CrossRef CAS.
- Z. W. Guo, F. Zhou, H. Wang, X. H. Liu, G. Y. Xu, Y. Zhang and Y. Fu, Highly selective conversion of natural oil to alcohols or alkanes over a Pd stabilized CuZnAl catalyst under mild conditions, Green Chem., 2019, 21, 5046–5052 RSC.
- K. K. Miao, X. L. Luo, W. Wang, J. L. Guo, S. F. Guo, F. J. Cao, Y. Q. Hu, P. M. Chang and G. D. Feng, One-step synthesis of Cu-SBA-15 under neutral condition and its oxidation catalytic performance, Microporous Mesoporous Mater., 2019, 289, 109640 CrossRef CAS.
- Y. Huang, D. K. Sarkar and X. G. Chen, Superhydrophobic aluminum alloy surfaces prepared by chemical etching process and their corrosion resistance properties, Appl. Surf. Sci., 2015, 356, 1012–1024 CrossRef CAS.
- D. D. Li, F. Xu, X. Tang, S. Dai, T. C. Pu, X. L. Liu, P. F. Tian, F. Z. Xuan, Z. Xu, I. E. Wachs and M. H. Zhu, Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol, Nat. Catal., 2022, 5(2), 99–108 CrossRef CAS.
- F. J. Lan, H. L. Zhang, C. Y. Zhao, Y. Shu, Q. X. Guan and W. Li, Copper clusters encapsulated in carbonaceous mesoporous silica nanospheres for the valorization of biomass-derived molecules, ACS Catal., 2022, 12(9), 5711–5725 CrossRef CAS.
- J. Luo, Y. Cheng, H. Niu, T. Wang and C. Liang, Efficient Cu/FeOx catalyst with developed structure for catalytic transfer hydrogenation of furfural, J. Catal., 2022, 413, 575–587 CrossRef CAS.
- J. Zhang, Y. M. Liu, Z. Jia, S. Yu, S. Liu, L. Li, Q. Wu, H. Yu, Y. X. Liu, X. Jiang, Y. Liu and C. Xu, Selective hydrogenation of furfural to furfuryl alcohol over copper-cobalt bimetallic catalyst, Chem. Eng. J., 2024, 490, 151677 CrossRef CAS.
- Q. Liu, H. Qin, J. A. Boscoboinik and G. Zhou, Comparative study of the oxidation of NiAl (100) by molecular oxygen and water vapor using ambient-pressure X-ray photoelectron spectroscopy, Langmuir, 2016, 32(44), 11414–11421 CrossRef CAS PubMed.
- L. Hu, M. Yang, N. Xu, J. Xu, S. Zhou, X. Chu and Y. Zhao, Selective transformation of biomass-derived 5-hydroxymethylfurfural into 2,5-dihydroxymethylfuran via catalytic transfer hydrogenation over magnetic zirconium hydroxides, Korean J. Chem. Eng., 2018, 35, 99–109 CrossRef CAS.
- M. Tu, D. T. Li, J. Y. Shen and Y. Chen, Microcalorimetric studies of surface acidity and basicity of metal oxides, Chem. J. Chin. Univ., 1998, 19, 946–949 CAS.
- S. Rajagopal, J. A. Marzari and R. Miranda, Silica-alumina-supported Mo oxide catalysts: Genesis and demise of Brønsted-Lewis acidity, J. Catal., 1995, 151(1), 192–203 CrossRef CAS.
- J. Zhang, C. Li, S. Hu, J. Gu, H. Yuan and Y. Chen, Mechanistic insights into copper oxides catalyzed bio-based furfural hydrogenation using methanol as in situ hydrogen donor, Renewable Energy, 2022, 200, 88–97 CrossRef CAS.
- S. Srivastava, G. C. Jadeja and J. Parikh, Synergism studies on alumina-supported copper-nickel catalysts towards furfural and 5-hydroxymethylfurfural hydrogenation, J. Mol. Catal. A:Chem., 2017, 426, 244–256 CrossRef CAS.
- K. A. Rodgers, M. R. Gregory and R. P. Cooney, Bayerite, Al(OH)3, from Raoul Island, Kermadec Group, South Pacific, Clay Miner., 1989, 24(3), 531–538 CrossRef CAS.
- G. Y. Wei, J. K. Qu, Y. D. Zheng, Q. I. Tao, G. U. O. Qiang, B. B. Han and H. X. Zhao, Crystallization behaviors of bayerite from sodium chromate alkali solutions, Trans. Nonferrous Met. Soc. China, 2014, 24(10), 3356–3365 CrossRef CAS.
- S. Musić, D. Dragčević, S. Popović and N. Vdović, Microstructural properties of boehmite formed under hydrothermal conditions, Mater. Sci. Eng., B, 1998, 52(2–3), 145–153 CrossRef.
- S. Musić, Đ. Dragčević, S. Popović and N. Vdović, Chemical and microstructural properties of Al-oxide phases obtained from AlCl3 solutions in alkaline medium, Mater. Chem. Phys., 1999, 59(1), 12–19 CrossRef.
- J. Zhang, X. Yang, Z. Jia, Q. Han, S. Yu, S. Liu, L. Li, Q. Wu, H. Yu, Y. X. Liu and Y. Liu, Bi-functional active sites Cu/MgO-La2O3 catalysts the selective hydrogenation of furfural to furfuryl alcohol, Chem. Eng. J., 2024, 500, 157400 CrossRef CAS.
- J. Tan, J. He, K. Gao, S. Zhu, J. Cui, L. Huang, Y. Zhu and Y. Zhao, Catalytic hydrogenation of furfural over Cu/CeO2 Catalyst: The effect of support morphology and exposed facet, Appl. Surf. Sci., 2022, 604, 154472 CrossRef CAS.
- N. Xi, S. Chen, R. Bao, Q. Wang, Y. Lin, J. Yue, R. Wang, C. Yang, W. Yin and T. Qiu, Layered carbon encapsulated CuOx nanoparticles for selective hydrogenation of furfural to furfuryl alcohol, Mol. Catal., 2024, 565, 114364 CrossRef CAS.
- Y. Ren, Y. Yang, L. Chen, L. Wang, Y. Shi, P. Yin, W. Wang, M. Shao, X. Zhang and M. Wei, Synergetic effect of Cu0-Cu+ derived from layered double hydroxides toward catalytic transfer hydrogenation reaction, Appl. Catal., B, 2022, 314, 121515 CrossRef CAS.
- F. Zaccheria, N. Scotti, M. Marelli, R. Psaro and N. Ravasio, Unravelling the properties of supported copper oxide: can the particle size induce acidic behaviour?, Dalton Trans., 2013, 42(5), 1319–1328 RSC.
- Y. Li, Q. Shen, Y. Nian, F. Wang, X. Zhang, Z. Zhang, C. Bing, X. Fan and R. Ahishakiye, Promoting effect of oxygen vacancies in Co/CoAl2O4 catalyst steered with a straightforward method on hydrogenation of furfural to 2-methylfuran, Appl. Catal., B, 2024, 343, 123529 CrossRef CAS.
- S. C. Chou, C. T. Yeh and T. H. Chang, Adsorption of Hydrogen on Dispersed Copper-Rhodium Bimetallic Crystallites, J. Phys. Chem. B, 1997, 101(30), 5828–5833 CrossRef CAS.
- F. Xu, W. An, A. E. Baber, D. C. Grinter, S. D. Senanayake, M. G. White, P. Liu and D. J. Stacchiola, Enhanced oxide reduction by hydrogen at cuprous oxide-copper interfaces near ascending step edges, J. Phys. Chem. C, 2022, 126(44), 18645–18651 CrossRef CAS.
- X. G. Wang, J. R. Smith and M. Scheffler, Adhesion of copper and alumina from first principles, J. Am. Ceram. Soc., 2003, 86(4), 696–700 CrossRef CAS.
- H. Niu, Y. Cheng, C. Li, S. Li, J. Luo and C. Liang, Construction of Cu-MOx (M= Zn or Al) interface in Cu catalysts for hydrogenation rearrangement of furfural, Ind. Eng. Chem. Res., 2021, 60(47), 16939–16950 CrossRef CAS.
- N. Wang, Y. Quan, J. Zhao, H. Li and J. Ren, Highly active CuZn/SBA-15 catalyst for methanol dehydrogenation to methyl formate: Influence of ZnO promoter, Mol. Catal., 2021, 505, 111514 CrossRef CAS.
- U. R. Pillai and S. Deevi, Copper-zinc oxide and ceria promoted copper-zinc oxide as highly active catalysts for low temperature oxidation of carbon monoxide, Appl. Catal., B, 2006, 65, 110–117 CrossRef CAS.
- L. Ban, H. Li, Y. Zhang, R. Wu, X. Huang, J. H. Zhao and Y. X. Zhao, Importance of zinc oxide in Cu-based catalysts for the ethynylation of formaldehyde to 1,4-Butynediol, J. Phys. Chem. C, 2021, 125(30), 16536–16549 CrossRef CAS.
- B. R. Strohmeier and D. M. Hercules, Surface spectroscopic characterization of the interaction between zinc ions and γ-alumina, J. Catal., 1984, 86(2), 266–279 CrossRef CAS.
- R. M. Palomino, P. J. Ramírez, Z. Liu, R. Hamlyn, I. Waluyo, M. Mahapatra, I. Orozco, A. Hunt, J. P. Simonovis, S. D. Senanayake and J. A. Rodriguez, Hydrogenation of CO2 on ZnO/Cu (100) and ZnO/Cu (111) catalysts: role of copper structure and metal–oxide interface in methanol synthesis, J. Phys. Chem. B, 2018, 122(2), 794–800 CrossRef CAS PubMed.
- W. Jeon, C. Ban, J. E. Kim, H. C. Woo and D. H. Kim, Production of furfural from macroalgae-derived alginic acid over Amberlyst-15, J. Mol. Catal. A:Chem., 2016, 423, 264–269 CrossRef CAS.
- J. Zhang, Z. Jia, S. Yu, S. Liu, L. Li, C. Xie, Q. Wu, Y. Zhang, H. Yu, Y. X. Liu, J. Pang and Y. Liu, Regulating the Cu0-Cu+ ratio to enhance metal-support interaction for selective hydrogenation of furfural under mild conditions, Chem. Eng. J., 2023, 468, 143755 CrossRef CAS.
- R. Tu, K. Liang, Y. Sun, Y. Wu, W. Lv, C. Q. Jia, E. Jiang, Y. Wu, X. Fan, B. Zhang, Q. Lu, B. S. Zhang and X. Xu, Ultra-Dilute high-entropy alloy catalyst with core-shell structure for high-active hydrogenation of furfural to furfuryl alcohol at mild temperature, Chem. Eng. J., 2023, 452, 139526 CrossRef CAS.
- W. Chen, Q. Peng, G. Fan, Q. Cheng, M. Tu and G. Song, Catalytic transfer hydrogenation of furfural to furfuryl alcohol over Al-containing ferrihydrite, J. Ind. Eng. Chem., 2023, 119, 574–585 CrossRef CAS.
- J. J. Musci, M. Montaña, A. B. Merlo, E. Rodríguez-Aguado, J. A. Cecilia, E. Rodriguez-Castellon, I. D. Lick and M. L. Casella, Supported ruthenium catalysts for the aqueous-phase selective hydrogenation of furfural to furfuryl alcohol, Catal. Today, 2022, 394, 81–93 CrossRef.
- W. Mao, J. Liu, B. Yin, D. Kong, S. Miao and F. Wang, Transfer hydrogenation of furfural catalyzed by multi-centers collaborative Ni-based catalyst and kinetic research, Appl. Catal., A, 2021, 623, 118247 CrossRef CAS.
- F. Li, S. Jiang, J. Huang, Y. Wang, S. Lu and C. Li, Catalytic transfer hydrogenation of furfural to furfuryl alcohol over a magnetic Fe3O4@C catalyst, New J. Chem., 2020, 44(2), 478–486 RSC.
- B. Gao, J. Zhang and J. H. Yang, Bimetallic Cu-Ni/MCM-41 catalyst for efficiently selective transfer hydrogenation of furfural into furfural alcohol, Mol. Catal., 2022, 517, 112065 CrossRef CAS.
- P. Weerachawanasak, P. Krawmanee, W. Inkamhaeng, F. J. C. S. Aires, T. Sooknoi and J. Panpranot, Development of bimetallic Ni-Cu/SiO2 catalysts for liquid phase selective hydrogenation of furfural to furfuryl alcohol, Catal. Commun., 2021, 149, 106221 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08609k |
| This journal is © The Royal Society of Chemistry 2025 |






