Open Access Article
Open Access ArticlePreparation and evaluation of the biosynthetic procedure of iron oxide and magnesium oxide nanoparticles using Hylocereus undatus fruit peel extract and their anticancer properties†
Sadia Adnin Oyshi *a,
Rumana A. Jahanb,
Fahima Aktar
*a,
Rumana A. Jahanb,
Fahima Aktar *c,
Md. Zakir Sultan
*c,
Md. Zakir Sultan b,
Abu Asad Chowdhuryc,
Jakir Ahmed Chowdhuryd,
Shaila Kabirc and
Md. Shah Amran*c
b,
Abu Asad Chowdhuryc,
Jakir Ahmed Chowdhuryd,
Shaila Kabirc and
Md. Shah Amran*c
aDepartment of Pharmacy, East West University, Dhaka, Bangladesh. E-mail: Sadia02021@gmail.com
bCentre for Advanced Research in Sciences (CARS), University of Dhaka, Bangladesh
cDepartment of Pharmaceutical Chemistry, University of Dhaka, Bangladesh. E-mail: fahima@du.ac.bd; amranms@du.ac.bd
dDepartment of Pharmaceutical Technology, University of Dhaka, Bangladesh
First published on 12th May 2025
Abstract
Nanoparticles offer enhanced interactions with other materials owing to their enlarged surface area. This property makes them stronger, more stable and ideal for biomedical applications. Among the various synthetic methods for nanoparticles, biosynthetic method stands out due to its cost-effectiveness and environmentally friendly nature. In this context, we developed novel biosynthetic procedures for iron oxide and magnesium oxide nanoparticles using the extract of Hylocereus undatus (dragon fruit) peel, which acted as a reducing agent and capping agent. The biosynthesized nanoparticles were characterized using different techniques, such as ultraviolet-visible (UV-VIS) spectrophotometry, transmission electron microscopy (TEM), energy dispersive X-ray spectroscopy (EDX), Fourier-transform infrared spectroscopy (FTIR) and X-ray diffractometry (XRD). To evaluate their anticancer properties, the nanoparticles were tested on HeLa cells (derived from human cervical carcinoma) and BHK-21 cells (obtained from baby hamster kidney fibroblasts) and compared with the negative control group (dimethyl sulfoxide) and standard group (Hylocereus undatus fruit peel extract). Results showed that only less than 5% HeLa cells survived in both cases, and less than 5% and 60% BHK-21 cells survived on administering magnesium oxide and iron oxide nanoparticles, respectively, which were quite better than the results obtained for the standard and negative control groups. This study reports a safe and rapid method for the biosynthesis of iron oxide and magnesium oxide nanoparticles using Hylocereus undatus fruit peel extract and demonstrates their potential as anticancer agents. These findings suggest that iron oxide and magnesium oxide nanoparticles warrant further investigation for the development of more effective and safe anticancer drug formulations.
Introduction
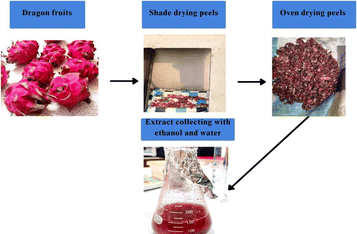
Nanotechnology is a crucial field of the modern world that deals with nanometer scale materials, which falls typically in the 1–100 nanometer range.1 It is applied for industrial, medicinal and energy purposes, exhibiting potential results in each of these sectors. Moreover, nanoparticles are noted for their small size, high surface area-to-volume ratio, and unique optical, magnetic, chemical, and mechanical properties.2 These characteristics make them promising candidates for biomedical applications, especially as antioxidant and anticancer agents.3 Although various chemical and physical methods are used to synthesize nanoparticles, they often involve hazardous and toxic chemicals. In contrast, biosynthesis strategies of metal nanoparticles are better than other strategies due to the low toxicity, cost-effectiveness, eco-friendliness, reduced time requirements and higher control ability in composition and distribution of the particle structure offered by these methods. Generally, the biosynthesis procedure includes a biological compound as a capping and reducing agent and a precursor.4,5 In our study, we used Hylocereus undatus fruit peel (HUFP) as the biological compound and iron chloride hexahydrate (FeCl3·6H2O) and magnesium nitrate hexahydrate (Mg(NO3)2·6H2O) as precursors to form iron oxide (Fe2O3NPs) and magnesium oxide nanoparticles (MgONPs). The reason behind choosing HUFP as the biological compound was because it contains chemical compounds, such as betanin, phyllocactin, hylocerenin, betacyanin, pectin, triterpenoids, and steroids,6,7 which exhibit various pharmacological effects against tumors, inflammation and oxidation.8,9 The antioxidant activity was particularly notable because these compounds could donate electrons to neutralize free radicals, indirectly suggesting that the organic compounds in dragon fruit peel might be capable of reducing metal salt ions in the synthesis of metal nanoparticles.Fe2O3NPs possess ferro-, ferri-, or super-magnetic properties that enhance their ability to reach specific organs using an external magnetic field. Their high reactivity can be localized within tumor cells through various methods and drive their potential use.10 When these NPs release ferrous or ferric ions in the acidic environment of lysosomes during endocytosis, it triggers Fenton and Haber–Weiss reactions, producing reactive oxygen species (ROS). This process leads to lipid peroxidation and damage to intracellular macromolecules in cancer cells.11,12 On the other hand, magnesium oxide (MgO) NPs exhibit anticancer properties through several key mechanisms. They induce ROS production, leading to the damage and death of cancer cells, which are particularly susceptible to oxidative stress. MgONPs trigger apoptosis in cancer cells, reducing their number. They show selective toxicity towards cancer cells over normal cells due to differences in cellular environments.13,14 Additionally, MgONPs disrupt mitochondrial function, causing loss of membrane potential and cell death, and can arrest the cancer cell cycle, preventing proliferation. These mechanisms make MgONPs promising for cancer therapy, with ongoing research aimed at optimizing their clinical use.15 Using HUFP extract here as biological agent we are trying to biosynthetically create iron oxide and magnesium oxide nanoparticles and study their anticancer effect (Fig. 1).
Experimental
Materials and chemicals
Dragon fruit peel, ferric chloride hexahydrate (FeCl3·6H2O), magnesium nitrate hexahydrate (Mg(NO3)2·6H2O), deionized water, sodium hydroxide (NaOH), dimethyl sulfoxide (DMSO), HeLa cell line and BHK21 Cell line from Centre for Advanced Research in Sciences (CARS), DMEM (Dulbecco's Modified Eagles' Medium).Preparation of the extract
For collecting their extract 5 g of the prepared powder was mixed into a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol and deionized water solution in two different 250 mL beakers. Then, the mixture was heated at 34 °C for 1 h. After cooling to room temperature, the solution was filtered through filter paper. Finally, the extract was stored at −4 °C for further use16 (Fig. 2).
1 ethanol and deionized water solution in two different 250 mL beakers. Then, the mixture was heated at 34 °C for 1 h. After cooling to room temperature, the solution was filtered through filter paper. Finally, the extract was stored at −4 °C for further use16 (Fig. 2).
Synthesis of NPs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
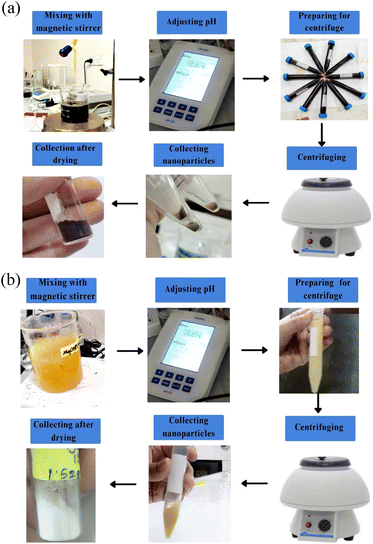
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio of extract and magnesium salt. Magnesium nitrate hexahydrate (Mg(NO3)2·6H2O) worked as a precursor and HUFP extract worked as a capping agent. Three hundred mL of 1 M of Mg(NO3)2·6H2O was taken in a 500 mL beaker and 60 mL of HUFP extract was added dropwise into it while magnetic stirring at 80 °C. To adjust the pH at 12, 1 M of NaOH was added and the resultant mixture was kept stirring for 4 h. As a result, the solution developed a yellowish colloidal appearance which indicated the formation of MgONPs.
5 ratio of extract and magnesium salt. Magnesium nitrate hexahydrate (Mg(NO3)2·6H2O) worked as a precursor and HUFP extract worked as a capping agent. Three hundred mL of 1 M of Mg(NO3)2·6H2O was taken in a 500 mL beaker and 60 mL of HUFP extract was added dropwise into it while magnetic stirring at 80 °C. To adjust the pH at 12, 1 M of NaOH was added and the resultant mixture was kept stirring for 4 h. As a result, the solution developed a yellowish colloidal appearance which indicated the formation of MgONPs.To separate the nanoparticles, the solution was centrifuged at 3000 rpm for 10 min. The collected nanoparticles were dried in a hot air oven at 50 °C. Later, the final product was calcined in a muffle furnace at 400 °C for 2 h to obtain the white powder of MgONPs18 (Fig. 3b).
Plausible chemical reaction
HUFP contains numerous phytochemicals, with betacyanin being the most abundant, contributing to its distinct reddish color.19 The precise mechanisms of the phytosynthesis of biogenic NPs are not fully understood, though several plausible pathways have been proposed. In biosynthesis, phytochemicals found in plant parts often play a crucial role in reducing particle size. Given the wide variety of phytochemicals, it is challenging to pinpoint the specific bio-reductant and stabilizing agents responsible for the production and stabilization of metallic nanoparticles (MNPs). Since betacyanin is the predominant phytochemical in HUFP, we hypothesize that it may act as a reducing agent, aiding in particle size reduction and facilitating the formation of nanoparticles. Notably, betacyanin contains a betalamic acid structure, which may contribute to size reduction by donating electrons through its functional –OH group20 and possibly through its nitrogen-containing groups as well. The exact stoichiometry and resulting products are likely to vary depending on conditions such as pH, temperature, and pressure. A plausible reaction involving the –OH functional group is illustrated in Fig. 4. The proposed mechanisms for the synthesis of Fe2O3 NPs and MgONPs from HUFP extracts are as follows:(1) An initial interaction occurs between the precursor salt solutions of FeCl3 and Mg(NO3)2 with the betacyanin-rich HUFP extract, particularly with the betalamic acids present in the extract. (2) As a result of this interaction, Fe–betalamic acid (Fig. 4(a)) and Mg–betalamic acid (Fig. 4(b)) complexes are formed. Here, betalamic acid functions as a reducing agent, with its –OH group contributing to the reduction and control of NP size. (3) Finally, Fe2O3 NPs (Fig. 4(a)) and MgONPs (Fig. 4(b)) are synthesized through the heating and calcination of the Fe–betalamic and Mg–betalamic acid complexes at elevated temperatures.
Characterization techniques
The biosynthesized Fe2O3NPs and MgONPs were characterized for their wavelength absorption, size, geometry, and structural consequences.UV-visible spectroscopy
UV-visible spectrophotometry was used to confirm NP formation. The formation of Fe2O3NPs and MgONPs was verified by monitoring the surface plasmon resonance band within the 200–800 nm range.X-ray diffraction (XRD) analysis
XRD was used to examine the crystalline nature of the biosynthesized Fe2O3NPs and MgONPs. Powdered samples were subjected to X-rays in scanning mode, operated at 30 mA current with 40 kV voltage and Cu/Kα radiation with 20–70° in 2θ angles the diffraction pattern was recorded at specific parameters. The resulting diffraction pattern allowed calculation of average crystalline size using the Debye–Scherrer equation:D = kλβ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ |
Fourier transform infrared (FTIR) spectroscopy
FTIR was employed to identify functional groups involved in Fe2O3NP and MgONP synthesis. This technique analyses the sample's infrared absorption spectrum in the 400–4000 cm−1 range, providing insight into capping, reduction, and stabilization mechanisms. Prior to analysis, synthesized Fe2O3NPs and MgONPs were mixed with KBr to make a transparent pellet.Transmission electron microscope analysis and energy dispersive X-ray analysis (TEM-EDX)
The size, shape, and morphology of the biosynthesized Fe2O3NPs and MgONPs were analysed using a TEM. To prepare the samples for TEM analysis, the Fe2O3NPs and MgONPs were dispersed in deionized water using an ultrasonic bath to form a uniform suspension. A drop of this suspension was then applied to a carbon-coated copper grid with a lacey carbon film and left to dry at room temperature. The elemental composition of the synthesized Fe2O3NPs and MgONPs was examined through EDX analysis using the same instrument used for capturing TEM images.Anticancer test
The anti-cancer effect of Fe2O3NPs and MgONPs was observed qualitatively and quantitatively. The qualitative test was done by checking cytotoxicity against HeLa and BHK21 cells and the quantitative test was done by checking cytotoxicity against HeLa cells. HeLa and BHK21 cell lines were maintained in DMEM containing 1% penicillin–streptomycin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 0.2% gentamycin, and 10% foetal bovine serum (FBS). HeLa cells (2 × 104/100 μL) and BHK21 cells (1.5 × 104/100 μL) were seeded onto a 96-well plate and incubated at 37 °C in a CO2 incubator (Nuaire, USA). A 50 μL (30 mg mL−1) sample of Fe2O3NPs and 50 μL (3 mg mL−1) of MgONPs (autoclaved) were added to each well for qualitative testing and a 25 μ sample from different doses of Fe2O3NPs and MgONPs was applied for quantitative testing. After 48 h of incubation, insoluble samples were washed out with fresh media and cytotoxicity was examined under a trinocular microscope with a camera (Optika, Italy). Duplicate wells were used for each sample.17,21
1), 0.2% gentamycin, and 10% foetal bovine serum (FBS). HeLa cells (2 × 104/100 μL) and BHK21 cells (1.5 × 104/100 μL) were seeded onto a 96-well plate and incubated at 37 °C in a CO2 incubator (Nuaire, USA). A 50 μL (30 mg mL−1) sample of Fe2O3NPs and 50 μL (3 mg mL−1) of MgONPs (autoclaved) were added to each well for qualitative testing and a 25 μ sample from different doses of Fe2O3NPs and MgONPs was applied for quantitative testing. After 48 h of incubation, insoluble samples were washed out with fresh media and cytotoxicity was examined under a trinocular microscope with a camera (Optika, Italy). Duplicate wells were used for each sample.17,21
Results and discussion
Analysis of nanoparticles
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and 478.35 cm−1, 601.79 cm−1, 1083.99 cm−1, 1477.47 cm−1, 1622.13 cm−1 and 3427.71 cm−1 for the 1
1 ratio and 478.35 cm−1, 601.79 cm−1, 1083.99 cm−1, 1477.47 cm−1, 1622.13 cm−1 and 3427.71 cm−1 for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio (Fig. 6). The peaks at 474.49 and 474.35 cm−1 ensured the presence of Fe–O bond in the sample.25,26 The peaks at 3427.71 cm−1 and 3435.22 cm−1 represent –OH bond stretching from the aqueous phase. Moreover, the peaks at 1477.47 cm−1 indicates C–H stretching, 1622.13 cm−1 and 1627.92 cm−1 indicates C
3 ratio (Fig. 6). The peaks at 474.49 and 474.35 cm−1 ensured the presence of Fe–O bond in the sample.25,26 The peaks at 3427.71 cm−1 and 3435.22 cm−1 represent –OH bond stretching from the aqueous phase. Moreover, the peaks at 1477.47 cm−1 indicates C–H stretching, 1622.13 cm−1 and 1627.92 cm−1 indicates C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching, 1084.70 cm−1 and 1083.99 cm−1 generally indicates C–O stretching, ensuring the presence of alkane, conjugated alkene and secondary alcohol in the HUFP extract as observed by a previous study.27
C stretching, 1084.70 cm−1 and 1083.99 cm−1 generally indicates C–O stretching, ensuring the presence of alkane, conjugated alkene and secondary alcohol in the HUFP extract as observed by a previous study.27
The peak at 464.84 cm−1 ensured the presence of the Mg–O bond in MgONPs (Fig. 6). The peak at 3452.58 cm−1 represents –OH bond stretching from the aqueous phase, the peak at 2927.94 cm−1 indicates the stretching vibrations of aliphatic C–H bonds, 1627.96 cm−1 indicates the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of alkenes and the peak at 1384.89 cm−1 indicates the bending vibrations of the methyl (–CH3) group. Additionally, the peak at 1082.07 cm−1 indicates the presence of functional groups involving C–O stretching vibrations of alcohol.28
O stretching of alkenes and the peak at 1384.89 cm−1 indicates the bending vibrations of the methyl (–CH3) group. Additionally, the peak at 1082.07 cm−1 indicates the presence of functional groups involving C–O stretching vibrations of alcohol.28
The shift in peak position in the range of 400–4000 cm−1 ensures that these functional groups contain compounds bound to the iron oxide and magnesium oxide surfaces (Fig. 6).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (1
1) and (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
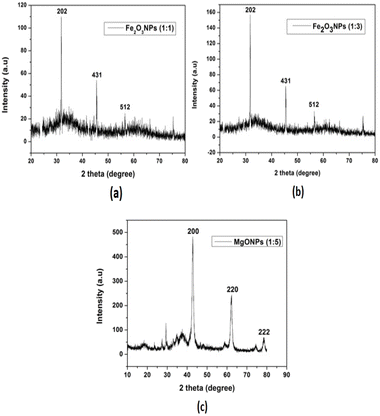
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (Fig. 7(b)). The intense and sharp peaks undoubtedly revealed that Fe2O3NPs formed by the reduction method using HUFP extract were crystalline in nature according to Te JCPDS file 019-0629.29 On the other hand, XRD data of MgONPs (1
3) (Fig. 7(b)). The intense and sharp peaks undoubtedly revealed that Fe2O3NPs formed by the reduction method using HUFP extract were crystalline in nature according to Te JCPDS file 019-0629.29 On the other hand, XRD data of MgONPs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) indicates that the crystal planes of (200), (220) and (222) correspond to that of 42, 62.19 and 78.47 which confirms the formation of MgONPs (Fig. 7(c)). They were verified using the JCPDS standard XRD data (no. 01-023-0074).30,31
5) indicates that the crystal planes of (200), (220) and (222) correspond to that of 42, 62.19 and 78.47 which confirms the formation of MgONPs (Fig. 7(c)). They were verified using the JCPDS standard XRD data (no. 01-023-0074).30,31
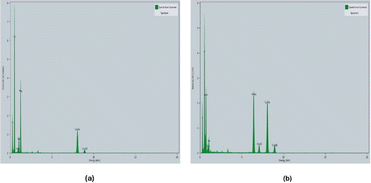
The EDX spectrum of the materials are depicted in Fig. 10(a) and (b), which show the elemental analysis of the synthesized Fe2O3NPs and MgONPs, respectively.34 This analysis demonstrated a prominent indication of a metallic iron and magnesium area at 6.5 keV and 1.5 keV, respectively and confirmed the formation of Fe2O3NPs and MgONPs synthesized through the utilization of HUFP. A high peak of oxygen and moderate signal of copper was observed. Furthermore, low signals for carbon and sodium were detected as a result of the chemicals used in Fe2O3NP preparation. Similar peaks were observed for MgONPs, which might be due to the use of different chemicals during sample preparation.
| Name of sample | Dose (μL) | Cell survival (%) | |
|---|---|---|---|
| HeLa cell | BHK-21 cell | ||
| Absence of solvent | 50 | 100 | 100 |
| In presence of solvent | 50 | >95% | >95% |
| HUFP extract (standard) | 50 | >95% | >95% |
| Fe2O3NPs | 50 (30 mg mL−1) | <5% | 60–70% |
| MgONPs | 50 (3 mg mL−1) | <5% | <5% |
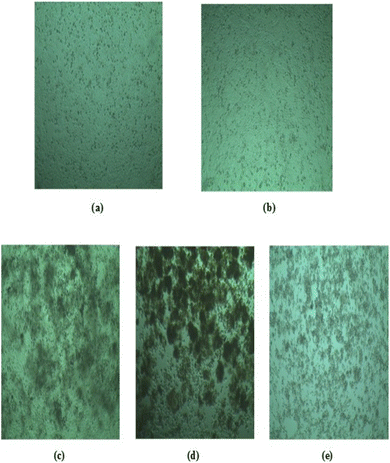
The data presented in the table and figures indicate that all cell lines survived without damage when no solvent (DMSO) was used. However, approximately 5% of cells are damaged after 48 h in the presence of DMSO. We considered HUFP extract as the standard which showed cell damage in less than 95% in both cells. Conversely, green-synthesized Fe2O3NPs and MgONPs exhibited significant toxicity towards HeLa cell lines, resulting in only 5% survival after 48 h. Additionally, BHK21 cell lines showed a similar survival rate in MgONPs (5%) but higher survival rates for Fe2O3NPs (around 60–70%) after 48 h at the same doses (Fig. 12). This increased toxicity is likely owing to higher nanoparticle doses, as observed in a previous study. The escalating nanoparticle concentration may induce excessive ROS-mediated oxidative stress, leading to DNA damage. This sensitivity to ROS suggests the potential for targeting cancer cells via ROS-mediated mechanisms, as cancer cells tend to be more vulnerable than normal cells.35 Iron and magnesium-based NPs have ROS inducing properties that could selectively eliminate tumor cells and hinder tumor growth. Also, Fe2O3NP damages cancer cells (HeLa cell) more than normal cells (BHK-21) whereas MgONP damages both cells equally. This indicates more selectivity of Fe2O3NPs than MgONPs towards cancer cells.
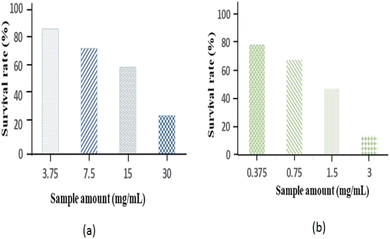 | ||
| Fig. 13 Quantitative test: survival rate of HeLa cells at different doses of (a) Fe2O3NPs and (b) MgONPs. | ||
 | ||
| Fig. 14 Quantitative test with Fe2O3 NPs: (1) 3.75 mg mL−1, (2) 7.5 mg mL−1, (3) 15 mg mL−1, and (4) 30 mg mL−1. | ||
Conclusions
This study reports a safe and rapid method for the synthesis and characterization of Fe2O3 and MgO nanoparticles using H. undatus fruit peel extract. The synthesized nanoparticles showed significant (p < 0.05) anticancer activities compared to standard drug and negative control in in vitro models. The Fe2O3NPs showed more selectivity towards cancer cells than MgONPs but it can be controlled with dose adjustments. The findings of this research indicate that Fe2O3 and MgO nanoparticles can be further studied for creating more effective and safe formulations as ideal anticancer drugs.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Sadia Adnin Oyshi: conceived and designed the experiments, performed the experiments, analyzed and interpreted the data and wrote the whole manuscript. Md. Shah Amran and Rumana A. Jahan: analyzed and interpreted the data; contributed reagents, materials, analysis tools or data. Md. Zakir Sultan: reviewed the analysis and interpreted the data. Fahima Aktar and Abu Asad Chowdhury: reviewed the study design and manuscript. Jakir Ahmed Chowdhury and Shaila Kabir: reviewed the analytical data and manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The author thanks to Dr Sheikh Ariful Hoque, Principal Scientist of Centre for Advanced Research in Sciences (CARS), University of Dhaka, Bangladesh for his support during anticancer study.References
- I. Khan, K. Saeed and I. Khan, Nanoparticles: Properties, applications and toxicities, Arabian J. Chem., 2017, 12(7), 908–931 CrossRef.
- K. A. Altammar, A review on nanoparticles: characteristics, synthesis, applications, and challenges, Front. Microbiol., 2023, 14, 1155622 CrossRef PubMed.
- J. Baranwal, B. Barse, A. Di Petrillo, G. Gatto, L. Pilia and A. Kumar, Nanoparticles in Cancer Diagnosis and Treatment, Materials, 2023, 16(15), 5354 CrossRef CAS PubMed.
- N. Saleh and Z. Yousaf, Tools and techniques for the optimized synthesis, reproducibility and scale up of desired nanoparticles from plant derived material and their role in pharmaceutical properties, Nanoscale Fabrication, Optimization, Scale-Up and Biological Aspects of Pharmaceutical Nanotechnology, ed. A. M. Grumezescu, Science-Direct: William Andrew Publishing, 2018, pp. 85–131 Search PubMed.
- C. Pandit, A. Roy, S. Ghotekar, A. Khusro, M. N. Islam and T. B. Emran, et al., Biological agents for synthesis of nanoparticles and their applications, J. King Saud Univ., Sci., 2022, 34(3), 101869 CrossRef.
- K. M. Herbach, F. C. Stintzing and R. Carle, Identification of heat-induced degradation products from purified betanin, phyllocactin and hylocerenin by high-performance liquid chromatography/electrospray ionization mass spectrometry, Rapid Commun. Mass Spectrom., 2005, 19(18), 2603–2616 CrossRef CAS PubMed.
- H. Luo, Y. Cai, Z. Peng, T. Liu and S. Yang, Chemical composition and in vitro evaluation of the cytotoxic and antioxidant activities of supercritical carbon dioxide extracts of pitaya (dragon fruit) peel, Chem. Cent. J., 2014, 8(1), 1 CrossRef PubMed.
- P. C. Dartsch, A. Kler and E. Kriesl, Antioxidative and antiinflammatory potential of different functional drink concepts in vitro, Phytother. Res., 2008, 23(2), 165–171 CrossRef PubMed.
- H. Kim, H. K. Choi, J. Y. Moon, Y. S. Kim, A. Mosaddik and S. K. Cho, Comparative antioxidant and antiproliferative activities of red and white pitayas and their correlation with flavonoid and polyphenol content, J. Food Sci., 2011, 76(1), C38–C45 CAS.
- Y. A. Koksharov, S. P. Gubin, I. V. Taranov, G. B. Khomutov and Y. V. Gulyaev, Magnetic Nanoparticles in Medicine: Progress, Problems, and Advances, J. Commun. Technol. Electron., 2022, 67(2), 101–116 CrossRef.
- F. Collin, Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases, Int. J. Mol. Sci., 2019, 20(10), 2407 CrossRef PubMed.
- R. Fernández-Acosta, C. Iriarte-Mesa, D. Alvarez-Alminaque, B. Hassannia, B. Wiernicki and A. M. Díaz-García, et al., Novel Iron Oxide Nanoparticles Induce Ferroptosis in a Panel of Cancer Cell Lines, Molecules, 2022, 27(13), 3970 CrossRef PubMed.
- B. Kumar, K. Smita, L. Cumbal, A. Debut and Y. Angulo, Biofabrication of copper oxide nanoparticles using Andean blackberry (Rubus glaucus Benth.) fruit and leaf, J. Saudi Chem. Soc., 2017, 21, S475–S480 CrossRef CAS.
- A. Pugazhendhi, R. Prabhu, K. Muruganantham, R. Shanmuganathan and S. Natarajan, Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii, J. Photochem. Photobiol., B, 2019, 190, 86–97 CrossRef CAS PubMed.
- V. Daniele, A. R. Volpe, P. Cesare and G. Taglieri, MgO Nanoparticles Obtained from an Innovative and Sustainable Route and Their Applications in Cancer Therapy, Nanomaterials, 2023, 13(22), 2975 CrossRef CAS PubMed.
- M. F. Ananta, S. A. Oyshi, M. M. A. Mim, R. N. Chowdhury, R. Akter and M. A. Rahman, et al., Multipurpose Drug from Rouwolfia serpentina and Nigella sativa: A Herbal Approach to Treat Hypertension and Hyperlipidemia in Experimental Rodent Model, Journal of Complementary and Alternative Medical Research, 2023, 24(4), 9–15 CrossRef.
- M. S. H. Bhuiyan, M. Y. Miah, S. C. Paul, T. D. Aka, O. Saha, M. M. Rahaman, M. J. I. Sharif, O. Habiba and M. Ashaduzzaman, Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity, Heliyon, 2020, 6(8), e04603 CrossRef CAS PubMed.
- R. B. Rotti, D. V. Sunitha, R. Manjunath, A. Roy, S. B. Mayegowda and A. P. Gnanaprakash, et al., Green synthesis of MgO nanoparticles and its antibacterial properties, Front. Chem., 2023, 11, 1–13 Search PubMed.
- L. Permana, P. Sriprom, K. Manamoongmongkol, L. Phumjan and P. Assawasaengrat, Optimization of betalain extraction from dragon fruit (Hylocereus undatus) peel and effect of pH on its properties, Biomass Convers. Biorefin., 2024, 15, 3545–3556 CrossRef.
- M. Ramezani Farani, M. Farsadrooh, I. Zare, A. Gholami and O. Akhavan, Green Synthesis of Magnesium Oxide Nanoparticles and Nanocomposites for Photocatalytic Antimicrobial, Antibiofilm and Antifungal Applications, Catalysts, 2023, 13(4), 642 CrossRef CAS.
- M. Rashid Khan, N. O. Alafaleq, A. K. Ramu, K. Alhosaini, M. S. Khan, T. A. Zughaibi and S. Tabrez, Evaluation of biogenically synthesized MgO NPs anticancer activity against breast cancer cells, Saudi J. Biol. Sci., 2024, 31(1), 103874 CrossRef CAS PubMed.
- Y. S. Kurniawan, A Fluorescence Study on the Extracts of Red Dragon Fruit Peel in Various Solvents, Indones. J. Nat. Pigm., 2021, 3(2), 47–51 Search PubMed.
- A. Hussain, M. Yasar, G. Ahmad, M. Ijaz, A. Aziz and M. G. Nawaz, et al., Synthesis, characterization, and applications of iron oxide nanoparticles, Int. J. Health Sci., 2023, 17(4), 3–10 Search PubMed.
- B. Boro, A. K. Nath, M. Barthakur and P. Kalita, Synthesis and characterization of MgO nanoparticle and its in vitro cytotoxic effect on erythrocytes, in Lecture Notes in Bioengineering, Springer, Singapore, 2020, pp. 199–207 Search PubMed.
- S. O. Aisida, N. Madubuonu, M. H. Alnasir, I. Ahmad, S. Botha, M. Maaza and F. I. Ezema, Biogenic synthesis of iron oxide nanorods using Moringa oleifera leaf extract for antibacterial applications, Appl. Nanosci., 2019, 10(1), 305–315 CrossRef.
- H. Liu, P. Li, B. Lu, Y. Wei and Y. Sun, Transformation of ferrihydrite in the presence or absence of trace Fe(II): The effect of preparation procedures of ferrihydrite, J. Solid State Chem., 2009, 182(7), 1767–1771 CrossRef CAS.
- S. O. Aisida, N. Madubuonu, M. H. Alnasir, I. Ahmad, S. Botha, M. Maaza and F. I. Ezema, Biogenic synthesis of iron oxide nanorods using Moringa oleifera leaf extract for antibacterial applications, Appl. Nanosci., 2019, 10(1), 305–315 CrossRef.
- B. Y. Hirphaye, N. B. Bonka, A. M. Tura and G. M. Fanta, Biosynthesis of magnesium oxide nanoparticles using Hagenia abyssinica female flower aqueous extract for characterization and antibacterial activity, Appl. Water Sci., 2023, 13, 175 CrossRef CAS.
- S. Lakshminarayanan, M. F. Shereen, K. L. Niraimathi, P. Brindha and A. Arumugam, One-pot green synthesis of iron oxide nanoparticles from Bauhinia tomentosa: Characterization and application towards synthesis of 1,3 diolein, Sci. Rep., 2021, 11, 8643 CrossRef PubMed.
- I. Y. Younis, S. S. El-Hawary, O. A. Eldahshan, M. M. Abdel-Aziz and Z. Y. Ali, Green synthesis of magnesium nanoparticles mediated from Rosa floribunda charisma extract and its antioxidant, antiaging and antibiofilm activities, Sci. Rep., 2021, 11, 16868 CrossRef CAS PubMed.
- S. Yousefi, B. Ghasemi, M. Tajally and A. Asghari, Optical properties of MgO and Mg(OH)2 nanostructures synthesized by a chemical precipitation method using impure brine, J. Alloys Compd., 2017, 711, 521–529 CrossRef CAS.
- N. Madubuonu, S. O. Aisida, A. Ali, I. Ahmad, T. Zhao, S. Botha, M. Maaza and F. I. Ezema, Biosynthesis of iron oxide nanoparticles via a composite of Psidium guavaja-Moringa oleifera and their antibacterial and photocatalytic study, J. Photochem. Photobiol., B, 2019, 199, 111601 CrossRef CAS PubMed.
- M. M. Abdel-Aziz, T. M. Emam and E. A. Elsherbiny, Bioactivity of magnesium oxide nanoparticles synthesized from cell filtrate of endobacterium Burkholderia rinojensis against Fusarium oxysporum, Mater. Sci. Eng., C, 2020, 109, 110617 CrossRef CAS PubMed.
- M. Kamaraj, T. Kidane, K. U. Muluken and J. Aravind, Biofabrication of iron oxide nanoparticles as a potential photocatalyst for dye degradation with antimicrobial activity, Int. J. Environ. Sci. Technol., 2019, 16(12), 8305–8314 CrossRef CAS.
- S. Mitra, L. N. Nguyen, M. Akter, G. Park, E. H. Choi and N. K. Kaushik, Impact of ROS generated by chemical, physical, and plasma techniques on cancer attenuation, Cancers, 2019, 11(7), 1030 CrossRef PubMed.
- U. S. Gaharwar, R. Meena and P. Rajamani, Biodistribution, clearance and morphological alterations of intravenously administered iron oxide nanoparticles in male Wistar rats, Int. J. Nanomed., 2019, 14, 9677–9692 CrossRef CAS PubMed.
- M. Amina, N. M. Al Musayeib, N. A. Alarfaj, M. F. El-Tohamy, H. F. Oraby and G. A. Al Hamoud, et al., Biogenic green synthesis of MgO nanoparticles using Saussurea costus biomasses for a comprehensive detection of their antimicrobial, cytotoxicity against MCF-7 breast cancer cells and photocatalysis potentials, PLoS One, 2020, 15(8), e0237567 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07411d |
| This journal is © The Royal Society of Chemistry 2025 |