 Open Access Article
Open Access ArticleEnhancement of biogas production using nanostructured magnetite (Fe3O4) in a biodigester fed with Peruvian guinea pig manure†
Rosa Flores Vargas ,
William Eduardo Gómez Hernández
,
William Eduardo Gómez Hernández ,
Karenina Ela Macazana López
,
Karenina Ela Macazana López ,
Clemente Alfredo Luyo Caycho
,
Clemente Alfredo Luyo Caycho ,
Yelstin Adrian Muñoz Baca,
Harry Anderson Rivera Tito and
María Esther Quintana Cáceda
,
Yelstin Adrian Muñoz Baca,
Harry Anderson Rivera Tito and
María Esther Quintana Cáceda *
*
Center for the Development of Advanced Materials and Nanotechnology, National University of Engineering, Av. Tupac Amaru 210, Lima 25, Peru. E-mail: mquintana@uni.edu
First published on 9th May 2025
Abstract
Magnetite nanoparticles were used to increase biogas production in a biodigester fed with Peruvian guinea pig (Cavia porcellus) manure (PGPM). The nanoparticles were synthesized via two different methods—coprecipitation and polyol—and thus showed different sizes of 410.7 nm and 34.03 nm, respectively. Likewise, various configurations were tested using three distinct Fe3O4 proportions, with each configuration tested in triplicate biodigesters. The coprecipitation trial with Fe3O4 was tested with an initial substrate of 5.57 g of chemical oxygen demand (COD) and 0.96 g of volatile solids (VSs) as inoculum. This ferrous additive led to a methane production increase of up to 9.61%, with a biodegradability of 57.91%. At the same time, the polyol trial with Fe3O4 was tested with an initial substrate of 34.47 g of COD and 0.80 g VS as inoculum, increasing methane production by up to 64.5% with a biodegradability of up to 8.56%. Moreover, the inhibitory effect of the synthesized Fe3O4, which was inconsequential for bacterial growth, was analyzed. Therefore, these nanoparticles have been shown to support methanogenic bacteria in enhancing methane production.
Introduction
Biogas is a gaseous mixture primarily composed of methane (CH4) and carbon dioxide (CO2); it also contains traces of other gases such as hydrogen (H2) and hydrogen sulfide (H2S) and is formed through the decomposition of natural organic matter (OM) in an anaerobic environment.1,2 Among these gases, CH4 stands out for its calorific value of around 7.0 kWh m−3 (considering an average 65% CH4 and 35% CO2 biogas composition).3 Furthermore, among the trace gases, H2S is noteworthy as it is responsible for the strong and unpleasant odor associated with biogas.4As its name suggests, biogas is naturally formed through a biological pathway and is therefore widely present in nature.1 It can be found in swamps, ruminants' stomachs,5 and sanitary landfills.6 In the latter, when there are high OM levels, such as in landfill leachate, its decomposition produces biogas due to the low solubility of oxygen (O2) in water. This gas is chemically similar to the natural gas found in fossil fuel deposits.4 In terms of energy content, 1 m3 of biogas is equivalent to 0.6–0.65 L of petroleum.3
Moreover, the European Union retrieves methane (CH4) from sanitary landfills. For example, Germany leads in biogas production owing to its positive legal framework established in 2000 for renewable energy sources, which has promoted the development of biogas facilities throughout the country.1 Currently, there are over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 facilities across Germany.7 Similarly, in Asia, thanks to programs that fomented the construction of digesters,3 China has over 100
000 facilities across Germany.7 Similarly, in Asia, thanks to programs that fomented the construction of digesters,3 China has over 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 biogas plants.7 This overview stands in stark contrast to Peru's efforts, as Peru is still far from reaching the renewable energy production goal set for 2030, which is 20%.8 As of 2022, Peru has converted only 5.9% of its energy matrix to unconventional renewable energy,9 of which only 0.1% is derived from biomass,10 despite the country's potential for renewable energy production (solar, wind, and biomass).11 Likewise, Peru generates a large volume of agricultural and commercial fishing waste, which is discarded,12 although it could be used for biogas production and thus tackle three major national needs: (1) improve organic waste disposal sanitation; (2) generate renewable energy and (2) supply stabilized materials, such as biofertilizer, that can be used in agriculture.3 Farm animal waste has already been used in biogas and energy production, with an array of different applications according to Monteros et al.13
000 biogas plants.7 This overview stands in stark contrast to Peru's efforts, as Peru is still far from reaching the renewable energy production goal set for 2030, which is 20%.8 As of 2022, Peru has converted only 5.9% of its energy matrix to unconventional renewable energy,9 of which only 0.1% is derived from biomass,10 despite the country's potential for renewable energy production (solar, wind, and biomass).11 Likewise, Peru generates a large volume of agricultural and commercial fishing waste, which is discarded,12 although it could be used for biogas production and thus tackle three major national needs: (1) improve organic waste disposal sanitation; (2) generate renewable energy and (2) supply stabilized materials, such as biofertilizer, that can be used in agriculture.3 Farm animal waste has already been used in biogas and energy production, with an array of different applications according to Monteros et al.13
Biogas production occurs through a complex biotechnological process of anaerobic digestion, where a consortium of bacteria degrades the OM in a coordinated manner.4 The essential condition to ensure the success of the process is the absence of O2. Biochemical and microbiological studies have thus far divided the process into four phases: hydrolysis, acidogenesis, acetogenesis, and methanogenesis.3 Anaerobic digestion has been extensively studied. However, despite being a promising method for treating large quantities of waste, it remains a low-efficiency process, which limits its ability to compete in the current fuel market.14 Since hydrolysis is the rate-limiting step of the process, several studies have focused on this phase to make the digestion process more efficient using chemical or physical pretreatments, such as the one reported by Hu et al.14 where oxidative methods were applied to enhance the degradation rate of sludge from a water treatment plant. Other studies evaluated the effect of component addition during digestion, as reported by Casals et al.15 who investigated the effect of adding magnetite (Fe3O4) nanoparticles of 7 nm into an anaerobic reactor for waste treatment.
Their results showed that biogas production per gram of OM increased by up to 180% when working with 100 ppm of Fe3O4, approximately 2.8 times the typical output. Huamán et al.16 also evaluated the effect of adding Fe3O4 particles sized 0.5–1.0 mm during anaerobic digestion. They used pig manure and evaluated the effect of two Fe3O4 dosages: 8 g and 12 g. The best results were obtained with 8 g of Fe3O4, yielding 3.82 × 10−2 N m3 of CH4 kg−1 of volatile solids (VSs). It is important to note that each OM source possesses different biogas production potentials. Generally, manure from polygastric animals generates less biogas than that from monogastric animals.17 According to the Food and Agriculture Organization (FAO),3 carbon (C) and nitrogen (N) are the main nutrients for methanogenic bacteria, both of which are present in larger quantities in PGPM compared with horse, cow, bird or pig manure.18
Villargaray et al.19,20 maintain that the average amount of guinea pig manure produced by females is 28.15 g per day and 31.03 g per day for males, which implies an average daily production of 801.19 tons of guinea pig excrement per day (considering the number of guinea pigs in 2021 reported by MIDAGRI: approximately 25820![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 guinea pigs).21
000 guinea pigs).21
Therefore, this study focuses on the effect of Fe3O4 particles on biogas production using PGPM under standard conditions, taking into account the geography and the diet of the animals in situ. The effect of the nanostructured particles was evaluated using a laboratory-scale biogas prototype reactor consisting of 500 mL bottles with hermetically sealed caps adapted for this purpose. They were used to directly quantify the amount of CH4 via volumetric displacement, produced through the synergy between the OM, microorganisms, and Fe3O4 nanoparticles. Finally, this study aims to contribute to the enhancement of biogas production in sectors dedicated to the use of guinea pig manure.
Materials and methods
Chemical reagents
The materials used for the synthesis and methanogenic activity tests are listed below: Iron nitrate (III) nonahydrate (Fe(NO3)3·9H2O, ≥98%), Sigma-Aldrich. Triethylene glycol (HO(CH2CH2O)3H, ≥98%), Merck. Ethyl acetate (CH3COOC2H5, ≥98), Merck. Ethanol (C2H6O, 99.5%), Spectrum Chemical. Iron chloride (II) (FeCl2, 99%), J. T. Baker. Iron chloride (III) (FeCl3, 97%), Central Drug House (CDH). Hydrochloric acid (HCl, 37%), Spectrum Chemical. Ammonium hydroxide (NH4OH, 30%), Merck. Sodium hydroxide (NaOH, ≥97%), Merck. Bromothymol blue (C27H28Br2O5S), Merck.Inoculum
The bacterial consortium (Fig. 1a) was collected from a biodigester of Universidad Nacional Agraria La Molina (UNALM). Moreover, the analyses of total solids (TS) and volatile suspended solids (VSS) were carried out at the SLab Peru testing and research laboratory (Table 1). The VSS/TS ratio was 0.38. This means that the amount of bacterial biomass in the inoculum was approximately 38% by mass.| Analysis | Results |
|---|---|
| TS | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 mg L−1 200 mg L−1 |
| VSS | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 mg L−1 200 mg L−1 |
Raw material
Dried guinea pig manure collected (Fig. 1b) from the UNALM cages was used as a feedstock bio-substrate providing MO to each experiment. Its physicochemical characteristics, such as TS, were analysed at the SLab Peru testing and research laboratory. At the same time, the percentages of carbon (C%), nitrogen (N%), carbon-to-nitrogen ratio (C/N), and moisture (H%) were determined at the Soil Laboratory of the Faculty of Agricultural Engineering of the UNALM (Table 2).| Analysis | Results (%) |
|---|---|
| TS | 79.42 |
| C | 44.95 |
| N | 2.04 |
| C/N | 22.03 |
| H | 25.04 |
Microbial growth support
The salts necessary for bacterial growth were prepared following the guidelines of Chernicharo et al.22,23 and the amounts reported by Cendales et al.24 using a Balch-type and reducing solution.Magnetite (Fe3O4) synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fe3+/Fe2+) is maintained in an alkaline medium (pH between 8 and 14) and an inert environment since Fe3O4 is unstable and sensitive to oxidation, which would lead to the formation of maghemite (γ-Fe2O3). However, oxygen is not the only oxidizing agent for Fe3O4, as shown in eqn (2); oxidation can also occur via electron or ion transfer, depending on the pH of the suspension.29
1 (Fe3+/Fe2+) is maintained in an alkaline medium (pH between 8 and 14) and an inert environment since Fe3O4 is unstable and sensitive to oxidation, which would lead to the formation of maghemite (γ-Fe2O3). However, oxygen is not the only oxidizing agent for Fe3O4, as shown in eqn (2); oxidation can also occur via electron or ion transfer, depending on the pH of the suspension.29| Fe2+ + 2Fe3+ + 8OH− → Fe3O4 + 4H2O | (1) |
| Fe3O4 + 2H+ → γ-Fe2O3 + Fe2+ + H2O | (2) |
The main advantage of this method is that a large number of particles can be synthesized albeit at the cost of a limited size distribution. In this study, Fe3O4 was synthesized following the method described by Puca et al.,29 with slight modifications. The experimental setup is shown in Fig. 2a. FeCl3 and FeCl2 were used as precursor salts, and 50 mL solutions were prepared, with each salt dissolved in HCl. Both solutions were poured into a three-necked flask equipped with a magnetic stirring bar. The reaction was conducted in an inert environment, with N2(g) bubbled into the system for over 5 min. The flask was then heated to about 80 °C, and synthesis started by dropwise adding 50 mL of NH4OH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Thereafter, Fe3O4 was washed with distilled water until the supernatant reached a pH of ≈7. Finally, the precipitate was poured into a Petri dish and stored in a desiccator for further use.
4). Thereafter, Fe3O4 was washed with distilled water until the supernatant reached a pH of ≈7. Finally, the precipitate was poured into a Petri dish and stored in a desiccator for further use.
In parallel, the precursor metal dissolves in the diol, thereby reducing the salt into iron complexes, which subsequently decompose and produce intermediate species (denoted as FeAx) (eqn (4)). These species act as nanoparticle cores in the subsequent step (eqn (5)) and serve as building blocks for their self-assembly and growth.
| Fe(NO3)3 + TEG ↔ Fe(NO3)2TEG + NO3− | (3) |
| Fe(NO3)2TEG → FeAx | (4) |
| FeAx ↔ Fe3O4 | (5) |
Specific methanogenic activity (SMA)
There are different methods for testing SMA. The one used as a basis for this work is described in the book Anaerobic Reactors23 and recommendations from the experiments developed by Córdova et al.34 and Cendales et al.24 To perform the tests, two glass bottles of 500 mL and 1000 mL were used. These were joined using a snap connector in the cap, through which a small hose allowed the biogas to flow from one container to the other. The digestion process was carried out inside the 500 mL bottle. In addition, 53 mL of inoculum, 32 mL of dissolved manure and 395 mL of mineral salt solution were added to each reactor.To measure gas production during the digestion process, the volumetric displacement method was used (Fig. 3). Each 1 L bottle was prepared with a 5% NaOH solution and a few drops of bromothymol blue. Additionally, the bottle cap contained a hose extended to the bottom of the bottle so that the displaced soda could flow due to the pressure of the generated biogas, while the other end of the hose rested on a glass collector. The tests were repeated three times under mesophilic conditions (35 °C). A total of five reactors were evaluated per run (the composition of each one is shown in Fig. 4). The first reactor was the target, the second was the pattern, and the rest were evaluated to assess the effect of different F3O4 quantities. Group 1 evaluated the performance of cop-Fe3O4 (magnetite synthesized via the coprecipitation method) with an initial organic loading of 5.57 g COD. Group 2 used pol-Fe3O4 (magnetite synthesized via the polyol method) and had an organic loading of 34.47 g COD, where higher performance was achieved when working up to 100 ppm16 (100 mg per litre of mixture).
Characterization equipment
Results and discussion
The quantities of Fe3O4 obtained from the synthesis routes are reported in Table 3. These measurements represent averages and do not account for the weight of small particles lost during the washing process.| Method | Quantity |
|---|---|
| Coprecipitation | 1.34 g |
| Polyol | 0.48 g |
Therefore, the average crystallite size was calculated from the analysis of the most intense diffraction peaks using the Debye–Scherrer equation (eqn (6)):
 | (6) |
| Miller index | 2θ (°) | d | a (Å) |
|---|---|---|---|
| (220) | 30.16 | 2.96 | 8.38 |
| (311) | 35.60 | 2.52 | 8.36 |
| (400) | 43.22 | 2.09 | 8.37 |
| (422) | 53.72 | 1.71 | 8.36 |
| (511) | 57.14 | 1.61 | 8.38 |
| (440) | 62.83 | 1.48 | 8.37 |
| Average | — | — | 8.37 |
| Miller index | 2θ (°) | d | a (Å) |
|---|---|---|---|
| (220) | 30.24 | 2.96 | 8.36 |
| (311) | 35.57 | 2.52 | 8.37 |
| (400) | 42.71 | 2.12 | 8.47 |
| (422) | 53.92 | 1.70 | 8.33 |
| (511) | 57.18 | 1.61 | 8.37 |
| (440) | 62.49 | 1.49 | 8.41 |
| Average | — | — | 8.38 |
K factor depends on the geometry, although a commonly used value is 0.89. This approximation is based on the assumption of perfectly spherical particles and is applicable to crystals with sizes up to 100 or 200 nm,38 as peak broadening decreases with an increasing particle size. In this regard, Bragg's law allows the determination of the lattice parameters of a crystal structure. cop-Fe3O4 and pol-Fe3O4 results are shown in Tables S1 and S2,† respectively. In addition, the Fe3O4 structure is a characteristic FCC of the inverse spinel type, featuring 8 tetrahedral sites occupied by Fe2+ and 16 octahedral sites equally occupied by Fe2+ and Fe3+.39 As an FCC structure, it has only one lattice parameter, which can be calculated from each crystallographic plane using Eq. (7) and (8).
| λCuKα = 2 × d × sin(θ) | (7) |
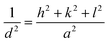 | (8) |
Moreover, it is well known in crystallography that h, k, and l are the Miller indices of a crystallographic plane. The calculated values for cop-Fe3O4 and pol-Fe3O4 are shown in Tables 4 and 5, respectively.
Both materials have a similar average lattice parameter of ≈8.39 Å, which was also reported by G. Peña et al.40
Measuring CH4 production content in biogas through the volumetric displacement method demonstrated daily biogas production by applying nanoparticles (Fig. 6a and b). In Group 2, the Fe3O4 effect is highly remarkable. Using 48 mg of pol-Fe3O4 (100 ppm) with a smaller particle size (according to DLS,41 Fig. S1 and eqn (S1)–(S3)†), CH4 production increased by up to 64.5% compared with the sample without Fe3O4. Nonetheless, the use of larger cop-Fe3O4 (100 ppm) (0.4 μm particle size) resulted in only a 9.61% increase. Therefore, both tests confirm that the optimal amount of Fe3O4 is around 100 ppm. Although, particle size also affects biogas production, when particles are too large (800 nm to 4.5 μm), they become inefficient.42 Thus, the increase in biogas is attributed to magnetite's role as a mediator between the donors and acceptors of chemical species involved in methanogenesis.42–44 According to the results, this suggests that increased biogas production was due to the symbiotic association between bacteria and iron nanoparticles, according to Baek et al.45 and Wang et al.,46 whose studies focused on investigating the role of iron compounds such as Fe3O4 in the performance of anaerobic digestion. Biogas production modelling can follow a sigmoid function.42,47 The most commonly used function is the modified Gompertz function (eqn (9)). Using this equation, the average daily gas production rate, the lag time, and the mass production estimate were obtained:
 | (9) |
Furthermore, Gompertz’s correlation in CH4 production (as shown in Fig. 7) from the corresponding reactors is shown in Fig. 6a. It is possible to determine the theoretical maximum CH4 that could be produced from a specific organic load. Theoretically, 2.61 g of COD can be converted into 1 L of CH4 under standard conditions if the organic matter is fully converted to methane.22 In the case of the trials with pol-Fe3O4, an additional amount of PGPM corresponding to 34.47 g COD was used, whereas the trials with cop-Fe3O4 used a corresponding amount of 5.57 g COD. The results for these trials are shown in Table 6.
| Analysis | Results | |
|---|---|---|
| Bd target (%) | Bd 100 ppm (%) | |
| Coprecipitation trial | 52.88% | 57.91% |
| Polyols trial | 5.30% | 8.56% |
Additionally, Table 8 provides a summary of various sources regarding the CH4 vol. Produced relative to volatile solids in the feed and the COD of the substrate.
The models are experimental extrapolation since most studies on biogas have test periods of 30 or 60 days. For this reason, the Gompertz mathematical model, commonly applied in such cases, was used for approximation (eqn (9) (S4) and (S5)†).
In the assays with pol-Fe3O4, an average increase of up to 64.5% in biogas production over 7 days was observed when 100 ppm of Fe3O4 nanoparticles were added, compared with a bioreactor without them. According to the Gompertz model, this increase would reach 45.81% after 40 days, indicating the point of maximum biogas production. In contrast, for the cop-Fe3O4 assays, an average increase of 9.61% was observed over 14 days with the addition of 100 ppm of Fe3O4 nanoparticles. Based on the Gompertz model, this increase would reach only 6.13% after 40 days.
Bacterial inoculum samples were taken and analyzed to determine the types of bacteria and to assess the potential inhibitory effect of Fe3O4 (Fig. 8). As shown in Table 7 and Fig. 9, the bacteria exhibited no inhibition in the presence of Fe3O4. These findings are corroborated by other studies, which confirm that Fe3O4 is not inhibitory but rather highly biocompatible with methanogenic bacteria.43
| Analysis | 108 UFC mL−1 | ||||
|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 96 h | 120 h | |
| Inoculum (control) | 9.7 | 9.5 | 9.0 | 8.0 | 7.0 |
| Inoculum plus Fe3O4 | 9.7 | 9.3 | 8.9 | 8.1 | 6.9 |
| Substrate | mL CH4/g VSs* | mL CH4/g COD** | Time (days) | Temperature (°C) |
|---|---|---|---|---|
| a *VSs: volatile solids, ** COD: chemical oxygen demand. | ||||
| Guinea pig manure (blank- pol-Fe3O4) - modeling | 1386 | 32 | 40 | 37.5 ± 0.5 |
| Guinea pig manure (blank- cop-Fe3O4) - modeling | 1583 | 274 | 40 | 37.5 ± 0.5 |
| Guinea pig manure (pol-Fe3O4) - modeling | 2021 | 47 | 40 | 37.5 ± 0.5 |
| Guinea pig manure (cop-Fe3O4) - modeling | 1680 | 291 | 40 | 37.5 ± 0.5 |
| Guinea pig manure (blank- pol-Fe3O4) | 869 | 20 | 7 | 37.5 ± 0.5 |
| Guinea pig manure (blank- cop-Fe3O4) | 1166 | 202 | 14 | 37.5 ± 0.5 |
| Guinea pig manure (pol-Fe3O4) | 1402 | 33 | 7 | 37.5 ± 0.5 |
| Guinea pig manure (cop-Fe3O4) | 1277 | 221 | 14 | 37.5 ± 0.5 |
| Cow manure48 | 168 | 90 to 180 | 37 ± 1 | |
| Mink manure48 | 512 | 90 to 180 | 37 ± 1 | |
| Cattle excrement49 | — | 300 | 7.3 | Mesophilic (30 to 38) |
| Domestic solid waste50 | — | 882 (mL biogas) | Continuous | 35 ± 1 |
| Municipal solid waste51 | 360 | Continuous | 35 ± 2 | |
| Rice straw52 | 178 | 356 | 40 | 37 |
| Corn straw53 | 217 | 35 | 37 ± 1 | |
| Wheat straw in co-digestion with fungi54 | 269 | 269 | 62 | 37 |
| Fallen leaves55 | 82 | 21 | 30 | 37 |
| Garden waste56 | 45 | 11 | 40 | 37 ± 1 |
| Pig manure57 | 127 | 212 | 30 | 32 |
| Pig manure58 | 247 | 412 | 60 | 32 |
 | ||
| Fig. 9 Benchmark of Methanosarcina bacteria in culture. (a) Bacteria Methanosarcina barkeri reported in the literature.58 (b) Methanosarcina colonies found in the experiment. | ||
Two main bacterial strains were identified, corresponding to methanogenic bacteria. Methanosarcina species were found, which are characteristic of ruminants. They primarily metabolize CH3COO−, C2H5OH and NH2CH3, converting these into CH4. They thrived at temperatures between 30 °C and 45 °C and at a pH between 5.0 and 7.4. They were observed to be immobile and grouped in cell aggregates, as shown in Fig. 9.58
The other strain identified was Methanothrix (Fig. 10). These are Gram-negative bacteria, filamentous in shape and measuring several micrometres in length. They are characterized by their ability to degrade CH3COO− into CH4 and CO2 and are incapable of degrading other substrates.44,59
 | ||
| Fig. 10 Benchmark of Methanothrix bacteria in culture. (a) Methanothrix sp. As reported in the literature.58 (b) Methanothrix colonies found in the experiment. | ||
Conclusions
To conclude, the trials clearly demonstrate the effect of adding Fe3O4 in 100 ppm on CH4 production. Smaller Fe3O4 particles (34 nm) showed an increase of up to 64.5% in total CH4 production compared with the Group 2 control reactor. Regarding cop-Fe3O4 (average size 0.4 μm), a slight increase of up to 9.61% was observed in the total CH4 production compared with the Group 1 control reactor. Notably, Fe3O4 also significantly affects lag time by reducing it. Although with cop-Fe3O4, the effect is negligible (a reduction of 0.13 days or 3 hours). When comparing trials with a smaller initial organic load over 7 days (Group 1 = 5.57 g COD initial), a higher biodegradability percentage was achieved compared with Group 2, which had a greater initial load (34.47 g). This highlights the importance of avoiding biodigester overloading with excessive substrate to ensure a more efficient COD removal process. Furthermore, nanoscale magnetite enhances biogas production regardless of the organic load, although its effects are best seen at higher loads (pol-Fe3O4). Finally, in the three regions of Peru (coast, Andes and Amazonia) household biogas production is feasible owing to abundant guinea pig farms, and the addition of Fe3O4 to guinea pig manure could help address energy needs in these remote areas.Data availability
https://www.drive.google.com/drive/folders/1k9sdaxQYGRHHUZfbE6r2ipNFfTMsWlto?usp=drive_link.Author contributions
Rosa Flores Vargas: formal analysis, investigation, methodology, validation, writing-original draft, and visualization; William Eduardo Gómez Hernández: investigation and methodology; Karenina Ela Macazana López: investigation, methodology, and manuscript correction; Clemente Alfredo Luyo Caycho: resources and investigation; Yelstin Adrian Muñoz Baca: resources and investigation; Harry Anderson Rivera Tito: investigation and manuscript correction and María Esther Quintana Cáceda: conceptualization, formal analysis, funding, and writing–review & editing.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
This research has been financed by PROCIENCIA, CONCYTEC and SENCICO through project 123-2018-FONDECYT. We extend our gratitude to the professors and researchers of the Science Faculty—Ronny Huamani, Bryan Córdova, Iván Luyo, Pilar García and Henry Huanca—for their contributions as well as Carmen Felipe-Morales and Jaime Villa for their guidance and expertise in biodigester development. Finally, we thank the Thin Films Lab, URPUNANO group and CEMAT for the facilities provided.References
- F. N. Rohstoffe e. V. (FNR), Guía sobre el Biogás: Desde la producción hasta el uso, 5a ed., Gülzow, 2010 Search PubMed.
- P. G. K. y I. Angelidaki, Biogas and its opportunities—A review, Front. Environ. Sci. Eng., 2018, 12, 14 Search PubMed.
- FAO, Manual de biogás, Ministerio de Energía del Gobierno de Chile, Programa de las Naciones Unidas para el Desarrollo, Organización de las Naciones Unidas para la Alimentación y la Agricultura, Global Environment Facility, 2011 Search PubMed.
- G. Casanovas, F. Della, F. Reymundo and R. Serafini, Guía teórico-práctica sobre el biogás y los biodigestores, Food and Agriculture Organization of the United Nations (FAO), Rome, 2019, Available from: https://openknowledge.fao.org/handle/20.500.14283/CA7005ES Search PubMed.
- V. García, Manual de Biogás: Conceptos básicos, beneficios de su producción y la aplicación de sus sub-productos, 9a ed., Dirección de Sustentabilidad, Medio Ambiente y Cambio Climático, Buenos Aires, 2015 Search PubMed.
- M. J. Asgari and K. S. y F. Mortazaeinezhad, Landfill Biogas Production Process, 2011 International Conference on Food Engineering and Biotechnology, IPCBEE, IACSIT Press, Singapore, 2011, 9 Search PubMed.
- M. Calero, V. Godoy, C. García Heras, E. Lozano and S. A. y M. A. Martín-Lara, Current state of biogas and biomethane production and its implications for Spain, Sustainable Energy Fuels, 2023, 7, 3584–3602 RSC.
- Propuesta Ciudadana, Balance de las políticas vinculadas a la transición energética en el Perú 2019-2022, Propuesta Ciudadana, Lima, 2023 [cited 2025 May 1], Available from: https://propuestaciudadana.org.pe/wp-content/uploads/2023/06/Balance-de-las-pol%C3%ADticas-vinculadas-a-la-transici%C3%B3n-energ%C3%A9tica-en-el-Per%C3%BA-2019-2022.pdf Search PubMed.
- E. Salazar Vega, «El lento avance del Perú para renovar su matriz energética por recursos limpios,» 2021. [En línea]. Available: https://www.ojo-publico.com/3106/el-lento-avance-del-peru-para-renovar-su-matriz-energetica Search PubMed.
- MINEM, Balance Nacional de Energía, 2022 Search PubMed.
- El Peruano, «Energías renovables: cambio necesario con visión de futuro para asegurar sostenibilidad,» 2022. [En línea]. Available: https://www.elperuano.pe/noticia/198353 Search PubMed.
- R. Orrego Moya, Estado del Arte y Novedades de la Bioenergía en el Perú, vol. Organización de las Naciones Unidas para la Alimentación y la Agricultura, 2011 Search PubMed.
- E. Monteros Curiel, L. C. Durand Moreno y and C. Martin del Campo Moreno, Nanopartículas de hierro magnetizado en la producción de biogás de excretas de ganado porcino, Tecnogestión, 2019, vol. 16(1), pp. 38–39, [cited 2025 May 1], Available from: https://revistas.udistrital.edu.co/index.php/tecges/article/download/14603/14643/72056 Search PubMed.
- Y. Hu, F. Wang and G. Lv y Y. Chi, Enhancing the Biogas Production of Sludge Anaerobic Digestion by a Combination of Zero-Valent Iron Foil and Persulfate, Energy Fuels, 2019, 33, 7436–7442 CrossRef CAS.
- E. Casals, R. Barrena, A. García, E. González, L. Delgado, M. Busquets-Fité, X. Font, J. Arbiol, P. Glatzel, K. Kvashnina and A. S. y. V. Puntes, Programmed Iron Oxide Nanoparticles Disintegration in Anaerobic Digesters Boosts Biogas Production, Small, 2014, 10, 2801–2808 CrossRef CAS PubMed.
- M. E. Huamán Córdova, H. L. de Castro e Silva, R. M. Barros, E. E. Silva Lora, I. F. Silva dos Santos, J. V. Rocha de Freitas, A. H. Moreira Santos, J. R. Pedreira and y B. K. Flauzino, Analysis of viable biogas production from anaerobic digestion of swine manure with the magnetite powder addition, Environ. Technol. Innovation, 2022, 25, 102207 CrossRef.
- J. E. Martí Herrero, Biodigestores Tubulares: Guía de diseño y Manual de instalación, RedBioLAC, Ecuador, 2019 [cited 2025 May 1], Available from: http://repositorio.ikiam.edu.ec/jspui/handle/RD_IKIAM/351 Search PubMed.
- T. Montes, Asistencia técnica dirigida en crianza tecnificada de cuyes., de 2.Trabajo presentado en Cajabamba por parte de la Universidad Nacional Agraria La Molina, Oficina Académica de Extensión y Proyección Socia, Cajamarca, 2015, vol. 1 Search PubMed.
- L. Villargaray and V. Alvares, Cantidad y calidad del estiércol producido por el cuy (cavia porcellus) alimentados con rye Grass y alfalfa, 1994.
- C. Felipe-Morales and U. Moreno, Biogas production with guinea pig manure, LEISA: ILEIA newsletter for low-external-input and sustainable agriculture, LEISA, 2005, vol. 21(1), p. 16, Available from: https://library.wur.nl/WebQuery/groenekennis/1756019 Search PubMed.
- Ministerio de Desarrollo Agrario y Riego, Cadena productiva de Cuy, vol. Dirección de estudios económicos. Dirección general de políticas agrarias., 2023 Search PubMed.
- C. A. de Lemos Chernicharo, Anaerobic Reactors, Biological Wastewater Treatment Series, IWA Publishing, London/New York, 2007, vol. 4 Search PubMed.
- C. A. de Lemos and C. y T. Bressani-Ribeiro ed., Anaerobic Reactors for Sewage Treatment: Design, Construction and Operation, IWA Publishing, 2019 Search PubMed.
- E. D. Cendales Ladino, Producción de biogás mediante la codigestión anaeróbica de la mezcla de residuos cítricos y estiércol bovino para su utilización como fuente de energía renovable, Tesis Doctoral, 2011 Search PubMed.
- S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. Vander Elst and R. N. Muller, Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications, Chem. Rev., 2008, 108, 2064–2110 CrossRef CAS PubMed.
- T. Ahn, J. H. Kim, H.-M. Yang, J. W. Lee and y J.-D. Kim, Formation Pathways of Magnetite Nanoparticles by Coprecipitation Method, J. Phys. Chem. C, 2012, 116(10), 6069–6076 CrossRef CAS.
- K. P. y A. Sirivat, Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method, Mater. Sci. Eng. B, 2012, 177, 421–427 CrossRef.
- A.-G. Niculescu and C. C. y A. M. Grumezescu, Magnetite nanoparticles: Synthesis methods-A comparative review, Methods, 2022, 199, 16–27 CrossRef CAS PubMed.
- M. Puca Pacheco, M. Guerrero Aquino, E. Tacuri Calanchi and R. G. Lopez Campos, Síntesis y caracterización de nanopartículas superparamagnéticas obtenidas por precipitación en microemulsión inversa para aplicaciones biomédicas, Rev. Soc. Quim. Peru, 2013, 79, 99–106 Search PubMed.
- M. Naito, T. Yokohama and K. H. y K. Nogi, Nanoparticle Technology Handbook, Elsevier, 2018 Search PubMed.
- W. Cai and J. Wan, Facile synthesis of superparamagnetic magnetite nanoparticles in liquid polyols, J. Colloid Interface Sci., 2007, 305, 366–370 CrossRef CAS PubMed.
- A. Kotoulas, C. Dendrinou-Samara and M. A. y O. Kalogirou, The Effect of Polyol Composition on the Structural and Magnetic Properties of Magnetite Nanoparticles for Magnetic Particle Hyperthermia, Materials, 2019, 12(17), 2663 CrossRef CAS PubMed.
- J. R. Vega Chacón, Influencia de los parámetros experimentales de síntesis en la formación de nanopartículas de magnetita preparadas por método poliol, Tesis de titulación, 2020 Search PubMed.
- V. E. Córdoba, D. G. Ibarlucía and E. M. Santalla, Desarrollo y validación de un mecanismo para remover CO2 y cuantificar la producción de CH4 en sistemas de digestión anaeróbica, RedBioLAC, 2022, 07, 2393–7394 Search PubMed.
- J. Vega, G. Picasso, L. Avilés Félix and A. López, Influencia de las variables experimentales de preparación en la obtención de nanopartículas de magnetita por el método de descomposición térmica, Rev. Soc. Quim. Peru, 2013, 79, 331–347 CAS.
- U. Schwertmann and y R. M. Cornell, Iron Oxides in the Laboratory, vol. 173, Weinheim: Wiley-VcH, 1991 Search PubMed.
- A. Ghosh and V. S. y R. Sundara, Comprehensive structural and magnetic properties of iron oxide nanoparticles synthesized through chemical routes, J. Alloys Compd., 2020, 818, 152931 CrossRef CAS.
- Nature Nanotechnology, "Broadening of diffraction peaks and its implications," Nat. Nanotechnol., 2011. [Online]. Available: https://www.nature.com/articles/nnano.2011.145 Search PubMed.
- B. Kalska-Szostko, D. Satula and W. Olszewski, Mössbauer spectroscopy studies of the magnetic properties of ferrite nanoparticles, Curr. Appl. Phys., 2015, 15, 226–231 CrossRef.
- G. Peña-Rodríguez, P. A. Rivera-Suárez, C. H. González-Gómez, C. A. Parra-Vargas, A. O. Garzón-Posada, D. A. Landínez-Téllez and J. Roa-Rojas, Efecto de la concentración de magnetita en la estructura, propiedades eléctricas y magnéticas de un material compuesto a base de resina de poliéster, Tecno Lógicas, 2018, 21(41), 13–27 CrossRef.
- B. J. Berne and R. Pecora, Dynamic Ligh Scattering with Applications to Chemistry, Biology, and Physics, Wiley - Interscience, New York, 1976 Search PubMed.
- E. Al-Essa, R. Bello-Mendoza and D. G. Wareham, The effect of magnetite particle size on methane production by fresh and degassed anaerobic sludge, Int. J. Environ. Ecol. Eng., 2020, 14(2), 63–66 Search PubMed.
- Y. Xu, Y. Lu, X. Dai, M. Liu, L. Dai and B. Dong, Spatial Configuration of Extracellular Organic Substances Responsible for the Biogas Conversion of Sewage Sludge, ACS Sustainable Chem. Eng., 2018, 6, 8308–8316 CrossRef CAS.
- C. Cruz Viggi, S. Rossetti, S. Fazi, P. Paiano, M. Majone and F. Aulenta, Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation, Environ. Sci. Technol., 2014, 48(13), 7536–7543 CrossRef CAS PubMed.
- G. Baek, J. Kim and C. Lee, A review of the effects of iron compounds on methanogenesis in anaerobic environments, Renewable Sustainable Energy Rev., 2019, 113, 109282 CrossRef CAS.
- Z. Wang, T. Wang, B. Si, J. Watson and Y. Zhang, Accelerating anaerobic digestion for methane production: Potential role of direct interspecies electron transfer, Renewable Sustainable Energy Rev., 2021, 145, 111069 CrossRef CAS.
- V. Rumyantceva, V. Rumyantceva, E. Koshel and V. Vinogradov, Biocide-conjugated magnetite nanoparticles as an advanced platform for biofilm treatment, Ther. Deliv., 2019, 10(4), 241–250 CrossRef CAS PubMed.
- V. Dubrovskis, I. Plume and I. Straume, Investigation of biogas production from mink and cow manure, Presented at Latvia University of Agriculture, Jelgava, Latvia, 2009 Search PubMed.
- L. Castrillón, I. Vázquez and E. M. y H. Sastre, Anaerobic thermophilic treatment of cattle manure in UASB reactors, Waste Manage. Res., 2002, 20, 350–356 CrossRef PubMed.
- J. Lebrato, J. L. Pérez-Rodríguez and y C. Maqueda, Domestic solid waste and sewage improvement by anaerobic digestion: a stirred digester, Resour. Conserv. Recycl., 1995, 13, 83–88 CrossRef.
- F. Cecchi and P. G. T. y P. Cescon, Anaerobic digestion of organic fraction of municipal solid wastes-digester performance, Sci. Total Environ., 1986, 56, 183–197 CrossRef CAS PubMed.
- Y. Gu, X. Chen, Z. Liu and X. Z. y Y. Zhang, Effect of inoculum sources on the anaerobic digestion of rice straw, Bioresour. Technol., 2014, 158, 149–155 CrossRef CAS PubMed.
- Z. Song, G. Yang, X. Liu, Z. Yan and Y. Y. y Y. Liao, Comparison of seven chemical pretreatments of corn straw for improving methane yield by anaerobic digestion, PLoS One, 2014, 9, e93801 CrossRef PubMed.
- J. Zhu and C. W. y Y. Li, Enhanced solid-state anaerobic digestion of corn stover by alkaline pretreatment, Bioresour. Technol., 2010, 101, 7523–7528 CrossRef CAS PubMed.
- Y. Lin and X. Ge y Y. Li, Solid-state anaerobic co-digestion of spent mushroom substrate with yard trimmings and wheat straw for biogas production, Bioresour. Technol., 2014, 169, 468–474 CrossRef CAS PubMed.
- J. Zhao and Y. Z. y Y. Li, Fungal pretreatment of yard trimmings for enhancement of methane yield from solid-state anaerobic digestion, Bioresour. Technol., 2014, 156, 176–181 CrossRef CAS PubMed.
- C. González-Fernández, C. León-Cofreces y and P. A. García-Encina, Different pretreatments for increasing the anaerobic biodegradability in swine manure, Bioresour. Technol., 2008, 99, 8710–8714 CrossRef PubMed.
- S. C. Lambie, W. J. Kelly, S. C. Leahy, D. Li, K. Reilly, T. A. McAllister, E. R. Valle, G. T. Attwood and E. Altermann, The complete genome sequence of the rumen methanogen Methanosarcina barkeri CM1, Stand. Genomic Sci., 2015, 10, 57 CrossRef PubMed.
- J. P. Touzel, G. Prensier, J. L. Roustan, I. Thomas, H. C. Dubourguier and G. Albagna, Description of a New Strain of Methanothrix soehngenii and Rejection of Methanothrix concilii as a Synonym of Methanothrix soehngenii, Int. J. Syst. Evol. Microbiol., 1988, 38, 30–36 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00102a |
| This journal is © The Royal Society of Chemistry 2025 |








