 Open Access Article
Open Access ArticleA sensitive and selective electrochemical detection and kinetic analysis of methyl parathion using Au nanoparticle-decorated rGO/CuO ternary nanocomposite†
N. I. Nayem a,
M. Sabbir Hossaina,
Md. A. Rashed
a,
M. Sabbir Hossaina,
Md. A. Rashed *a,
K. M. Anis-Ul-Haqueb,
Jahir Ahmedcd,
M. Faisalcd,
Jari S. Algethami
*a,
K. M. Anis-Ul-Haqueb,
Jahir Ahmedcd,
M. Faisalcd,
Jari S. Algethami cd and
Farid A. Harraz*ce
cd and
Farid A. Harraz*ce
aDepartment of Chemistry, Faculty of Science, Mawlana Bhashani Science and Technology University, Santosh, Tangail, 1902, Bangladesh. E-mail: abu.rashed@mbstu.ac.bd
bDepartment of Chemistry, Jessore University of Science and Technology, Jessore, 7408, Bangladesh
cAdvanced Materials and Nano-Research Centre (AMNRC), Najran University, Najran, 11001, Saudi Arabia. E-mail: faharraz@nu.edu.sa
dDepartment of Chemistry, Faculty of Science and Arts, Najran University, Najran, 11001, Saudi Arabia
eDepartment of Chemistry, Faculty of Science and Arts at Sharurah, Najran University, Sharurah, 68342, Saudi Arabia
First published on 9th May 2025
Abstract
Detecting organophosphorus pesticide (OP) residues is essential for maintaining ecological integrity and monitoring public health concerns. This research developed a novel electrochemical sensor that employed composite materials based on copper oxide (CuO) nanostructures and reduced graphene oxide (rGO), customized with Au nanoparticles (AuNPs), to detect methyl parathion (MP) pesticide with high selectivity and sensitivity. The nanocomposite was synthesized in two facile steps, without the use of stabilizers or dispersants, utilizing a simple ultrasonication and photo-reduction process. Morphological analysis revealed a uniform distribution of AuNPs and rGO within the CuO nanostructure. Kinetic studies demonstrated that the electro-reduction of MP on a glassy carbon electrode (GCE) modified with Au@rGO/CuO exhibited irreversible, diffusion-controlled kinetics, with a transfer coefficient (α) value of 0.485. A sensing study employing the square wave voltammetry (SWV) technique exhibited exceptional sensitivity (3.46 μA μM−1 cm−2), with a limit of detection (LOD) of 0.045 μM. Moreover, the Au@rGO/CuO-based sensor electrode exhibited exceptional selectivity for MP in the presence of various organic and inorganic species, along with notable reproducibility, repeatability, and stability. Overall, this electrochemical method for effective MP detection suggests that the prepared nanocomposite could contribute to the development of viable electrocatalysts.
1. Introduction
A growing population has led to the extensive use of pesticides to control insects and plant diseases, aiming to meet global food demands. However, the excessive use of pesticides may affect food quality. Overuse and misuse of pesticides can have detrimental effects on soil, water, human health, and ecological balance.1–4 Organophosphorus pesticides (OPs), such as methyl parathion (MP), have been used in agriculture for decades, demonstrating potent effectiveness against a wide range of pests in fruit trees, cotton, rice, tea, and vegetables.5 MP works by inhibiting acetylcholineesterase, disrupting the transmission of nerve signals in pests, which leads to paralysis and rapid death.6,7 Consequently, even at low concentrations, MP residues are universally recognized as a significant concern. Acute poisoning from OP compounds can lead to respiratory paralysis and death.8 Therefore, it is increasingly important for public health and environmental safety to rapidly identify and accurately quantify trace levels of OP pesticides.Currently, several established analytical techniques are employed to detect environmental pollutants, including OP pesticides. These include gas chromatography-mass spectrometry (GC-MS),9 high-performance liquid chromatography (HPLC),10 liquid chromatography-mass spectrometry (LC-MS),11 thin-layer chromatography (TLC),12 fluorogenic and colorimetric methods,13 and capillary electrochromatography (CEC).14 Although these methods offer excellent precision and accuracy, they also present notable drawbacks, such as complicated pre-treatment steps, high costs, the need for skilled personnel, extended detection times, poor selectivity, lack of portability, and labor-intensive procedures. In light of these challenges, considerable research efforts have focused on developing inexpensive, portable sensors for OP detection.
Recently, electrochemical techniques have garnered significant interest due to their affordability, ease of use, wide detection range, outstanding sensitivity, superior selectivity, fast reaction kinetics, and minimal operating time in MP determination.6,15 Electrochemical sensors often utilize the glassy carbon (GC) electrode as the working electrode due to its minimal reactivity, chemical resistivity, broad operating potential window, and effective conductive properties. However, the GC electrode suffers from electrode poisoning/fouling due to analyte adsorption or by-product generation, which significantly damages the electrode surface and blocks the active sites. This results in reduced repeatability and catalytic performance of the electrode.16,17 Consequently, electrochemical measurements of biological and environmental species have increasingly focused on chemically modifying electrodes instead of relying on bare electrodes, which tend to be less effective. It is widely acknowledged that the use of functional nanomaterials for electrode modification significantly enhances the performance of electrochemical sensors.18–20 The electrodes modified with nanomaterials facilitate electron transport by accelerating charge transfer, which ultimately improves sensitivity.21,22 In contrast, the unmodified GC electrode exhibits a limited response to electrochemical MP detection. By tailoring the GC electrode surface with different nanomaterials, either individually or in a combination form (composite form), such as conducting polymers,23 carbon nanomaterials,24 and metal oxide nanostructures,25 sensitivity for MP detection can be significantly increased. Research indicates that electrodes functionalized with nanomaterials may greatly increase the reaction rate and enhance the anti-interference capabilities of the sensor.26,27 Moreover, it is well-documented that nanomaterials have unique electronic, optical and mechanical properties.28 Additionally, noble metal nanoparticles, including Ag, Au, and Pt, have also attracted much interest when doping these onto a nanostructured matrix because of their remarkable conductivity and catalytic properties, which improve sensor performance.29,30
CuO, recognized as a P-type semiconductor, has been the subject of extensive investigation as a promising material in biosensing, which is attributed to its remarkable catalytic activity, chemical stability, and minimal overpotential for electron transport. The material exhibits a small bandgap of 1.20 eV and a nearly negligible redox potential (approximately 0.0 V vs. Ag/AgCl (KCl)).31,32 However, metallic Cu nanoparticles are easily oxidized by air or dissolved oxygen, making Cu(0) less durable for electrochemical processes. As such, much research has been done on chemically stable CuO nanoparticles for various electrochemical sensor applications, including MP sensors.25,33,34 A significant limitation of using CuO nanoparticles as efficient electrocatalysts is their propensity to agglomerate during electrode modification. Consequently, a supporting matrix is necessary to avert this aggregation.35 As the surface-to-volume ratio decreases due to nanoparticle aggregation, the efficacy of sensing also diminishes. To address these challenges, CuO is combined with high surface area containing materials such as conducting polymers31 and carbon nanomaterials (e.g., carbon nanotube, graphene oxide, porous carbon, etc.) in chemical and biological sensor applications.
Recent studies highlight the frequent use of rGO in the development of electrochemical sensors, attributed to its unique catalytic properties, remarkable conductivity, outstanding mechanical strength, and significant surface-to-volume ratio.36,37 rGO can significantly inhibit the aggregation of CuO nanostructures and increase the effective surface area of the sensor electrode. In addition, rGO is often mixed with metal oxide nanostructures and novel metal nanoparticles to produce composite materials that enhance the catalytic performance of electrochemical sensors.38
Among these novel metals, gold nanoparticles (AuNPs) are ideal for sensing applications because of their distinctive optical and electrical characteristics, which enhance selectivity, sensitivity, and reproducibility, ultimately boosting the sensor's performance.39 AuNPs are doped onto a CuO/rGO composite, which has been demonstrated to be useful in sensing applications. AuNPs significantly increase both the selectivity and sensitivity of the sensor. Furthermore, AuNPs are capable of setting apart rGO sheets to inhibit aggregation via π–π stacking interactions, while the limited contact among the surrounding rGO segments is reduced due to its hydrophilicity.40
Recently, CuO nanorod41 and CuO nanostructure anchored with conductive polymer42 were employed to develop an electrochemical sensor platform for OP pesticide detection. Literature reveals that in the presence of OP pesticides (such as MP), CuO forms a coordination bond with the thionate or oxonate functional group,41 which ultimately enhances the adsorption of the targeted OP pesticides onto the CuO surface. In addition, zirconia nanofiber-loaded rGO nanocomposite-modified stainless steel43 and electrochemically reduced graphene oxide modified GCE44 electrodes were successfully used for the lower limit detection of MP, where the RGO provides high surface area and electrical conductivity as well as high electrocatalytic performance. In addition, outstanding electrocatalytic activity of AuNPs, MIP/AuNPs/GCE,45 and AuNPs/Nafion/GCE46 were employed for the sensitive detection of OP pesticides (phosmet and parathion). This sensitivity could be attributed to the enlargement of the catalytic active sites and the synergistic coupling effect between AuNPs and matrix moieties, which ultimately enhanced the electron transfer between the surface of the indicator electrode and bulk (pesticide) solution.
Using the motivation of our anticipation of the positive characteristics of CuO, rGO, and AuNPs, in this study, we developed an AuNPs@rGO/CuO ternary nanocomposite in order to effectively detect and quantify MP in a neutral aqueous solution. Although the previously described MP sensor was impressive, the development of commercial sensors is hindered by high costs and intricate catalyst production processes. Therefore, we offer a simple and inexpensive method for fabricating electrocatalysts, and the current study addresses these challenges. Our demonstrated technique for catalyst fabrication is ecologically friendly because no stabilizer or dispersion was used during the nanocomposite's synthesis. Previous literature has already documented that AuNPs and CuO composite have been successfully employed for sensing applications involving methylglyoxal (MGO) and glyoxalase (GLO)47 as well as metformin (MET).48
Herein, the Au@rGO/CuO ternary nanocomposite was created using a simple synthesis route involving ultrasonication followed by photo-reduction. Numerous spectroscopic and microscopic techniques were employed to thoroughly characterize the resultant Au@rGO/CuO composite. The catalytic and sensing properties of Au@rGO/CuO-modified GCEs were evaluated via cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and square wave voltammetry (SWV) methods. The sensor electrode exhibited significant selectivity despite the presence of various common interfering species. Additionally, a successful recovery experiment was conducted using the newly adjusted sensor electrode to identify MP in river water samples collected from the river banks adjacent to agricultural fields. Finally, to our knowledge, this is the first report on the fabrication of an MP sensor using the Au@rGO/CuO ternary nanocomposite materials.
2. Experimental
2.1 Materials
The materials needed to fabricate the sensor electrode CuO nanopowder (99%+ pure; APS 30–50 nm) were procured from Otto Chemie, India, and used as received. rGO, HAuCl4·3H2O (containing at least 49.0% of Au), and Nafion solution (5 wt%) were obtained from Sigma-Aldrich (USA). The experimental reagent for the sensors (MP) was also sourced from Sigma-Aldrich (USA). In order to make a phosphate buffer solution (PBS) with a concentration of 0.1 M, Na2HPO4·2H2O and NaH2PO4·2H2O salts were used. A combination of 0.1 M NaOH and 0.1 M HCl was used to regulate the pH of the buffer solution. These chemicals were used without purification after being purchased from Merck, India. The anti-interference investigation also made use of the following chemicals: K2CO3, Mg(NO3)2, CaCl2, Na2SO4, KCl along with the organic species 4-NP, glucose (Glu), sucrose (Suc), chlorpyriphos (Chp) and H2O2, all of which were purchased and utilized in their original forms from Merck, India. The solutions used in the experiment were prepared using deionized water (DI-H2O).2.2 AuNPs@rGO/CuO nanocomposite fabrication
A simple ultrasonication method was employed to fabricate the reduced graphene oxide/copper oxide (rGO/CuO) nanocomposite. In this process, 470 mg CuO + 25 mg of rGO was typically sonicated continuously using an ultrasonic frequency of 40 kHz as well as ultrasonic power of 180 W for 30 minutes at room temperature in 90 mL of ethanol. To develop a 5 wt% rGO/CuO nanocomposite, the freshly prepared blend was repeatedly rinsed with a mixture of ethanol and water and then heated in an oven at 60 °C overnight. For the fabrication of the 1 wt% AuNPs@5 wt% rGO/94 wt% CuO nanocomposite, a specified mass of HAuCl4·3H2O salt and 1 mL of methanol was added to the as-prepared rGO/CuO nanocomposite. The mixture was then exposed to illumination for 21 hours with an Osram Hg lamp with an intensity of 2.0 mW cm−2 at a wavelength λ = 350 nm in a homemade closed chamber. Finally, the mixture was rinsed and dried to get the required Au@rGO/CuO electrocatalyst.2.3 Fabricated nanocomposite characterization
The morphology and structure of the synthesized nanocomposite were methodically examined using modern microscopy and spectroscopic techniques. The structural investigation was carried out using X-ray diffraction (XRD) with Bruker D4 Endeavour utilizing Cu Kα1/2 as the applied radiation source (λα1 = 154.06 pm, λα2 = 154.44 pm). The elemental composition and oxidation states of the components in the developed nanocomposite were investigated using X-ray photoelectron spectroscopy (XPS) using an AlKα (−10 to 1350 eV) monochromatic radiation source, Thermo Fisher, USA. Open-source XPSPEAK41 packaged software was used for XPS fine-scan peak deconvolution using a linear background with the energy calibration of C 1s at 284.5 eV. The investigation of surface morphology was carried out using techniques such as field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM) and high-resolution TEM (HR-TEM) technique. A scanning electron microanalyzer (JEOL-6300 F, 5 kV) connected to energy dispersive spectroscopy (EDS) was used to capture images and elemental composition. In addition, TEM and HR-TEM images were acquired using a JEOL JEM-2100F-UHF transmission electron microscope operating at an accelerating voltage of 200 kV. The microscope was equipped with a Gatan GIF 200 energy filter, and images were recorded using a 1k-CCD camera. Finally, Fourier transform infrared spectroscopy (FTIR) was conducted using Parkin-Elmer, USA, with the KBr plate method.2.4 Assembly of the sensor electrodes and electrochemical study
The working electrode (GCE; BAS Inc. Japan) with a diameter of 3.0 mm was used for the deposition of the newly synthesized nanocomposite, which was ultimately employed as a sensor electrode for MP detection. The GCE electrode was precisely tuned before modification with the ternary Au@rGO/CuO nanocomposite. The electrode surface was rubbed using 1.0 μm diamond paste and 0.05 μm aluminum slurry and then successively washed with a water–ethanol solution to achieve a shiny surface. The electrode surface underwent modification through the established drop-cast technique. During this process, 10 mg of the nanocomposite was dissolved in 900 μL of isopropanol along with 100 μL of a 5% Nafion solution as a binder. The active catalytic layer was generated by slowly injecting 7.5 μL of the aforementioned suspension onto the surface of the electrode, using 1.5 μL of each drop, and then allowing it to dry at room temperature. The last step was to heat the Au@rGO/CuO/GC electrode for 30 min at 60 °C. Additionally, rGO/CuO/GCE and CuO/GCE electrodes were prepared for the comparative analysis with the same modification conditions. An electrochemical workstation (CHI 660E, USA) with a three-electrode system was used to investigate the electrocatalytic and sensing events. The setup included a spiral Pt, Ag/AgCl electrode saturated with KCl, and GCE modified by ternary nanocomposite as the counter, reference, and working electrodes, respectively. Ambient conditions were maintained during the electrochemical tests.3. Results and discussion
3.1 Physicochemical characterization of the nanocomposite
The structural characteristics of the as-synthesized nanocomposite were thoroughly examined using XRD. The usual XRD patterns for CuO, rGO/CuO, and Au@rGO/CuO are shown in Fig. 1. The CuO XRD diffraction peaks were seen at 2θ values of 32.40°, 35.48°, 38.72°, 48.60°, 53.50°, 58.17°, 61.49°, 66.23°, 67.90°, 72.43° and 75.10° corresponding to the (110), (002), (111), (112), (020), (202), (113), (310), (220), (311) and (004) planes, respectively. The acquired diffraction patterns align closely with the monoclinic crystal structure of CuO (C2/c space group, JCPDS # 45-0937).49 XRD pattern of rGO/CuO shows the diffraction peaks for CuO with an additional broad peak at peak centered 2θ = 25.15° for rGO attributed to (002) crystalline plane, indicating the interlayer distance (d-spacing) of 3.53 Å.50 However, four additional diffraction peaks were observed in the case of the ternary composite at 2θ values of 38.16°, 44.26°, 64.68° and 77.42°, which correspond to the (111), (200), (220), and (311) crystalline planes, respectively, for the cubic structure of Au according to the previously reported literature29,51 and the standard diffraction pattern of Au (JCPDS # 04-0784).20 | ||
| Fig. 1 Combined XRD patterns of Au@rGO/CuO, rGO/CuO, and CuO. Standard diffraction cards for Au (JCPDS # 04-0784) and CuO (JCPDS # 45-0937) are included for indexing. | ||
Using the Debye–Scherrer equation, the size of the CuO crystallites in both undoped CuO nanostructure and Au@rGO/CuO composite was determined.52
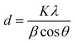 | (1) |
Further investigation into the catalyst surface composition and chemical states of AuNPs decorated rGO/CuO was carried out using XPS measurements. In Fig. S1,† the survey scan XPS spectra display Cu, O, C, and Au typical peaks at the defined binding energies (eV) with atomic % of 29.29, 44.0, 26.63, and 0.07, respectively. This finding clearly validates the successful assembly of a ternary nanocomposite. In order to examine the fine scan spectra of C, O, Cu, and Au, energy calibration was performed using a binding energy reference value of 284.5 eV for C 1s (Fig. 2(a)–(d)). As seen in Fig. 2(a), the high-resolution peak for C 1s splits into four peaks during deconvolution. The significant peak observed at the lowest binding energy of 284.5 eV is attributed to the C–C/C–H interaction.53 The peak at 286.1 eV is indicative of C–O bonds from functional groups that contain oxygen, such as hydroxyl (–OH) and epoxy (C–O–C), in the XPS spectra addressed for reduced rGO.54,55 Although GO is reduced to form rGO, some oxygen groups are usually retained. Meanwhile, the peaks at 287.1 eV binding energies are ascribed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O56 and the peak at 288.6 eV is attributed to the O
O56 and the peak at 288.6 eV is attributed to the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O link associated with the carboxyl group.57
C–O link associated with the carboxyl group.57
 | ||
| Fig. 2 XPS fine scan spectra of the Au@rGO/CuO nanocomposite: (a) C 1s, (b) O 1s, (c) Cu 2p, and (d) Au 4f. | ||
The high-resolution XPS spectra of O 1s are shown in Fig. 2(b). The presence of O2− in CuO (529.8 eV) is shown by fitting the O 1s XPS spectra into distinct components,58 whereas the peaks at 531.5 eV and 533.1 eV correspond to (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and C–OH59,60 in the nanocomposite, respectively.
O) and C–OH59,60 in the nanocomposite, respectively.
Fig. 2(c) unveils the Cu 2p core level XPS spectrum, illustrating a binding energy separation of 20 eV between the doublet Cu 2p3/2 (934.1 eV) and Cu 2p1/2 (954.1 eV).61 Nonetheless, a subtle positive shift in binding energies was noted concerning the prominent peaks of the reported CuO spectrum.62 The observed shifting may occur as a result of the interaction between CuO and other precursors within the nanocomposite. Furthermore, satellite peaks were detected at elevated binding energies in comparison to the principal peaks. Two satellite peaks were observed at 940.9 eV and 943.3 eV, alongside the principal peak Cu 2p3/2 at 934.1 eV. Additionally, a satellite peak at 962.3 eV was noted for the main peak Cu 2p1/2 at 954.1 eV. These satellite peaks within the energy ranges of 940–945 eV and 960–965 eV serve as a clear indication of the CuO phase characterized by a d9 electron configuration at the lower energy level.63,64
Finally, Fig. 2(d) reveals the high-resolution Au 4f spectrum, showcasing a doublet at 84.0 eV and 86.8 eV, which corresponds to Au 4f7/2 and Au 4f5/2, respectively. This outcome indicates the presence of AuNPs in their metallic form (Au0) within the developed nanocomposite, as evidenced by the binding energies recorded at 83.9 eV and 87.6 eV.65 Nevertheless, two shoulder-like peaks detected at binding energies of 85.2 and 88.0 eV may be linked to the cationic form of gold nanoparticles. The aforementioned results unequivocally validate the successful synthesis of the Au@rGO/CuO ternary nanocomposite.
The SEM technique was used to analyze the nanocomposite's morphology. Analysis of the undoped CuO and Au@rGO/CuO by SEM and EDS is shown in Fig. 3. The primary component of the nanocomposite, CuO, forms nanoflower-like structures characterized by a high surface area due to their petal-like nanosheets (Fig. 3(a)), which are advantageous for catalytic and adsorption applications. This extensive surface area also provides numerous sites for interaction with other components to enhance the composite's overall performance. In Fig. 3(b), the rGO, with its layered, wrinkled structure, acts as a conductive network within the composite, wrapping around or intercalating with the CuO nanoflowers to improve electrical conductivity. Furthermore, rGO plays a crucial role in stabilizing the dispersion of CuO nanoflowers, preventing their aggregation. Despite being present in a small quantity, the AuNPs contribute significantly due to their high catalytic activity and strong interactions with both CuO and rGO. These bright, spherical AuNPs are strategically dispersed throughout the composite, often attaching to the surfaces of CuO nanoflowers or residing within the rGO layers. The presence of AuNPs not only enhances the composite's catalytic activity but also facilitates charge transfer. The element composition of the undoped CuO and Au@rGO/CuO nanocomposite from EDS are represented in Fig. 3(c) and (d), respectively. The characteristic peaks of elements C, O, Cu, and Au certainly confirm the nanocomposite formation (Fig. 3(d)). The EDS compositional analysis (inset of Fig. 3(d)) indicates the wt% of Au is 1.32%, which is slightly higher than the theoretical 1% estimated based on the initial amount of the gold precursor (HAuCl4·3H2O) used during the nanocomposite synthesis. This minor deviation may arise due to experimental factors such as variations in precursor deposition, the localized nature of EDS analysis, and sampling in a specific area of the nanomaterial rather than an entirely homogeneous bulk composition.
 | ||
| Fig. 3 SEM image of CuO (a) and Au@rGO/CuO nanocomposite (b). Corresponding EDS compositional analysis for (c) CuO and (d) Au@rGO/CuO. | ||
The TEM analysis in Fig. 4(a) confirms the successful integration of AuNPs within the rGO/CuO matrix, where AuNPs appear as well-dispersed high-contrast spots over the matrix surface. The high-resolution TEM (HRTEM) image in Fig. 4(b) indicates the crystalline nature of the components, with measured lattice spacings of 0.229 nm and 0.249 nm, corresponding to the (111) plane of Au and the (002) plane of CuO, respectively.19,66 These findings indicate the presence of well-defined crystalline domains, reinforcing the structural integrity and stability of the composite. Additionally, the selected area electron diffraction (SAED) pattern in Fig. 4(c) exhibits clearly ordered centric rings, corroborating the polycrystalline nature of the as-synthesized nanocomposite. The observed lattice fringes and SAED results collectively confirm that the crystallinity of the CuO nanostructure and AuNPs are retained after the nanocomposite formation.
 | ||
| Fig. 4 TEM image of (a) the Au@rGO/CuO nanocomposite, (b) HR-TEM image of the Au@rGO/CuO nanocomposite, and (c) the SAED pattern of the Au@rGO/CuO nanocomposite. | ||
In the FTIR spectra of the 1% AuNPs@5% rGO/CuO nanocomposite (shown in Fig. S2†), distinct peaks were observed in comparison with those of individual rGO, CuO, and rGO/CuO samples, providing evidence of effective component integration and possible interactions. In the FTIR spectrum of rGO, bands at 1595 cm−1 and 3435 cm−1 corresponded to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching and O–H stretching, respectively. CuO displayed characteristic peaks associated with Cu–O stretching, typically appearing at 580 cm−1. In the rGO/CuO composite, both CuO and rGO peaks were present with slight shifts, indicating interactions between the two components, likely through π–π stacking and metal–oxygen bonds.
C stretching and O–H stretching, respectively. CuO displayed characteristic peaks associated with Cu–O stretching, typically appearing at 580 cm−1. In the rGO/CuO composite, both CuO and rGO peaks were present with slight shifts, indicating interactions between the two components, likely through π–π stacking and metal–oxygen bonds.
Upon the addition of AuNPs to form Au@rGO/CuO, further shifts and intensity changes in the characteristic peaks of CuO and rGO were noted. These spectral modifications suggest interactions among Au, CuO, and rGO, possibly due to electron delocalization and the plasmonic effect of AuNPs. These observations support successful composite formation with synergistic properties that are appropriate for electrochemical applications. This analysis effectively showcases structural integration and component interactions crucial for sensor efficacy.
In order to investigate the chemical stability of the sensory nanomaterials, the as-fabricated ternary nanocomposite was treated with 1 M HCl and 1 M NaOH for 3 days. After 3 days, the treated nanocomposite was separated and dried completely in an oven, and the nanostructure's morphology and structural properties were characterized using the FESEM and FT-IR studies. The FESEM images after the acid-base treatment of Au@rGO/CuO showed no significant morphological changes (Fig. S3†). In the case of the FTIR study (Fig. S4†), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching and O–H stretching were observed in all cases near 1600 cm−1 and 3450 cm−1 wavenumbers, respectively. Moreover, a characteristic Cu–O stretching band was also found near 580 cm−1. Other characteristic bands are in identical wavenumbers except for the changes in peak intensities.
C stretching and O–H stretching were observed in all cases near 1600 cm−1 and 3450 cm−1 wavenumbers, respectively. Moreover, a characteristic Cu–O stretching band was also found near 580 cm−1. Other characteristic bands are in identical wavenumbers except for the changes in peak intensities.
Thus, FESEM and FTIR analyses demonstrated that Au@rGO/CuO has excellent chemical stability, as its functional groups and overall chemical structure remained intact even after exposure to harsh conditions (1 M HCl and 1 M NaOH for three days). This makes Au@rGO/CuO a robust composite suitable for practical applications in aqueous environmental monitoring and pollutant detection.
Moreover, the structural characterization of the sensory nanocomposite was performed using FTIR (Fig. S4†), which also revealed that the chemical structure of the desired nanocomposite remained intact after prolonged electrolysis.
3.2 Investigation of the electrochemical properties of MP on the Au@rGO/CuO/GC electrode
Fig. 5 illustrates two consecutive cycles of cyclic voltammogram observed with 30 μM MP in 0.1 M PBS (pH = 7.0) on the Au@rGO/CuO/GC electrode. During the first cathodic scan, two reduction peaks were observed at −0.042 V and −0.62 V potentials. The small peak at the (Epc1 −0.042 V) potential results for the reduction of Cu+ from Cu2+ and the sharp irreversible reduction peak (Epc –0.62 V) corresponds to the reduction of the nitro group (–NO2) to hydroxylamine group (–NHOH) via a four-electron process (reaction (1)). This is then oxidized to the nitroso compound (–NO) during the anodic scan at (Epa1 −0.017 V) (reaction (2 forward)) and another small peak was observed at 0.16 V potential, addressed as Cu+ re-oxidized to Cu+. In the 2nd cycle, the current intensity of the reduction peak at (Epc1 –0.042 V) was increased compared to the 1st cycle as the nitroso group (–NO) was reversibly reduced to hydroxylamine (–NHOH) along with Cu+ from Cu2+ (reaction (2 backward)), and this step is facilitated by the reduction of Cu2+ to Cu+ at the same potential as well. The reversible redox peak (reaction (2)) can be ascribed to a two-electron redox process, aligning with various studies on nitroaromatic compounds.67–69 The reduction peak (Epc −0.62 V) decreased, which should be due to the minute quantity of MP present on the Au@rGO/CuO/GC electrode surface.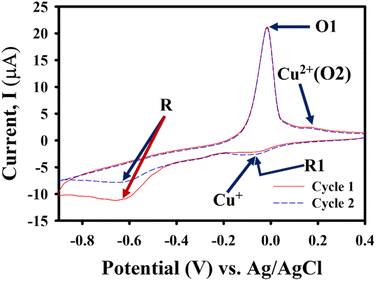 | ||
| Fig. 5 Cyclic voltammograms of 2 consecutive cycles at a scan rate of 50 mV s−1 for 30 μm MP in 0.1 M PBS at pH = 7.0, starting from the potential of 0.4 V. | ||
This result demonstrated that the Au@rGO/CuO/GCE ternary nanocomposite revealed good electroactivity toward MP. It has been reported that the noble metal Au can reduce 4-nitrophenol (4-NP) to 4-aminophenol (4-AP).70 The irreversible reduction peak (R; Epc = −0.62 V) resulted from the reduction of the nitro group (–NO2) of MP to hydroxylamine (–NHOH) using a four-electron reduction process (reaction (1)), and the reversible redox peak O1/R1 (at Epa1 and Epc1) having a peak splitting value of 25 mV was related to the interexchange between hydroxylamine (–NHOH) and the nitroso group (–NO) in a two-electron redox process (reaction (2)).71 The relevant chemical reactions are shown in Scheme 1.
The electrocatalytic investigation of MP was performed by the CV and EIS methods (30 μM MP in 0.1 M PBS; pH = 7.0). Fig. 6(a) displays the voltammograms of both the unmodified GCE and the CuO/GCE in the presence of MP at a scan rate of 50 mV s−1 with a potential window ranging from +0.4 to −0.9 V. A small irreversible reduction peak (Epc = −0.73 V) as well as oxidation peak (Epa1 = 0.040 V) were observed for the unmodified GCE. However, after modification with CuO, the irreversible reduction peak (Epc = −0.70 V), as well as a reversible oxidation (Epa1 = 0.071 V) and reduction Epc1 = 0.042 V peaks were observed, where the potential difference for the reversible peak was estimated as ΔE(p(CuO/GCE)) = 25 mV. As it is well documented, the catalytic ability is quantitatively enhanced when the ΔEp value for the redox reaction decreases. CuO acts as a reducing agent towards the reduction of MP in this case. Furthermore, CuO significantly increased the intensity of current in both irreversible (R) and reversible (O1 and R1) regions, as shown in (Fig. 6(a)). This suggests that the CuO/GCE system facilitates efficient electron exchange through faradaic redox processes at the electrode/electrolyte interface. The reduction of Cu2+ to Cu+ and the oxidation of Cu+ to Cu2+ are the sources of the redox signal seen in CuO/GC electrodes (Epc1 = −0.042 V).72,73
 | ||
| Fig. 6 Responses of CVs in the presence of 30 μm MP in 0.1 M PBS: (a) bare GCE and CuO/GCE; (b) bare GCE, CuO/GCE, rGO/CuO/GCE, and Au@rGO/CuO/GCE at a scan rate of 50 mV s−1. | ||
Voltammograms using bare GCE, CuO/GCE, rGO/CuO/GCE, and Au@rGO/CuO/GCE in the presence of MP are shown in (Fig. 6(b)). The rGO/CuO/GCE and Au/rGO/CuO/GCE shows Epa1 (O1) as well as Epc1 (R1) at the same potential as CuO/GCE, but the intensity of current at Epa1 was higher in the case of the ternary nanocomposite rather than the binary one, where the peak Epa1 (O1) represents the oxidation of hydroxylamine to the nitroso group and the peak at the Epc1 region is attributed to the reduction of Cu2+ to Cu+, combined with the reduction of the nitroso group to hydroxylamine. However, in the case of both binary and ternary composite systems, the peak potential and current intensity of Epa2 were the same as those of CuO/GCE. This also supports that the peak of Epa2 is significantly visible only for the oxidation of Cu+ to Cu2+.
In the CuO/GCE system, the cathodic irreversible peak (R) for MP electro-reduction appeared at (−0.70 V with Ipc = −8.82 μA). Furthermore, the reduction current exhibited a notable enhancement at the lower potential (−0.67 V with Ipc = −9.72 μA) when utilizing the rGO/CuO-modified GCE. Meanwhile, the Au@rGO/CuO/GC electrode exhibits a notable cathodic peak with a comparatively higher reduction current than its counterparts at a lower overpotential (Ipc = −11.09 μA, at −0.62 V). Table 1 illustrates the evaluation of the MP reductive voltammetric response, focusing on the cathodic peak current (Ipc) and peak potential (Epc) as determined by the examined electrodes. This observation indicates that the Au@rGO/CuO/GCE exhibited superior electrocatalytic activity compared to the other electrodes assessed for MP electro-reduction.
| Electrode | Epc (V) | Ipc (μA) |
|---|---|---|
| GCE | −0.73 | −5.36 |
| CuO/GCE | −0.70 | −8.82 |
| rGO/CuO/GCE | −0.67 | −9.72 |
| Au@rGO/CuO/GCE | −0.62 | −11.09 |
In order to explore in detail the electrochemical properties, EIS was used to investigate the variations in charge transfer resistance (Rct) among unmodified GCE, CuO/GCE, rGO/CuO/GCE, and Au@rGO/CuO/GCE.51 Under the following conditions, −0.6 V applied potential, 1 to 103 Hz frequency range, and 0.05 V signal amplitude, the EIS Nyquist plot was obtained for bare GCE, CuO/GCE, rGO/CuO/GCE, and Au@rGO/CuO/GCE with respect to the MP (30 μM) test analyte in 0.1 M PBS (pH = 7.0) (Fig. S5†). The semicircle diameter of impedance spectroscopy corresponds to the interfacial electron transfer resistance (Rct), which governs the electron transfer kinetics at the electrode surface.74 The electron transfer restricted process is shown by a semicircle in the high-frequency part of the EIS Nyquist plot, while the diffusion process is shown by a straight line in the low-frequency zone.75 Furthermore, the electrode-solution interface's lowest charge transfer resistance (Rct) is indicated by the lowest semicircle diameter in the high-frequency region, which also entails the highest conductivity.76 In this instance, a nearly straight line was seen for both unmodified GCE and CuO/GCE under the same experimental circumstances. The rGO/CuO/GCE and Au@rGO/CuO/GCE electrodes, on the other hand, exhibited a semicircle form at higher frequencies, with the Au@rGO/CuO/GCE electrode appearing to have a smaller semicircle diameter than rGO/CuO/GCE itself. Compared to the intended ternary composite modified GCE, this result shows that the unmodified GCE, CuO/GCE, and rGO/CuO/GCE electrodes produced greater Rct values, i.e., lower conductivity or catalytic efficiency with slow charge transfer kinetics. In conclusion, the calculated quantitative Rct values for GCE, CuO/GCE, rGO/CuO/GCE, and Au@rGO/CuO/GCE were 9600 Ω, 5233 Ω, 3610 Ω, and 257 Ω, respectively. In the simulation, a simplified Randles equivalent circuit incorporating Warburg impedance (W) was used, as shown in the inset of (Fig. S5†). In comparison to GCE, CuO/GCE, and rGO/CuO/GCE, the Au@rGO/CuO nanocomposite-modified GC electrode exhibited the fastest and greatest electron transfer kinetics for MP electrochemical reduction, as confirmed by this finding.
The exceptional electrocatalytic performance of the developed ternary nanocomposite regarding MP can be attributed to the synergistic coupling effect and the possible enhancement of the catalytic active surface area. CuO is used in nanocomposites for electrochemical applications due to its unique properties. It is a semiconductor material with a high surface area.77 CuO-based nanocomposites have also shown higher electrical conductivity and better catalytic performance.78 The incorporation of rGO sheets into CuO markedly diminishes the inherent aggregation of CuO nanostructures and improves the availability of active sites on the electrode surface, facilitating electrochemical reactions. The presence of these extra active sites enhances the conductivity and catalytic performance of rGO/CuO by establishing new pathways for electron transfer. Consequently, the electrochemical performance of rGO/CuO is enhanced when compared to the pure CuO electrode material.72 The combination of CuO and rGO results in a synergistic effect that provides rapid electron transfer kinetics, while the inclusion of AuNPs plays a crucial role owing to their outstanding catalytic properties.
In order to determine the enhancement of the active surface area, the ternary (Au@rGO/CuO) nanocomposite-modified GCE has been compared to bare GCE, and the redox probe 3.0 mM K3[Fe(CN)6] in 0.1 M KCl was utilized. The CVs obtained using the potential scan variation approach are shown in (Fig. S6(a) and (c)†) for the GC electrode modified with Au@rGO/CuO nanocomposite and the GCE electrode alone, ranging from 5 mV s−1 to 100 mV s−1. The Au@rGO/CuO/GCE exhibits a higher redox current when compared to the bare GCE. The cathodic and anodic peak currents for Au@rGO/CuO/GCE and bare GCE electrodes exhibit a linear relationship with the square root of the scan rate (ν1/2), as illustrated in (Fig. S6(b) and (d)†). The K3[Fe(CN)6]3−/4− redox system could be influenced by diffusion, as indicated by the linear relationship of the anodic peak current (Ipa) and cathodic peak current (Ipc) as a function of ν1/2. The next step is to determine the effective surface area (Aeff) of the unmodified GC electrode and the nanocomposite-modified electrode using the Randles–Sevcik equation (eqn (2)) for the reversible process.
| Ipa = (2.69 × 105)n3/2AeffD1/2ν1/2C0 | (2) |
The electro-reduction performance of MP on the Au@rGO/CuO/GCE sensor electrode is influenced by the pH of the electrolyte (PBS). Fig. 7(a) shows the SWV response in the presence of 10 μM of MP with the variation of the electrolyte solution (PBS 0.1 M) pH from 5.8 to 8.8. From the voltammogram, the cathodic peak current response (Fig. 7(a)) first increased when the pH was raised from 5.8 to 7.0. After that, the SWV current response gradually decreased with increasing pH (7.0–8.8) of the solution. According to this, Au@rGO/CuO/GCE shows the highest catalytic activities in MP electro-reduction in a neutral electrolyte condition (pH = 7.0). Because of participation in resonance, the nitrogen lone pair in the nitro group of MP's π-bonding system stays less reactive, hindering the reduction process in an acidic environment.81 Additionally, under basic conditions, the presence of hydroxide ions at higher pH causes the hindrance of the nitro group.81 Finally, we determined the optimal value of PBS's pH at 7.0 for further electrochemical and sensing studies of MP. Fig. 7(b) illustrates the relationship between peak current (Ip) vs. solution pH, with the highest current intensity observed at pH = 7.0.
The relationship between the reduction peak potential (Ep) vs. solution pH is depicted in Fig. 7(c). The cathodic peak exhibited a shift towards a more negative potential as the pH of the electrolyte increased. Thus, the MP electro-reduction process appears to be proton-dependent, as indicated by the straight line (r2 = 0.998) with respect to the Ep vs. pH relationship (Fig. 7(c)). The same phenomena were observed for nitro group reduction in imidacloprid pesticide and 4-NP cases.20,21,82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eqn (3), which is based on the Nernst equation, illustrating the correlation between Ep and pH.
Eqn (3), which is based on the Nernst equation, illustrating the correlation between Ep and pH.
 | (3) |
3.3 MP reduction: a kinetics investigation
The potential scan rate variation effect provides a convenient explanation for some facts related to the electrochemical reaction process. As a result, altering the scan rate to determine the electrochemical parameters can have the following effects presumed from the link between the scan rate (ν, Vs−1), peak current (Ipc), and peak potential (Ep) (Fig. 8(a)–(d)). By varying the scan rates from 5 mV s−1 to 100 mV s−1 in 0.1 M PBS, the electrochemical reduction of MP (30 μM) on the Au@rGO/CuO/GCE electrode was investigated (Fig. 8(a)). The irreversible electrochemical process of the MP electro-reduction (Peak “R”) shows the shifting of the peak towards the negative potential direction with increasing the scan rate. A linear equation with regression value r2 = 0.991, eqn (4), is obtained by plotting the cathodic peak current (Ipc) (for peak R) against the square root of the scan rate (ν1/2), which suggests that the kinetics of the MP reduction process is diffusion-controlled,86 as shown in (Fig. 8(b)).
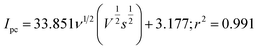 | (4) |
Additionally, a projected slope value of 0.279 was derived from the plot log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ipc vs. log
Ipc vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν, shown in (Fig. 8(c)). The literature indicates that for processes controlled solely by diffusion and those controlled entirely by adsorption, the slope values of log
ν, shown in (Fig. 8(c)). The literature indicates that for processes controlled solely by diffusion and those controlled entirely by adsorption, the slope values of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I against log
I against log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν are expected to be 0.5 and 1.0, respectively.87,88 If the slope value falls between 0.2 and 0.6, the literature states that the electrode reaction follows the diffusion-controlled process; if it falls between 0.75 and 1.0, the surface adsorption process controls the reaction kinetics; and if the value falls between 0.60 and 0.75, it is considered mixed adsorption-diffusion controlled.89 Hence, the slope value for log
ν are expected to be 0.5 and 1.0, respectively.87,88 If the slope value falls between 0.2 and 0.6, the literature states that the electrode reaction follows the diffusion-controlled process; if it falls between 0.75 and 1.0, the surface adsorption process controls the reaction kinetics; and if the value falls between 0.60 and 0.75, it is considered mixed adsorption-diffusion controlled.89 Hence, the slope value for log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ipc vs. log
Ipc vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν at 0.279 indicates that the electrochemical reduction of MP at the Au@rGO/CuO/GC electrode adhered to diffusion-controlled kinetics in a neutral pH medium.
ν at 0.279 indicates that the electrochemical reduction of MP at the Au@rGO/CuO/GC electrode adhered to diffusion-controlled kinetics in a neutral pH medium.
Next, from scan-rate dependent voltammograms (Fig. 8(a)), the cathodic peak potential (Epc) is estimated and displayed as a function of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν (Fig. 8(d)), revealing a linear relationship characterized by the following regression eqn (5).
ν (Fig. 8(d)), revealing a linear relationship characterized by the following regression eqn (5).
Epc = 0.061![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν −0.718 (ν, V s−1, r2 = 0.998) ν −0.718 (ν, V s−1, r2 = 0.998)
| (5) |
Determining the Tafel slope (b) is crucial for evaluating the electron transfer coefficient (α) value. The Tafel slope was estimated from eqn (6) to be 122 mV dec−1 using the slope value of eqn (5).
 | (6) |
The literature indicates that the rate-determining steps (RDS) for the transfer of two electrons (2e−) and a single electron (e−) are represented by the Tafel slope values of 60 mV dec−1 and ≥120 mV dec−1, respectively.90 This discovery allows us to propose that the MP reduction follows a single electron (e−) transfer in the RDS.
Subsequently, by applying the Tafel slope (b) value in addition to eqn (7), it is possible to estimate the electron transfer coefficient (α) for MP reduction utilizing the Au@rGO/CuO/GCE electrode.
 | (7) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485C mol−1. This equation leads to an estimate of α as 0.485, with the assumption that the electron in the rate-determining step (nα) is 1.
485C mol−1. This equation leads to an estimate of α as 0.485, with the assumption that the electron in the rate-determining step (nα) is 1.
3.4 Analytical performance for the detection of MP
The electrochemical sensing capabilities of the newly developed ternary nanocomposite-modified GCE were evaluated using the extremely sensitive SWV. The combination of high sensitivity and low background noise makes SWV particularly promising for electrochemical sensor study.91 Its ability to detect trace concentrations of organic or inorganic species in complex samples and perform rapid analysis makes it a versatile and powerful technique for a wide range of electrochemical applications.Square wave voltammetry (SWV) and differential pulse voltammetry (DPV) serve as essential techniques for electroanalysis, primarily in aqueous solvents. These methods generally exhibit sensitivity that is an order of magnitude greater than that of linear sweep voltammetry (LSV) and cyclic voltammetry (CV). For instance, SWV typically achieves detection limits in the nanomolar range, demonstrating a significant advantage over LSV. The primary concept that enhances the sensitivity of pulse techniques compared to CV/LSV lies in the varying decay rates of the faradaic and non-faradaic currents.91 The analytical performance study depicted in Fig. 9(a) shows the SWV of the Au@rGO/CuO/GCE-based electrochemical sensor for the determination of MP at a concentration window of 0.40–39.0 μM in 0.1 M PBS (pH = 7.0) solution. The duration of accumulation frequently plays a crucial role in influencing the sensing capabilities of the developed sensor for the assessment of MP. Fig. S7† illustrates that the cathodic peak current response does not exhibit a linear relationship with the accumulation time, even as the incubation/deposition time is altered between 30 s and 180 s. However, as the deposition time varied from 120 s to 180 s, the cathodic peak current remained constant. This is because as the accumulation time was longer than 120 s, the entire effective surface area of the electrode was covered, and the peak current did not change. Therefore, a deposition time or accumulation time of 120 s was considered as optimal for SWV measurement. This result demonstrates that the optimal deposition time is essential for enhancing the determination efficiency of MP using the Au@rGO/CuO/GCE sensor. Finally, the SWV was recorded using the experimental conditions: potential window −0.3 V to −0.9 V, the pulse amplitude 0.025 V, pulse increment 0.004 V, frequency 15 Hz, and accumulation time of 120 s. It is shown that with the increase in MP concentration, the reduction peak current is upraised. Additionally, Fig. 9(b) indicates a linear relationship between the peak current and [MP], ranging from 0.40 μM to 39.0 μM. The regression equation is as follows:
| I(μA) = −0.325[MP] − 4.174; (r2 = 0.996) | (8) |
The slope value, 0.325 μA μM−1, is derived from eqn (8). After dividing this slope by the effective surface area of the electrode (0.094 cm2), the sensor sensitivity was calculated, and the obtained value was 3.46 μA μM−1 cm−2. Lastly, the following eqn (9) can be used to estimate the detection limit (LOD)18,82 of the fabricated sensor:
| LOD = 3Sb/m(S/N = 3) | (9) |
In conclusion, the newly developed electrochemical sensor has demonstrated a commendable detection limit (LOD) and detection range (LRD), coupled with rapid sensing capabilities for MP detection. Table 2 presents a comparative analysis of the developed sensor alongside previously reported relevant MP sensors, focusing on LOD and LRD.25,34,43,44,68,69,86,92–99 The credible reason for enhancing the MP sensing performance in the case of the as-fabricated ternary nanocomposite is proposed as follows: (i) large surface area containing rGO hindered the aggregation of the CuO nanostructure, provides higher adsorption sites for forming coordination bonds between CuO and thionate or oxonate functional groups of MP.41 (ii) The small-sized AuNPs nicely dispersed over the rGO/CuO surface provide high catalytic activity towards MP electroreduction with a high surface-to-volume ratio. (iii) Doping of AuNPs and rGO into CuO tailored the catalytic properties of CuO as well as led to a synergistic effect, which in turn could improve the catalytic behavior and facilitate electron transfer between Au@rGO/CuO and the MP bulk solution.
| Electrode | Method | Linear range (μm) | LOD (μm) | Ref. |
|---|---|---|---|---|
| a Chi-GNs = chi-graphene nanosheets; ERGO-CS/Hb/FTO = electrochemically reduced graphene oxide-chitosan/hemoglobin based biosensor; YSZ = yttrium-stabilized zirconia@reduced graphene oxide; HCP5@AuNPs-ERGO = hydroxylatopillar[5]arene@gold nanoparticles and electrochemical reduced graphene oxide; CuO NWs = copper oxide nanowires; NanoPt-LDHs = nanosized Pt intercalated Ni/Al layered double hydroxides. | ||||
| AuNPs-chi-GNs/GCE | SWV | 0.76–3.79 | 0.0023 | 92 |
| ERGO/GCE | SWV | 0.002–0.03 | 0.00088 | 44 |
| ERGO-CS/Hb/FTO | SWV | 0.076–0.988 | 0.079 | 93 |
| PEDOT/YSZ@rGO/SS | SWV | 0.019–15.19 | 0.0059 | 43 |
| HCP5@AuNPs-ERGO/GCE | DPV | 0.005–30 | 0.001 | 86 |
| Nafion/CuONWs-SWCNT/GCE | DPV | 0.011–7.6 | 0.0000038 | 34 |
| NanoPt-LDHs/GCE | SWV | 0.001–0.0038 | 0.002 | 94 |
| Zr/Au | SWV | 0.019–0.38 | 0.011 | 95 |
| CPE | SWV | 1–60 | 0.05 | 68 |
| Pd/MWCNTs/GCE | DPV | 0.37–51.8 | 0.19 | 96 |
| Au–TiO2/Chit/GCE | DPV | 0.0038–26.60 | 0.0019 | 97 |
| Au/MWCNTs/GCE | DPV | 1.9–60.79 | 0.19 | 98 |
| Au/CNTs/GCE | LSV | 0.5–60 | 0.1 | 99 |
| CuO–TiO2/GCE | DPV | 0–0.0076 | 0.0000046 | 25 |
| AuNPs/Nafion/GCE | SWV | 0.5–120 | 0.1 | 69 |
| Au@rGO/CuO/GCE | SWV | 0.4–39.0 | 0.045 | This work |
The CV response in Fig. 6(a) depicts the current response noticeably enhanced in the CuO/GCE electrode compared to bare GCE. This observation indicates that the MP sensing mechanism is dominantly governed by the semiconductor-based metal oxide (CuO). This could happen because CuO plays a critical role in preferentially binding MP molecules through the coordination interaction as well as its catalytic activity towards MP.41 Moreover, the current response is sequentially enhanced after doping 5 wt% rGO and 1 wt% of AuNPs into the CuO nanostructure. rGO provides a high surface area, improving MP adsorption vs. π–π interaction, while also increasing the electrical conductivity. Finally, AuNPs catalyzed the MP electro-reduction process by significantly lowering the over-potential of 110 mV compared to bare GCE. It is well-documented that a combination of metal and metal oxide nanoparticles plays a crucial role in the electro-reduction of aromatic nitro compounds.100 Thus, an excellent sensing performance observed for the Au@rGO/CuO/GCE sensor is likely related to the brilliant combination of the nanomaterials. CuO acts as an outstanding matrix with large surface area and adsorption sites, rGO can hinder the aggregation of the CuO nanostructure as well as facilitate the electron transport process, and homogenously dispersed minute amount of AuNPs enhance the significant catalytic performance as well as sensing. Finally, we summarize that the newly developed nanocomposite combined the advantages of different single components and therefore, exhibited new properties beyond their single constituents.101 Furthermore, the enrichment of the adsorption-diffusion by the synergistic effect ultimately enhanced the sensing performance.
3.5 Interference, stability, and reproducibility studies
In order to ensure the validation of the suggested sensor electrode in practical applications, the selectivity test is an essential characteristic. As a result, we used the SWV approach to assess the selectivity of the as-fabricated sensor electrode in 0.1 M PBS (pH = 7.0) in the presence of a variety of typical organic and inorganic interfering species (Fig. 10). In comparison to the test analyte, MP (10 μM), five times higher concentration of interfering species were used during this selectivity study. Inorganic species, such as K2CO3, Mg(NO3)2, CaCl2, KCl, and Na2SO4, along with the organic species 4-nitrophenol (4-NP), glucose (Glu), sucrose (Suc), chlorpyriphos (Chp) and H2O2 were chosen for the selectivity test. The anticipated sensor Au@rGO/CuO/GCE exhibited minimal to barely noticeable current response in the case of interfering species with MP. Furthermore, the same current response was obtained for MP both before and after the addition of the interfering species. The excellent selective behaviour of this sensor can be attributed to the unique molecular interactions between MP and the nanocomposite, as well as the synergistic effects of its components. MP contains a nitro (–NO2) group and a phosphorus–sulfur (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) moiety, which play crucial roles in its electrochemical response. The aromatic nitro group undergoes a well-defined electrochemical reduction at a characteristic potential, minimizing interference from other analytes.44,102 Additionally, the strong interaction between the P
S) moiety, which play crucial roles in its electrochemical response. The aromatic nitro group undergoes a well-defined electrochemical reduction at a characteristic potential, minimizing interference from other analytes.44,102 Additionally, the strong interaction between the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S moiety and CuO contributes to selective adsorption and charge transfer, as copper-based nanomaterials exhibit an inherent affinity for organophosphorus compounds.103 The Au@rGO/CuO nanocomposite further enhances this selectivity through its distinct physicochemical properties. AuNPs act as excellent electron mediators, facilitating charge transfer and catalysing the electrochemical reduction of MP.104,105 The reduced graphene oxide (rGO) matrix provides a high surface area, improving MP adsorption via π–π interactions with its aromatic structure while also ensuring efficient electron transport.106 Furthermore, the electrochemical potential of MP is distinct from those of common interfering species, enabling selective detection. The adsorption–driven interaction between MP and the Au@rGO/CuO surface leads to an enhanced electrochemical signal, differentiating it from other pesticides and common organic/inorganic interfering species. As such, these findings unequivocally demonstrate that under the given experimental circumstances, the analyzed sensor electrode exhibits good selectivity for the measurement of MP. Fig. S8(a)† shows the results of an investigation into the repeatability and stability of the anticipated sensor, which was carried out using 25 repeated CV cycles. In 0.1 M PBS, CVs were recorded at 30 μM MP with the scan rate of 50 mV s−1. The variation in the intensity of the cathodic peak current as a function of the number of CV cycles is shown in Fig. S8(b).† The outcome showed that the relative standard deviation (RSD), is 1.70%. This %RSD value is exceedingly accommodating for electrochemical sensor applications. For the suggested sensor's reproducibility experiment, four GC electrodes that had been identically modified were investigated under the same experimental settings and their SWVs were recorded (Fig. S9†). According to the voltammogram responses, the peak current has a %RSD of 2.25%. Finally, the durability of the nanocomposite-modified sensor electrode during long-term storage was examined by leaving the electrode in an ambient environment for 12 days. With the exception of a slight decrease in current intensity from day 1 to day 12, the results showed no discernible change in peak shape (Fig. S10†). An acceptable %RSD of 3.88% was determined from four separate voltammograms by using the deviation value, as shown in the sensor analysis. It can be inferred from the above experimental results that the proposed sensor electrode has excellent sensing characteristics with respect to sensitivity, selectivity, repeatability, and storage stability.
S moiety and CuO contributes to selective adsorption and charge transfer, as copper-based nanomaterials exhibit an inherent affinity for organophosphorus compounds.103 The Au@rGO/CuO nanocomposite further enhances this selectivity through its distinct physicochemical properties. AuNPs act as excellent electron mediators, facilitating charge transfer and catalysing the electrochemical reduction of MP.104,105 The reduced graphene oxide (rGO) matrix provides a high surface area, improving MP adsorption via π–π interactions with its aromatic structure while also ensuring efficient electron transport.106 Furthermore, the electrochemical potential of MP is distinct from those of common interfering species, enabling selective detection. The adsorption–driven interaction between MP and the Au@rGO/CuO surface leads to an enhanced electrochemical signal, differentiating it from other pesticides and common organic/inorganic interfering species. As such, these findings unequivocally demonstrate that under the given experimental circumstances, the analyzed sensor electrode exhibits good selectivity for the measurement of MP. Fig. S8(a)† shows the results of an investigation into the repeatability and stability of the anticipated sensor, which was carried out using 25 repeated CV cycles. In 0.1 M PBS, CVs were recorded at 30 μM MP with the scan rate of 50 mV s−1. The variation in the intensity of the cathodic peak current as a function of the number of CV cycles is shown in Fig. S8(b).† The outcome showed that the relative standard deviation (RSD), is 1.70%. This %RSD value is exceedingly accommodating for electrochemical sensor applications. For the suggested sensor's reproducibility experiment, four GC electrodes that had been identically modified were investigated under the same experimental settings and their SWVs were recorded (Fig. S9†). According to the voltammogram responses, the peak current has a %RSD of 2.25%. Finally, the durability of the nanocomposite-modified sensor electrode during long-term storage was examined by leaving the electrode in an ambient environment for 12 days. With the exception of a slight decrease in current intensity from day 1 to day 12, the results showed no discernible change in peak shape (Fig. S10†). An acceptable %RSD of 3.88% was determined from four separate voltammograms by using the deviation value, as shown in the sensor analysis. It can be inferred from the above experimental results that the proposed sensor electrode has excellent sensing characteristics with respect to sensitivity, selectivity, repeatability, and storage stability.
3.6 Recovery analysis of methyl parathion in the river water sample
The developed electrochemical sensor's reliability and accuracy were carefully investigated using the conventional addition technique to recover MP from river water. The surface water was collected from the river banks adjacent to the cultivated lands (at Latitude (24.251917); Longitude (89.989555)) and (Latitude(24.162239); Longitude (89.850120)) at Bangshi and Dhaleswari rivers, respectively, in Tangail, Bangladesh. After removing all solid contaminants with filter paper (D = 0.45 μm), the real water samples were spiked with varying concentrations of standard MP (30, 20, and 15 μM). Each MP concentration was analyzed three times, and the average result was shown together with the standard deviation. The comprehensive experimental results are shown in Table S1.† Quantitative spiking recoveries of MP in the river water samples ranged from 96.03% to 103.88%, and the concentrations of MP found by the aforementioned approach were in agreement with the added values. This method's accuracy was further validated by the low %RSDs, which ranged from 1.31% to 3.54%. This indicates that the electrochemical sensor is highly reliable for the detection of MP in real samples.4. Conclusion
In the present study, we focused on developing an electrochemical sensor to determine the organophosphate (OP) class MP pesticide, which is widely used in the agricultural sector. To achieve this, we used a simple and cost-effective synthesis route to fabricate an AuNPs-doped rGO/CuO ternary nanocomposite. Experiments showed that even a small amount of AuNPs might dramatically boost the catalytic activity for the electro-reduction of MP, and the newly designed nanocomposite Au@rGO/CuO showed excellent catalytic performance, probably because of the synergistic coupling effect of the components' combined catalytic properties rather than individually. Kinetic analysis showed that the electro-reduction of MP at the Au@rGO/CuO-modified GCE was an irreversible diffusion-controlled process. The sensor demonstrated a broad linear concentration range of (0.40–39.0 μM), together with a lower LOD of 0.045 μM and a high sensitivity of 3.46 μA μM−1 cm−2, respectively. Additionally, during the detection of MP, the suggested sensor electrode showed a greater capacity to counteract the presence of common interfering species (both organic and inorganic). Finally, the satisfactory results of the MP recovery test conducted using surface-water samples highlight the potential benefits of using this proposed sensor electrode as an MP electrochemical sensor for real-world applications.Data availability
The data supporting the findings of this study are available from the author upon reasonable request.Author contributions
N. I. Nayem: conceptualization, methodology, software, formal analysis, investigation, data curation, writing – original draft. M. Sabbir Hossain: software, formal analysis, writing – original draft. Md. A. Rashed: supervision, project administration, funding acquisition, resources, validation, visualization, writing – review & editing. K. M. Anis-Ul-Haque: resources, software, data curation, writing – review & editing. Jahir Ahmed: resources, data curation, writing – review & editing. M. Faisal: data curation, formal analysis, data curation, writing – review & editing. Jari S. Algethami: resources, validation, writing – review & editing. Farid A. Harraz: project administration, validation, visualization, funding acquisition, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The research team thanks the Deanship of Graduate Studies and Scientific Research at Najran University for supporting the research project through the Nama'a program, with project code (NU/GP/SERC/13/425-1). The authors also acknowledge the Grants for Advanced Research in Education (GARE), Bangladesh Bureau of Educational Information & Statistics (BANBEIS) (Project ID: PS20232552), Ministry of Education, Bangladesh, for their financial assistance in this work.References
- A. M. Aloizou, V. Siokas, C. Vogiatzi, E. Peristeri, A. O. Docea and D. Petrakis, et al., Pesticides, cognitive functions and dementia: a review, Toxicol. Lett., 2020, 326, 31–51 CrossRef CAS PubMed.
- G. K. Sidhu, S. Singh, V. Kumar, D. S. Dhanjal, S. Datta and J. Singh, Toxicity, monitoring and biodegradation of organophosphate pesticides: a review, Crit. Rev. Environ. Sci. Technol., 2019, 49(13), 1135–1187 CrossRef CAS.
- W. Mandal, S. Fajal, P. Samanta, S. Dutta, M. M. Shirolkar and Y. D. More, et al., Selective and sensitive recognition of specific types of toxic organic pollutants with a chemically stable highly luminescent porous organic polymer (POP), ACS Appl. Polym. Mater., 2022, 4(11), 8633–8644 CrossRef CAS.
- S. Dutta, W. Mandal, A. V. Desai, S. Fajal, G. K. Dam and S. Mukherjee, et al., A luminescent cationic MOF and its polymer composite membrane elicit selective sensing of antibiotics and pesticides in water, Mol. Syst. Des. Eng., 2023, 8(12), 1483–1491 RSC.
- A. Kumaravel and M. Chandrasekaran, A novel nanosilver/nafion composite electrode for electrochemical sensing of methyl parathion and parathion, J. Electroanal. Chem., 2010, 638(2), 231–235 CrossRef CAS.
- C. S. Pundir, A. Malik and Preety, Bio-sensing of organophosphorus pesticides: a review, Biosens. Bioelectron., 2019, 140, 111348 CrossRef CAS PubMed.
- J. Yuan, L. Jiang, X. Tan, Y. Yue, H. Shi and S. Feng, Fabrication of Zr(IV) modified gold electrode via layer-by-layer self-assembly for methyl parathion, J. Electrochem. Soc., 2020, 167(2), 027519 CrossRef CAS.
- L. G. Costa, Organophosphorus compounds at 80: some old and new issues, Toxicol. Sci., 2018, 162(1), 24–35 CrossRef CAS PubMed . Available from: https://academic.oup.com/toxsci/article/162/1/24/4706006.
- N. Alvarenga, W. G. Birolli, E. B. Meira, S. C. O. Lucas, I. L. de Matos and M. Nitschke, et al., Biotransformation and biodegradation of methyl parathion by Brazilian bacterial strains isolated from mangrove peat, Biocatal. Agric. Biotechnol., 2018, 13, 319–326 CrossRef.
- L. Chen, X. Dang, Y. Ai and H. Chen, Preparation of an acryloyl β-cyclodextrin-silica hybrid monolithic column and its application in pipette tip solid-phase extraction and HPLC analysis of methyl parathion and fenthion, J. Sep. Sci., 2018, 41(18), 3508–3514 CrossRef CAS PubMed.
- R. Moreno-González, M. E. Juan and J. M. Planas, Table olive polyphenols: a simultaneous determination by liquid chromatography–mass spectrometry, J. Chromatogr. A, 2020, 1609 Search PubMed.
- R. Akkad and W. Schwack, Determination of organophosphorus and carbamate insecticides in fresh fruits and vegetables by high-performance thin-layer chromatography-multienzyme inhibition assay, J. AOAC Int., 2012, 95(5), 1371–1377 CrossRef CAS PubMed.
- G. K. Dam, S. Fajal, S. Dutta, S. Let, A. V. Desai and S. K. Ghosh, Hydrolytically stable luminescent cationic MOF for selective detection of toxic organic arsenic in water, ACS Appl. Opt. Mater., 2023, 1(7), 1217–1226 CrossRef CAS.
- W. Wu, Y. Wu, M. Zheng, L. Yang, X. Wu and X. Lin, et al., Pressurized capillary electrochromatography with indirect amperometric detection for analysis of organophosphorus pesticide residues, Analyst, 2010, 135(8), 2150–2156 RSC.
- H. Zhao, B. Liu, Y. Li, B. Li, H. Ma and S. Komarneni, One-pot green hydrothermal synthesis of bio-derived nitrogen-doped carbon sheets embedded with zirconia nanoparticles for electrochemical sensing of methyl parathion, Ceram. Int., 2020, 46(12), 19713–19722 CrossRef CAS.
- A. C. Schmidt, L. E. Dunaway, J. G. Roberts, G. S. McCarty and L. A. Sombers, Multiple scan rate voltammetry for selective quantification of real-time enkephalin dynamics, Anal. Chem., 2014, 86(15), 7806–7812 CrossRef CAS PubMed.
- M. Stoytcheva, R. Zlatev, V. Gochev, Z. Velkova, G. Montero and M. T. Beleño, Amperometric biosensors precision improvement. Application to phenolic pollutants determination, Electrochim. Acta, 2014, 147, 25–30 CrossRef CAS.
- M. A. Rashed, N. Nayem, M. H. Rahman, M. Faisal, J. S. Algethami and S. Alsareii, et al., A sensitive, selective non-enzymatic electrochemical detection and kinetic study of glucose over Pt nanoparticles/SWCNTs/NiO ternary nanocomposite, J. Taiwan Inst. Chem. Eng., 2023, 151, 105113 CrossRef CAS.
- M. H. Rahman, M. A. Rashed, N. I. Nayem, M. A. Rahaman, J. Ahmed and M. Faisal, et al., Nanogold-decorated reduced graphene oxide/chitosan composite for electrochemical sensing of N-acetyl-4-aminophenol, Mater. Chem. Phys., 2024, 314, 128915 CrossRef CAS.
- F. A. Harraz, M. A. Rashed, M. Faisal, M. Alsaiari and S. A. Alsareii, Sensitive and selective electrochemical sensor for detecting 4-nitrophenole using novel gold nanoparticles/reduced graphene oxide/activated carbon nanocomposite, Colloids Surf., A, 2022, 654, 130068 CrossRef CAS.
- M. A. Rashed, M. Faisal, S. A. Alsareii, M. Alsaiari, M. Jalalah and F. A. Harraz, Highly sensitive and selective electrochemical sensor for detecting imidacloprid pesticide using novel silver nanoparticles/mesoporous carbon/hematite ore ternary nanocomposite, J. Environ. Chem. Eng., 2022, 10(5), 108364 CrossRef CAS.
- M. A. Rashed, J. Ahmed, M. Faisal, S. A. Alsareii, M. Jalalah and F. A. Harraz, Highly sensitive and selective thiourea electrochemical sensor based on novel silver nanoparticles/chitosan nanocomposite, Colloids Surf., A, 2022, 644, 128879 CrossRef CAS.
- F. R. Wang, G. J. Lee, N. Haridharan and J. J. Wu, Electrochemical sensor using molecular imprinting polymerization modified electrodes to detect methyl parathion in environmental media, Electrocatalysis, 2018, 9(1), 1–9 CrossRef.
- H. Karimi-Maleh, R. Darabi, M. Baghayeri, F. Karimi, L. Fu and J. Rouhi, et al., Recent developments in carbon nanomaterials-based electrochemical sensors for methyl parathion detection, J. Food Meas. Charact., 2023, 17(5), 5371–5389 CrossRef.
- X. Tian, L. Liu, Y. Li, C. Yang, Z. Zhou and Y. Nie, et al., Nonenzymatic electrochemical sensor based on CuO-TiO2 for sensitive and selective detection of methyl parathion pesticide in ground water, Sensors Actuators, B, 2018, 256, 135–142 CrossRef CAS.
- K. P. Gannavarapu, V. Ganesh, M. Thakkar, S. Mitra and R. B. Dandamudi, Nanostructured diatom-ZrO2 composite as a selective and highly sensitive enzyme free electrochemical sensor for detection of methyl parathion, Sensors Actuators, B, 2019, 288, 611–617 CrossRef CAS.
- A. Othmani, Electrodes Coated with Nanomaterials and Their Use for Environmental and Electrochemical Applications, 2022, pp. 13–33 Search PubMed.
- S. Dutta, A. Sinelshchikova, J. Andreo and S. Wuttke, Nanoscience and nanotechnology for water remediation: an earnest hope toward sustainability, Nanoscale Horiz., 2024, 9(6), 885–899 RSC.
- M. A. Rashed, F. A. Harraz, M. Faisal, A. M. El-Toni, M. Alsaiari and M. S. Al-Assiri, Gold nanoparticles plated porous silicon nanopowder for nonenzymatic voltammetric detection of hydrogen peroxide, Anal. Biochem., 2021, 615, 114065 CrossRef CAS PubMed.
- A. R. Rajamani and S. C. Peter, Novel nanostructured Pt/CeO2@Cu2O carbon-based electrode to magnify the electrochemical detection of the neurotransmitter dopamine and analgesic paracetamol, ACS Appl. Nano Mater., 2018, 1(9), 5148–5157 CrossRef CAS.
- M. A. Rashed, J. Ahmed, M. Faisal, S. A. Alsareii, M. Jalalah and V. Tirth, et al., Surface modification of CuO nanoparticles with conducting polythiophene as a non-enzymatic amperometric sensor for sensitive and selective determination of hydrogen peroxide, Surf. Interfaces, 2022, 31, 101998 CrossRef CAS.
- N. R. Dhineshbabu, V. Rajendran, N. Nithyavathy and R. Vetumperumal, Study of structural and optical properties of cupric oxide nanoparticles, Appl. Nanosci., 2016, 6(6), 933–939 CrossRef CAS.
- N. Wannasri, P. Uppachai, N. Butwong, S. Jantrasee, I. M. Isa and S. Loiha, et al., A facile nonenzymatic electrochemical sensor based on copper oxide nanoparticles deposited on activated carbon for the highly sensitive detection of methyl parathion, J. Appl. Electrochem., 2022, 52(3), 595–606 CrossRef CAS.
- D. Huo, Q. Li, Y. Zhang, C. Hou and Y. Lei, A highly efficient organophosphorus pesticides sensor based on CuO nanowires-SWCNTs hybrid nanocomposite, Sensors Actuators, B, 2014, 199, 410–417 CrossRef CAS.
- H. Siddiqui, M. R. Parra, P. Pandey, M. S. Qureshi and F. Z. Haque, Utility of copper oxide nanoparticles (CuO-NPs) as efficient electron donor material in bulk-heterojunction solar cells with enhanced power conversion efficiency, J. Sci. Adv. Mater Devices, 2020, 5(1), 104–110 CrossRef.
- Z. Wang, F. Zhang, H. Zou, Y. Yuan, H. Wang and J. Xia, et al., Preparation of a Pt/NiFe layered double hydroxide/reduced graphene oxide composite as an electrocatalyst for methanol oxidation, J. Electroanal. Chem., 2018, 818, 198–203 CrossRef CAS.
- V. Mani, A. P. Periasamy and S. M. Chen, Highly selective amperometric nitrite sensor based on chemically reduced graphene oxide modified electrode, Electrochem. Commun., 2012, 17(1), 75–78 CrossRef CAS.
- N. Demir, K. Atacan, M. Ozmen and S. Z. Bas, Design of a new electrochemical sensing system based on MoS2-TiO2/reduced graphene oxide nanocomposite for the detection of paracetamol, New J. Chem., 2020, 44(27), 11759–11767 RSC.
- L. Tu, Gold nanomaterials for biochemical sensing, Gold Bull., 2022, 55(2), 169–185 CrossRef CAS.
- Z. Guo, Z. Y. Wang, H. H. Wang, G. Q. Huang and M. M. Li, Electrochemical sensor for Isoniazid based on the glassy carbon electrode modified with reduced graphene oxide-Au nanomaterials, Mater. Sci. Eng., C, 2015, 57, 197–204 CrossRef CAS PubMed.
- P. Sakdarat, J. Chongsuebsirikul, C. Thanachayanont, S. Prichanont and P. Pungetmongkol, Development of a nonenzymatic electrochemical sensor for organophosphate pesticide detection using copper(II) oxide nanorod electrodes, J. Nanomater., 2021, 2021, 1–11 CrossRef.
- S. Sudharsan, R. Rajaram, S. Kumar, P. Swaminathan, K. Ramanujam and L. Neelakantan, Copper oxide anchored polyaniline modified glassy carbon electrode: a new sensor platform for the amperometric determination of chlorpyrifos, Electrochim. Acta, 2023, 471, 143305 CrossRef CAS.
- Y. Lv, T. Yang, X. Hou, Z. Fang, K. Rajan and Y. Di, et al., Zirconia nanofibers-loaded reduced graphene oxide fabrication for specific electrochemical detection of methyl parathion, J. Alloys Compd., 2022, 904, 163798 CrossRef CAS.
- T. Jeyapragasam, R. Saraswathi, S. M. Chen and B. S. Lou, Detection of methyl parathion at an electrochemically reduced graphene oxide (ERGO) modified electrode, Int. J. Electrochem. Sci., 2013, 8(11), 12353–12366 CrossRef CAS.
- Y. Zhong, Z. Li, A. Zhang, Y. Peng, H. Zhou and Y. Guo, et al., Gold nanoparticle-mediated molecularly imprinted electrochemical sensor MIP/AuNPs/GCE for highly sensitive and selective detection of neutral phosmet residues in fruits and vegetables, Microchem. J., 2024, 201, 110728 CrossRef CAS.
- H. Alwael, S. H. Al-Sedran, M. Oubaha, N. A. A. Asiri, A. S. Bashammakh and A. S. Alharthy, et al., Probe-integrated electrochemical sensing platform for measuring trace levels of parathion pesticides residues in water using Au- nanoparticles anchored Nafion nano composite modified glassy carbon electrode, J. Food Compos Anal., 2023, 124, 105649 CrossRef CAS.
- A. S. Rajpurohit, N. S. Punde and A. K. Srivastava, An electrochemical sensor with a copper oxide/gold nanoparticle-modified electrode for the simultaneous detection of the potential diabetic biomarkers methylglyoxal and its detoxification enzyme glyoxalase, New J. Chem., 2019, 43(42), 16572–16582 RSC.
- M. H. Ghanbari, P. Sharafi, S. Nayebossadr and Z. Norouzi, Utilizing a nanocomposite consisting of zinc ferrite, copper oxide, and gold nanoparticles in the fabrication of a metformin electrochemical sensor supported on a glassy carbon electrode, Microchim. Acta, 2020, 187(10), 557 CrossRef CAS PubMed.
- M. Ahamed, H. A. Alhadlaq, M. A. M. Khan, P. Karuppiah and N. A. Al-Dhabi, Synthesis, characterization, and antimicrobial activity of copper oxide nanoparticles, J. Nanomater., 2014, 2014, 401–409 Search PubMed.
- X. Jiao, Y. Qiu, L. Zhang and X. Zhang, Comparison of the characteristic properties of reduced graphene oxides synthesized from natural graphites with different graphitization degrees, RSC Adv., 2017, 7(82), 52337–52344 RSC.
- M. A. Rashed, M. Faisal, J. Ahmed, S. A. Alsareii, M. Jalalah and F. A. Harraz, Highly sensitive and selective amperometric hydrazine sensor based on Au nanoparticle-decorated conducting polythiophene prepared via oxidative polymerization and photo-reduction techniques, J. Saudi Chem. Soc., 2022, 26(3), 101480 CrossRef CAS.
- M. Faraji, Y. Yamini and N. Salehi, Characterization of magnetic nanomaterials, Magn. Nanomater. Anal. Chem., 2021, 39–60 CrossRef CAS.
- M. T. Chen, Z. X. Huang, X. Ye, L. Zhang, J. J. Feng and A. J. Wang, Caffeine derived graphene-wrapped Fe3C nanoparticles entrapped in hierarchically porous Fe[sbnd]N[sbnd]C nanosheets for boosting oxygen reduction reaction, J. Colloid Interface Sci., 2023, 637, 216–224 CrossRef CAS PubMed.
- C. R. Yang, S. F. Tseng and Y. T. Chen, Characteristics of graphene oxide films reduced by using an atmospheric plasma system, Nanomaterials, 2018, 8(10), 802 CrossRef.
- K. Dave, K. H. Park and M. Dhayal, Two-step process for programmable removal of oxygen functionalities of graphene oxide: functional, structural and electrical characteristics, RSC Adv., 2015, 5(116), 95657–95665 RSC.
- P. F. Li, Y. Xu and X. H. Cheng, Chemisorption of thermal reduced graphene oxide nano-layer film on TNTZ surface and its tribological behavior, Surf. Coat. Technol., 2013, 232, 331–339 CrossRef CAS.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes and Y. Jia, et al., Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide, Carbon N. Y, 2007, 45(7), 1558–1565 CrossRef CAS.
- W. K. Han, J. W. Choi, G. H. Hwang, S. J. Hong, J. S. Lee and S. G. Kang, Fabrication of Cu nano particles by direct electrochemical reduction from CuO nano particles, Appl. Surf. Sci., 2006, 252(8), 2832–2838 CrossRef CAS.
- Y. Wang, J. Liu, L. Liu and D. D. Sun, High-quality reduced graphene oxide-nanocrystalline platinum hybrid materials prepared by simultaneous co-reduction of graphene oxide and chloroplatinic acid, Nanoscale Res. Lett., 2011, 6(1), 241 CrossRef PubMed.
- R. Al-Gaashani, A. Najjar, Y. Zakaria, S. Mansour and M. A. Atieh, XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods, Ceram. Int., 2019, 45(11), 14439–14448 CrossRef CAS.
- P. Kulkarni, S. Mahamuni, M. Chandrachood, I. S. Mulla, A. P. B. Sinha and A. S. Nigavekar, et al., Photoelectron spectroscopic studies on a silicon interface with Bi 2Sr2CaCu2BO8+δ high T c superconductor, J. Appl. Phys., 1990, 67(7), 3438–3442 CrossRef CAS.
- J. Yuan, J. J. Zhang, M. P. Yang, W. J. Meng, H. Wang and J. X. Lu, Cuo nanoparticles supported on TiO2 with high efficiency for CO2 electrochemical reduction to ethanol, Catalysts, 2018, 8(4), 171 CrossRef CAS.
- K. Gupta, M. Bersani and J. A. Darr, Highly efficient electro-reduction of CO2 to formic acid by nano-copper, J. Mater. Chem. A, 2016, 4(36), 13786–13794 RSC.
- P. Gao, Y. Gong, N. P. Mellott and D. Liu, Non-enzymatic amperometric detection of hydrogen peroxide using grass-like copper oxide nanostructures calcined in nitrogen atmosphere, Electrochim. Acta, 2015, 173, 31–39 CrossRef CAS.
- M. Nemakal, S. Aralekallu, I. Mohammed, M. Pari, K. R. Venugopala Reddy and L. K. Sannegowda, Nanomolar detection of 4-aminophenol using amperometric sensor based on a novel phthalocyanine, Electrochim. Acta, 2019, 318, 342–353 CrossRef CAS.
- B. Abebe, D. Tsegaye, C. Sori, R. C. K. Renuka Prasad and H. C. A. Murthy, Cu/CuO-Doped ZnO nanocomposites via solution combustion synthesis for catalytic 4-nitrophenol reduction, ACS Omega, 2023, 8(10), 9597–9606 CrossRef CAS PubMed.
- D. Pan, S. Ma, X. Bo and L. Guo, Electrochemical behavior of methyl parathion and its sensitive determination at a glassy carbon electrode modified with ordered mesoporous carbon, Microchim. Acta, 2011, 173(1–2), 215–221 CrossRef CAS.
- G. Liu and Y. Lin, Electrochemical stripping analysis of organophosphate pesticides and nerve agents, Electrochem. Commun., 2005, 7(4), 339–343 CrossRef CAS.
- T. F. Kang, F. Wang, L. P. Lu, Y. Zhang and T. S. Liu, Methyl parathion sensors based on gold nanoparticles and Nafion film modified glassy carbon electrodes, Sensors Actuators, B, 2010, 145(1), 104–109 CrossRef CAS.
- T. Ma, W. Yang, S. Liu, H. Zhang and F. Liang, A comparison reduction of 4-nitrophenol by gold nanospheres and gold nanostars, Catalysts, 2017, 7(2), 104 CrossRef.
- D. Du, X. Ye, J. Zhang and D. liu, Cathodic electrochemical analysis of methyl parathion at bismuth-film-modified glassy carbon electrode, Electrochim. Acta, 2008, 53(13), 4478–4484 CrossRef CAS.
- A. R. Ansari, S. A. Ansari, N. Parveen, M. O. Ansari and Z. Osman, Silver nanoparticles embedded on reduced graphene oxide@copper oxide nanocomposite for high performance supercapacitor applications, Materials, 2021, 14(17), 5032 CrossRef CAS.
- W. Xu, S. Dai, G. Liu, Y. Xi, C. Hu and X. Wang, CuO Nanoflowers growing on carbon fiber fabric for flexible high-performance supercapacitors, Electrochim. Acta, 2016, 203, 1–8 CrossRef CAS.
- C. Ruan, L. Yang and Y. Li, Immunobiosensor chips for detection of Escherichia coli O157:H7 using electrochemical impedance spectroscopy, Anal. Chem., 2002, 74(18), 4814–4820 CrossRef CAS.
- C. Erdem, D. K. Zeybek, G. Aydoǧdu, B. Zeybek, Ş. Pekyardimci and E. Kiliç, Electrochemical glucose biosensor based on nickel oxide nanoparticle-modified carbon paste electrode, Artif. Cells, Nanomed., Biotechnol., 2014, 42(4), 237–244 CrossRef CAS PubMed.
- J. Hao, L. Ji, K. Wu and N. Yang, Electrochemistry of ZnO@reduced graphene oxides, Carbon N. Y., 2018, 130, 480–486 CrossRef CAS.
- J. R. Choudhuri, Metal oxide–carbon nanocomposites for electrochemical storage, Energy, Environ. Sustainability, 2022, 49–67 Search PubMed.
- D. Majumdar and S. Ghosh, Recent advancements of copper oxide based nanomaterials for supercapacitor applications, J. Energy Storage, 2021, 34, 101995 CrossRef.
- M. A. Rashed, M. Faisal, F. A. Harraz, M. Jalalah, M. Alsaiari and M. S. Al-Assiri, rGO/ZnO/Nafion nanocomposite as highly sensitive and selective amperometric sensor for detecting nitrite ions (NO2−), J. Taiwan Inst. Chem. Eng., 2020, 112, 345–356 CrossRef CAS.
- L. Luo, L. Zhu and Z. Wang, Nonenzymatic amperometric determination of glucose by CuO nanocubes-graphene nanocomposite modified electrode, Bioelectrochemistry, 2012, 88, 156–163 CrossRef CAS PubMed.
- C. K. Chua and M. Pumera, Influence of methyl substituent position on redox properties of nitroaromatics related to 2,4,6-trinitrotoluene, Electroanalysis, 2011, 23(10), 2350–2356 CrossRef CAS.
- N. Kazemifard, A. A. Ensafi and M. P. Shirani, Fabrication of a highly sensitive and selective electrochemical imidacloprid sensor using a glassy carbon electrode modified with MWCNTs/SBA-15@Si-CDs nanocomposite, IEEE Sens. J., 2021, 21(8), 9763–9770 CAS.
- H. Zhao, H. Ma, X. Li, B. Liu, R. Liu and S. Komarneni, Nanocomposite of halloysite nanotubes/multi-walled carbon nanotubes for methyl parathion electrochemical sensor application, Appl. Clay Sci., 2021, 200, 105907 CrossRef CAS.
- R. Liu, Y. Wang, B. Li, B. Liu, H. Ma and D. Li, et al., VXC-72R/ZrO2/GCE-based electrochemical sensor for the high-sensitivity detection of methyl parathion, Materials, 2019, 12(21), 3637 CrossRef CAS PubMed.
- X. Yue, P. Han, W. Zhu, J. Wang and L. Zhang, Facile and sensitive electrochemical detection of methyl parathion based on a sensing platform constructed by the direct growth of carbon nanotubes on carbon paper, RSC Adv., 2016, 6(63), 58771–58779 RSC.
- X. Hou, X. Liu, Z. Li, J. Zhang, G. Du and X. Ran, et al., Electrochemical determination of methyl parathion based on pillar[5]arene@AuNPs@reduced graphene oxide hybrid nanomaterials, New J. Chem., 2019, 43(33), 13048–13057 RSC.
- Z. Pourghobadi and R. Pourghobadi, Electrocatalytic alfuzosin oxidation on electrochemically oxidized glassy carbon modified with multiwalled carbon nanotubes and nickel oxide nanoparticles, J. Electrochem. Soc., 2019, 166(2), B76–B83 CrossRef CAS.
- P. S. Ganesh and B. E. K. Swamy, Simultaneous electroanalysis of norepinephrine, ascorbic acid and uric acid using poly(glutamic acid) modified carbon paste electrode, J. Electroanal. Chem., 2015, 752, 17–24 CrossRef CAS.
- N. Jaiswal, I. Tiwari, C. W. Foster and C. E. Banks, Highly sensitive amperometric sensing of nitrite utilizing bulk-modified MnO2 decorated graphene oxide nanocomposite screen-printed electrodes, Electrochim. Acta, 2017, 227, 255–266 CrossRef CAS.
- C. Song and J. Zhang. Electrocatalytic oxygen reduction reaction, in PEM Fuel Cell Electrocatalysts and Catalyst Layers, 2008, pp. 89–134 Search PubMed.
- A. Chen and B. Shah, Electrochemical sensing and biosensing based on square wave voltammetry, Anal. Methods, 2013, 5(9), 2158–2173 RSC.
- J. Gong, X. Miao and T. Zhou, An enzymeless organophosphate pesticide sensor using Au nanoparticle-decorated graphene hybrid nanosheet as solid-phase extraction, Talanta, 2011, 175(3), 1344–1349 CrossRef.
- R. Kaur, S. Rana and K. Lalit, Electrochemical detection of methyl parathion via a novel biosensor tailored on highly biocompatible electrochemically reduced graphene oxide-chitosan-hemoglobin coatings, Biosens. Bioelectron., 2020, 167, 112486 CrossRef CAS.
- J. Gong, L. Wang and X. Miao, Efficient stripping voltammetric detection of organophosphate pesticides using NanoPt intercalated Ni/Al layered double hydroxides as solid-phase extraction, Electrochem. Commun., 2010, 1658–1661 CrossRef CAS.
- G. Liu and Y. Lin, Electrochemical sensor for organophosphate pesticides and nerve agents using zirconia nanoparticles as selective sorbents., Anal. Chem., 2005, 77(18), 5894–5901 CrossRef CAS PubMed.
- B. Huang, W. D. Zhang and C. H. Chen, Electrochemical determination of methyl parathion at a Pd/MWCNTs-modified electrode., Microchim. Acta., 2010, 171(1), 57–62 CrossRef CAS.
- Y. Qu, H. Min and Y. Wei, Au-TiO2/Chit modified sensor for electrochemical detection of trace organophosphates insecticides, Talanta, 2008, 76(4), 758–762 CrossRef CAS PubMed.
- J. C. Ma and W. D. Zhang, Gold nanoparticle-coated multiwall carbon nanotube-modified electrode for electrochemical determination of methyl parathion, Microchim. Acta, 2011, 175, 309–314 CrossRef CAS.
- Y. Zhang, T. F. Kang and Y. W. Wan, Gold nanoparticles-carbon nanotubes modified sensor for electrochemical determination of organophosphate pesticides, Microchim Acta., 2009, 165, 307–311 CrossRef CAS.
- A. Kumaravel and M. Chandrasekaran, Electrochemical determination of imidacloprid using nanosilver Nafion®/nanoTiO2 Nafion® composite modified glassy carbon electrode, Sensors Actuators, B, 2011, 158(1), 319–326 CrossRef CAS.
- S. Ivanov, U. Lange, V. Tsakova and V. M. Mirsky, Electrocatalytically active nanocomposite from palladium nanoparticles and polyaniline: oxidation of hydrazine, Sensors Actuators, B, 2010, 150(1), 271–278 CrossRef CAS.
- P. Manisankar, C. Vedhi and G. Selvanathan, Electrochemical determination of methyl parathion using a modified electrode, Toxicol. Environ. Chem., 2003, 85(4–6), 233–241 CrossRef CAS.
- Y. Zhao, L. Yu, C. Song, Z. Chen, F. Meng and M. Song, Selective degradation of electron-rich organic pollutants induced by CuO@Biochar: the key role of outer-sphere interaction and singlet oxygen, Environ. Sci. Technol., 2022, 56(15), 10710–10720 CrossRef CAS.
- H. W. Chang, C. L. Chen, Y. H. Chen, Y. M. Chang, F. J. Liu and Y. C. Tsai, Electrochemical organophosphorus pesticide detection using nanostructured gold-modified electrodes, Sensors, 2022, 22(24), 9938 CrossRef CAS PubMed.
- Y. Mao, H. Fa, Y. Cheng, Y. Du, W. Yin and C. Hou, et al., An electrode modified with AuNPs/graphene nanocomposites film for the determination of methyl parathion residues, Nano, 2014, 9(8), 1450096 CrossRef.
- X. Tan, Y. Liu, T. Zhang, S. Luo, X. Liu and H. Tian, et al., Ultrasensitive electrochemical detection of methyl parathion pesticide based on cationic water-soluble pillar[5]arene and reduced graphene nanocomposite, RSC Adv., 2019, 9(1), 345–353 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00765h |
| This journal is © The Royal Society of Chemistry 2025 |





