Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Binuclear Ni catalyzed ethylene copolymerization with short chain alkenol monomers†
Yuan
Lu
,
Gang
Ji
,
Shuyang
Yu
,
Xiaoshan
Ning
,
Xiu-Li
Sun
 *,
Yanshan
Gao
*,
Yanshan
Gao
 *,
Xiaoyan
Wang
*,
Xiaoyan
Wang
 and
Yong
Tang
*
and
Yong
Tang
*
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China. E-mail: xlsun@sioc.ac.cn.cn; gaoyanshan@sioc.ac.cn; tangy@sioc.ac.cn
First published on 31st January 2025
Abstract
Ethylene coordination copolymerization with vinyl polar monomers, particularly short chain alkenols, offers an attractive method for controlled synthesis of important hydroxy-functionalized polyethylenes under mild conditions. However, reports on short-chain alkenol copolymerization are limited due to issues like chelating coordination and β-O elimination. Here, we report the synthesis and characterization of binuclear Ni complexes for ethylene copolymerization with various alkenol monomers such as allyl-OH, 3-buten-1-ol, 4-penten-1-ol and 9-decen-1-ol. These complexes, upon activation with Et2AlCl, achieved notable activity (as high as 592 kg (mol cat h atm)−1) in ethylene/3-buten-1-ol copolymerization, producing copolymers with 1.7 mol% comonomer incorporation and a high molecular weight (Mn = 64.2 kg mol−1). The activity and comonomer content were influenced by Et2AlCl loading, reaction temperature, and alkenol monomer length, with longer alkenols such as 9-decen-1-ol yielding higher activity, comonomer incorporation and molecular weight. Activities up to 169 kg (mol cat h atm)−1 were also achieved in ethylene/allyl-OH copolymerization with reduced molecular weight (Mn = 17.2 kg mol−1). Microstructural analysis revealed predominant in-chain and chain-end polar monomer incorporation in all cases. Notably, ethylene/allyl-OH copolymers exhibited unique olefinic end groups and microstructures assignable to Friedel–Crafts reactions, which is likely due to an alternative chain termination pathway associated with the short chain length between the O atom and the active Ni center. For comparison, ethylene/allyl-OAc copolymers showed exclusively olefinic groups, indicating a β-OAc elimination mechanism. This process resulted in lower activity and molecular weight, suggesting catalyst poisoning from rapid chain termination.
Introduction
Hydroxy-functionalized polyolefins, which bear hydroxyalkyl branches, often exhibit superior properties and performances to traditional non-polar polyolefins, such as adhesion and barrier properties.1–4 They can be used as macroinitiators for the synthesis of polar–nonpolar graft block copolymers5–8 and have recently been used for the synthesis of high performance reprocessable polyolefin vitrimers.9–14. Thus, hydroxy-functionalized polyolefins have been a unique target in catalytic and synthetic polyolefin research.15–21 Ethylene coordination copolymerization with polar monomers, especially α,ω-alkenol monomers, provides an important pathway for the synthesis of functional polyolefins with well-defined structures under mild conditions.3,22 Compared with long chain alkenol monomers, which are usually copolymerized with good activity and comonomer incorporation (early23–31 and late32–37 transition metal catalysis), short chain alkenols are abundant, cheap, industrially more relevant, and hence more attractive monomer candidates.Despite the advantages of synthesizing hydroxy-functionalized polyolefins via coordination copolymerization, short chain alkenols are still challenging polar comonomers. Our laboratory reported27,28 [ONX]Ti-catalyzed (ONX is a monoanionic tridentate ligand; X = S and P) ethylene copolymerization with 4-penten-1-ol and achieved an activity of 2800 kg (mol h atm)−1 when the comonomer incorporation was 1.4 mol%. Duchateau et al. reported29 CGCTiCl2-catalyzed (CGC, constrained geometry catalyst) ethylene copolymerization with 3-buten-1-ol and allyl-OH and achieved activities of 792 kg (mol h atm)−1 and 860 kg (mol h atm)−1, when the comonomer incorporations were approximately 1.7 mol% and 3.1 mol%, respectively. Mu et al. reported38 amine bis(phenolate) Zr catalyzed ethylene/3-buten-1-ol copolymerization and achieved an activity of 26 kg (mol h atm)−1 when the comonomer incorporation was 1.1 mol%. Cui et al. reported39 (Flu-CH2CH2-NHC-C6H4Me)Sc(CH2SiMe3)2 catalyzed ethylene/3-buten-1-ol copolymerization and achieved an activity of 1168 kg (mol h atm)−1 when the comonomer incorporation was 0.7 mol%. Imuta et al. reported40 ethylene/allyl-OH copolymerization with an ansa-zirconocene catalyst and achieved an activity of 91.2 kg (mol h atm)−1 when the comonomer incorporation was 1.2 mol%. Besides early transition metal catalysis, late transition metal catalysis was also reported in ethylene copolymerization with short chain alkenol comonomers in the absence of trialkylaluminum. Nozaki et al.41 and Chen et al.42 reported ethylene/allyl-OH copolymerization with a Pd/phosphine-sulfonate catalyst and a Pd/α-diimine catalyst and achieved activities of 1 and 15.6 g (mol h atm)−1 when the comonomer incorporations were 5.7 mol% and 6.9 mol%, respectively. Overall, reports on ethylene copolymerization with short chain alkenol monomers are still very limited. Our group recently reported a series of tetranuclear Ni catalysts for ethylene copolymerizations with short chain protic monomers (vinyl acetic acid, acrylic acid, allyl-OH, 3-buten-1-ol, etc.), exhibiting great activity and comonomer incorporation.43 Here, we further designed and synthesized a series of binuclear Ni complexes with varied substitutions and probed their ethylene copolymerization with various alkenol monomers in terms of activity, molecular weight, branch density, and comonomer incorporation, and probed the chain termination processes that may be associated with catalyst poisoning/low activity, especially in copolymerization with allyl-OH.
Results and discussion
Synthesis and characterization of the ligands and Ni complexes
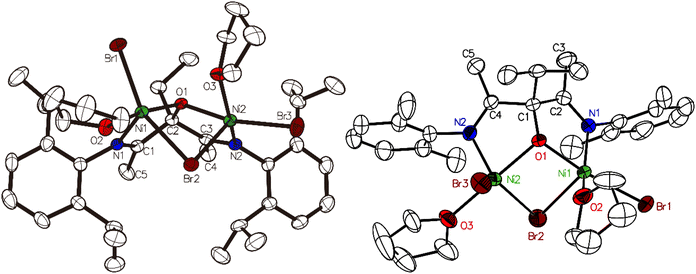
The synthesis of complex 4a was recently reported by our group.43 Ni complexes 4b–4d were synthesized following the same procedure (Scheme 1), i.e. ligand deprotonation with 2.0 equiv. of KH, followed by the reaction with NiBr2(DME),44 all giving good yields, 73%, 65% and 46% for 4b, 4c and 4d, respectively. Single crystals of 4c and 4d suitable for X-ray diffraction studies were grown from THF/n-hexane solution with the solid-state structure shown in Fig. 1. The two Ni atoms in the bimetallic Ni complex adopt a distorted trigonal bipyramidal configuration. The central O1 and Br2 atoms are equally shared by both nickel atoms. The Ni1⋯Ni2 distance for 4c is 3.101 Å, much shorter than that in complex 4a (3.259 Å), reflecting the dramatic effects of the R2 substituent on the steric environment surrounding both Ni atoms.Ethylene/3-buten-1-ol copolymerization
The four precatalysts are differentiated by the substituents R1 and R2. We first used 4a as the primary precatalyst and Et2AlCl (DEAC) as the cocatalyst for ethylene/3-buten-1-ol copolymerization studies (Table 1). The activity reached 474 kg (mol cat h atm)−1 when 0.2 M of alkenol monomer was used for ethylene copolymerization at 1 atm and 30 °C, and 1.5 equiv. DEAC was used for pretreating the comonomer immediately before the reaction. The resulting copolymer shows a high molecular weight (Mn = 53.9 kg mol−1) and 1.3 mol% alkenol comonomer content (entry 4). Here, we studied the effects of cocatalyst loading, comonomer amount, reaction temperature and time on the copolymerization. Note that hydroxy-functionalized PEs were acetylated using glacial acetic acid in the presence of H2SO4 at 120 °C before running GPC measurement (see the ESI for more details†).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Comon. (M) | DEAC/comon. ratio | Temp. (°C) | t (min) | Yield (g) | Act.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (kg mol−1) c (kg mol−1) |
Đ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
Comon. content![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (mol%) d (mol%) |
FG/chain | Branch![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (CH3/1000C) d (CH3/1000C) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Conditions: cat. 4a (5 μmol), toluene (total volume 50 mL), water bath, and ethylene (1 atm). All experiments were performed in duplicate. b kg (mol cat h atm)−1. c Determined by high temperature GPC. d Calculated from 1H NMR. | |||||||||||
| 1 | 0.2 | 0.8 | 30 | 10 | None | — | — | — | — | — | — |
| 2 | 0.2 | 1.0 | 30 | 10 | 0.405 | 486 | 46.3 | 2.4 | 1.4 | 23 | 75 |
| 3 | 0.2 | 1.25 | 30 | 10 | 0.393 | 472 | 46.7 | 2.4 | 1.4 | 23 | 81 |
| 4 | 0.2 | 1.5 | 30 | 10 | 0.395 | 474 | 53.9 | 2.2 | 1.3 | 25 | 75 |
| 5 | 0.2 | 2.0 | 30 | 10 | 0.306 | 367 | 38.3 | 2.4 | 1.4 | 19 | 82 |
| 6 | 0.2 | 2.5 | 30 | 10 | 0.314 | 377 | 35.5 | 3.1 | 1.2 | 15 | 82 |
| 7 | 0.1 | 1.5 | 30 | 10 | 0.700 | 840 | 79.0 | 2.3 | 0.4 | 11 | 79 |
| 8 | 0.3 | 1.5 | 30 | 10 | 0.220 | 264 | 27.0 | 2.3 | 2.9 | 28 | 74 |
| 9 | 0.4 | 1.5 | 30 | 10 | 0.143 | 172 | 16.7 | 2.2 | 4.7 | 28 | 70 |
| 10 | 0.2 | 1.5 | 20 | 10 | 0.523 | 628 | 68.1 | 1.9 | 1.1 | 27 | 70 |
| 11 | 0.2 | 1.5 | 40 | 10 | 0.256 | 307 | 27.2 | 2.9 | 1.9 | 18 | 80 |
| 12 | 0.2 | 1.5 | 50 | 10 | 0.146 | 175 | 15.9 | 2.9 | 2.7 | 15 | 80 |
| 13 | 0.2 | 1.5 | 30 | 5 | 0.259 | 622 | 34.0 | 1.9 | 1.6 | 19 | 78 |
| 14 | 0.2 | 1.5 | 30 | 15 | 0.434 | 347 | 52.7 | 3.2 | 1.3 | 24 | 81 |
| 15 | 0.2 | 1.5 | 30 | 20 | 0.489 | 293 | 56.3 | 3.1 | 1.3 | 26 | 78 |
| 16 | 0.2 | 1.5 | 30 | 30 | 0.510 | 204 | 56.3 | 3.3 | 1.2 | 24 | 74 |
On reducing the DEAC loading to 1 equiv./comonomer, the catalysis was not influenced in terms of activity, comonomer content, or product molecular weight (entry 2), but further reduction to 0.8 equiv. led to inactive catalysis (entry 1), suggesting the important stoichiometry requirement in pretreating the protic alkenol right before the polymerization. Further increasing the DEAC loading seems to have minor effects on catalysis, and thus we adopted 1.5 equiv. DEAC/comonomer for the subsequent studies. Upon increasing the alkenol amount from 0.1 to 0.2, 0.3 and then 0.4 M, the comonomer content increased from 0.4 mol% to 1.3 mol%, 2.9 mol% and then 4.7 mol% at the expense of activity, which dropped from 840 to 474, 264 and then 172 kg (mol cat h atm)−1, respectively (Fig. 2A). When the reaction temperature was gradually increased from 20 °C to 50 °C (entries 10, 4, 11 and 12), the activity was gradually reduced from 628 to 175 kg (mol cat h atm)−1, but the comonomer content increased from 1.1 to 2.7 mol% (Fig. 2B). This may be due to the reduced ethylene solubility at higher temperatures and limited thermal stability of the active species. When the reaction time gradually increased from 5 to 30 min (entries 13, 4 and 14–16), the activity was also reduced from 622 to 204 kg (mol cat h atm)−1 and the comonomer content was slightly reduced from 1.6 to 1.2 mol%. Interestingly, the molecular weight of the resulting copolymer increased from 34.0 to 56.3 kg mol−1 with a slightly broadened Đ, suggesting reduced catalytic efficiency over reaction time (Fig. 2C and D).
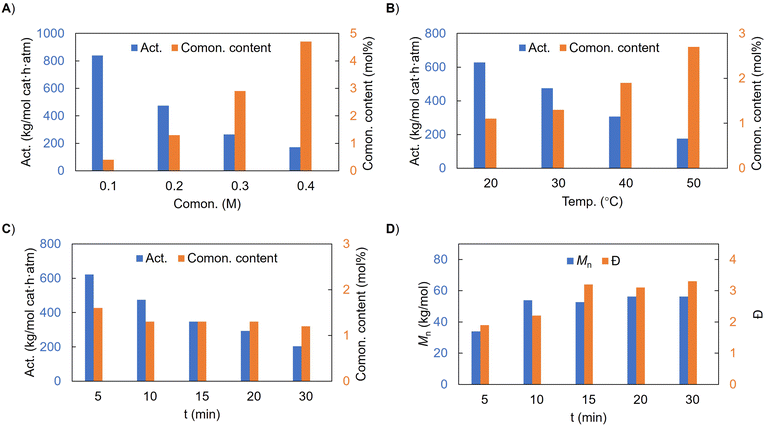 | ||
| Fig. 2 Effects of comonomer amount (A), reaction temperature (B) and time (C and D) on 4a catalyzed ethylene/3-buten-1-ol copolymerization. | ||
Effects of the alkenol comonomer length and catalyst structure
Next, we explored using all the catalysts 4a–4d for ethylene copolymerization with various alkenol monomers such as allyl-OH, 3-buten-1-ol, 4-penten-1-ol and 9-decen-1-ol (Table 2). We first analyzed the effects of the polar monomer linker length, and then the catalyst on the copolymerization activity, comonomer content, and molecular weight as well as the introduced FG other than –OH.| Entry | Cat. (μmol) | Comon. | Yield (g) | Act.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (kg mol−1) c (kg mol−1) |
Đ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
Comon. content![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (mol%) d (mol%) |
Branch![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (CH3/1000C) d (CH3/1000C) |
|---|---|---|---|---|---|---|---|---|
| a Conditions: alkenol monomer (0.2 M), DEAC (0.3 M), toluene (total volume 50 mL), water bath (30 °C), ethylene (1 atm), and 10 min. All experiments were performed in duplicate. b kg (mol cat h atm)−1. c Determined by high temperature GPC. d Calculated from 1H NMR. e Olefinic end group was observed in the polymer samples; please see the ESI† for detailed analysis. f Allyl-OH, 0.4 M; DEAC, 0.6 M. | ||||||||
| 1 | 4a (10) | Allyl-OH | 0.136 | 82 | 7.1 | 2.9 | 0.4e | 82 |
| 2 | 4b (10) | Allyl-OH | 0.062 | 37 | 3.8 | 1.9 | 0.6e | 89 |
| 3 | 4c (10) | Allyl-OH | 0.281 | 169 | 17.2 | 2.3 | 0.4e | 84 |
| 4 | 4d (10) | Allyl-OH | 0.204 | 122 | 13.9 | 1.9 | 0.4e | 96 |
| 5f | 4a (10) | Allyl-OH | 0.028 | 17 | 1.7 | 1.9 | 1.6e | 85 |
| 6 | 4a (5) | 3-Buten-1-ol | 0.395 | 474 | 53.9 | 2.2 | 1.3 | 75 |
| 7 | 4b (5) | 3-Buten-1-ol | 0.086 | 103 | 14.1 | 2.5 | 2.0 | 75 |
| 8 | 4c (5) | 3-Buten-1-ol | 0.493 | 592 | 64.2 | 1.6 | 1.7 | 76 |
| 9 | 4d (5) | 3-Buten-1-ol | 0.267 | 320 | 42.9 | 1.7 | 1.7 | 87 |
| 10 | 4a (5) | 4-Penten-1-ol | 0.414 | 497 | 57.0 | 2.3 | 1.1 | 76 |
| 11 | 4b (5) | 4-Penten-1-ol | 0.117 | 140 | 20.1 | 5.0 | 1.8 | 71 |
| 12 | 4c (5) | 4-Penten-1-ol | 0.481 | 577 | 65.2 | 1.5 | 1.7 | 75 |
| 13 | 4d (5) | 4-Penten-1-ol | 0.269 | 323 | 47.1 | 1.6 | 1.7 | 88 |
| 14 | 4a (5) | 9-Decen-1-ol | 0.489 | 587 | 71.0 | 2.7 | 1.3 | 74 |
| 15 | 4b (5) | 9-Decen-1-ol | 0.154 | 185 | 25.7 | 4.3 | 1.5 | 69 |
| 16 | 4c (5) | 9-Decen-1-ol | 0.590 | 708 | 79.5 | 1.4 | 2.9 | 67 |
| 17 | 4d (5) | 9-Decen-1-ol | 0.337 | 404 | 49.7 | 1.4 | 1.7 | 87 |
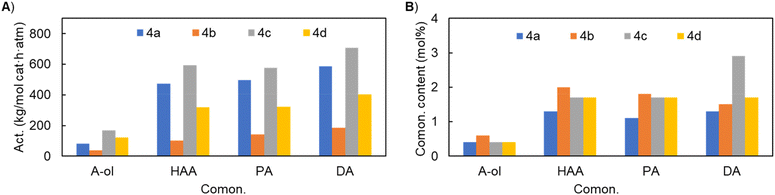
The length of the alkenol monomer exhibited significant effects on catalysis, and overall similar trends were observed in terms of activity, comonomer content and product molecular weight, regardless of the Ni catalyst used. Here, we used catalyst 4a as an example. Upon switching the monomer from allyl-OH to 3-buten-1-ol, 4-penten-1-ol and then 9-decen-1-ol, the activity increased from 82 to 474, 497 and then 587 kg (mol cat h atm)−1 (Fig. 3A), and the molecular weight (Mn) of the resulting copolymer also increased from 7.1 to 53.9, 57.0 and then 71.0 kg mol−1 (entries 1, 6, 10 and 14). Besides the remarkable effects of the allyl-OH monomer on activity and Mn, it also exhibited unique effects on the comonomer content and introduced FG (Fig. 3B). The comonomer content is much lower (0.4 mol% for allyl-OH) than those of other longer alkenol monomers (1.0–1.3 mol%); copolymer 1H NMR suggested that some of the –OH groups were converted to olefinic groups in the case of allyl-OH, likely resulting from β-OAl elimination.45–48 In contrast, copolymerization with other longer alkenol monomers leads to exclusively –OH groups.
The catalysts bear different R1 and R2 substituents, which exhibited varied effects on copolymerization. For all the alkenol monomers, the activity decreases in the order of 4c > 4a, 4d > 4b, i.e. the bulkier the R1 and R2 groups of the catalyst, the higher the activity. Overall, the four catalysts’ comonomer enchainment capabilities are similar. It is worth noting that the resulting copolymers (entries 11 and 15, Table 2) derived from catalyst 4b showed non-uniform comonomer composition through GPC traces and DSC thermograms (Fig. S80 and S84†), likely reflecting the formation of non-uniform catalytically active centers.
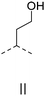
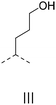
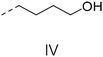
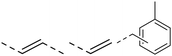
We also inspected the microstructure of ethylene copolymers with various alkenol comonomers. Representative samples derived from catalysis with catalyst 4c were chosen for a qualitative comparison (Table 3). Besides the hydroxyalkyl branch generated from regular alkenol insertion without chain walking (in-chain incorporation), i.e. (CH2)nOH from CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)nOH (n = 1, 2, and 3) enchainment, a chain-end hydroxyl group, –(CH2)nOH (n > 3), was observed in all the copolymer products. Structures I, II, III and IV were assigned by combined 1H, 13C and 2D NMR characterization, and according to the literature.40–43
CH(CH2)nOH (n = 1, 2, and 3) enchainment, a chain-end hydroxyl group, –(CH2)nOH (n > 3), was observed in all the copolymer products. Structures I, II, III and IV were assigned by combined 1H, 13C and 2D NMR characterization, and according to the literature.40–43
| Entry | Monomer | |||||
|---|---|---|---|---|---|---|
| a The samples are from entries 3, 8, 12 and 16 in Table 2 for the ethylene copolymers with allyl-OH, 3-buten-1-ol, 4-penten-1-ol and 9-decen-1-ol, respectively. The microstructures were identified by polymer 1H NMR and 13C NMR. Please see detailed assignments in the ESI.† | ||||||
| 1 |

|
✓ | n.d. | n.d. | ✓ | ✓ |
| 2 |
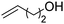
|
n.d. | ✓ | n.d. | ✓ | n.d. |
| 3 |
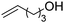
|
n.d. | n.d. | ✓ | ✓ | n.d. |
| 4 |
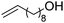
|
n.d. | n.d. | n.d. | ✓ | n.d. |
The in-chain incorporation was observed in the ethylene copolymerizations with all the types of alkenol comonomers, likely occurring via path a and path b during the catalysis (Fig. 4). The chain-end incorporation was observed in all the cases as well, regardless of the alkenol comonomer, suggesting a strong tendency to undergo path c in all the copolymerizations, i.e. 2,1-insertion of the polar comonomer followed by chain walking before the next monomer insertion. In contrast to other alkenols, ethylene copolymers with allyl-OH showed additional functional groups such as olefinic end groups41,49,50 and even microstructures assignable to the Friedel–Crafts reaction of alkenes with arenes51–54 (path d). It is likely associated with the short chain length between the olefin double bond and the –OH group, which leads to chelating coordination and other chain termination pathways. Note that the GPC traces of several polymer samples, especially for allyl-OH copolymers, exhibited broadened or even multiple peaks, likely reflecting the formation of non-uniform catalytically active centers and/or complicated chain transfer/termination processes.
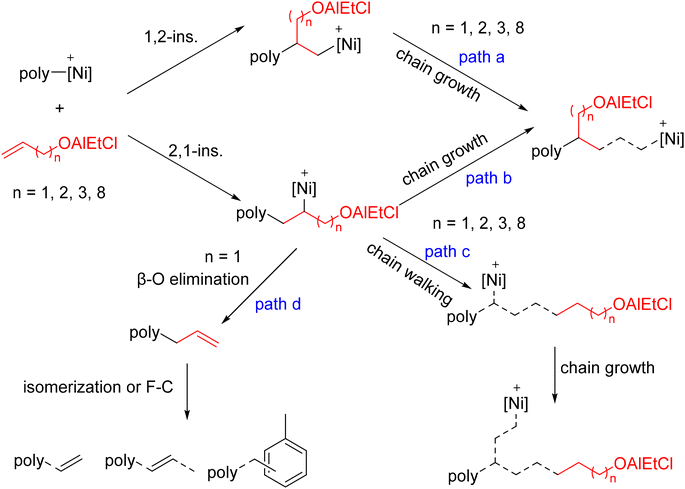 | ||
| Fig. 4 Possible polar monomer enchainment pathways (insertion, chain walking, and chain transfer). The active species is shown as [Ni]+ for clarity purposes. | ||
Ethylene/allyl-OAc copolymerization
On the other hand, we were curious about the chemistry associated with the functional group during the copolymerization as it is related to catalyst poisoning by back-biting and β-X elimination of the introduced functional group. Thus, we studied allyl-OAc in the copolymerization with DEAC as the masking reagent and cocatalyst. Copolymerization afforded polymers with exclusively olefinic groups and no –OAc group was detected by 1H NMR (Table 4). An olefin double bond and aromatic protons were detected instead. Due to the complexity of the reaction product and difficulty in analysis of the trace peaks, their quantitative analysis was not pursued. The polymer Mn ranged from 4.0 kg mol−1 to 18.2 kg mol−1, which is lower than that of the corresponding ethylene/alkenol copolymers (Table 2), reflecting a disadvantaged chain propagation vs. chain transfer. Thus, β-OAc elimination is invoked whenever an allyl acetate monomer is enchained,55 which affords olefin-ended PE. The lower activity in Ni catalyzed ethylene copolymerization with either allyl-OH or allyl-OAc vs. ethylene copolymerization with longer chain alkenols is likely associated with the facile chain transfer/termination and poisoning of the Ni species.| Entry | Cat. (μmol) | Allyl-OAc (M) | Temp. (°C) | Yield (g) | Act.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (kg mol−1) c (kg mol−1) |
Đ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
|---|---|---|---|---|---|---|---|
| a Conditions: toluene (total volume 50 mL), DEAC/comon. = 1.5, water bath, ethylene (1 atm), and 10 min. All experiments were performed in duplicate. b kg (mol cat h atm)−1. c Determined by high temperature GPC. | |||||||
| 1 | 4a (5) | 0.2 | 30 | 0.150 | 180 | 11.9 | 2.1 |
| 2 | 4b (5) | 0.2 | 30 | 0.098 | 118 | 6.1 | 2.1 |
| 3 | 4c (5) | 0.2 | 30 | 0.170 | 204 | 18.2 | 2.1 |
| 4 | 4d (5) | 0.2 | 30 | 0.124 | 149 | 10.0 | 1.8 |
| 5 | 4a (10) | 0.4 | 20 | 0.178 | 107 | 7.8 | 1.9 |
| 6 | 4a (10) | 0.4 | 30 | 0.136 | 82 | 5.8 | 2.0 |
| 7 | 4a (10) | 0.4 | 40 | 0.071 | 43 | 4.0 | 2.0 |
Conclusion
Binuclear Ni complexes paired with DEAC offer a great catalyst system for ethylene coordination copolymerization with short-chain alkenols and controlled synthesis of hydroxy-functionalized polyethylenes. Despite challenges like chelating coordination and β-O elimination, these complexes exhibit notable activity, producing copolymers with high molecular weights. Microstructural analysis reveals insights into copolymerization mechanisms, highlighting the influence of the catalyst structure and reaction conditions on activity, comonomer incorporation and polymer molecular weight. Understanding these factors is crucial for optimizing activity and mitigating catalyst poisoning, thereby advancing the development of functional polyolefins. Further investigations focusing on the elucidation and modulation of the polar monomer enchainment process and novel catalyst design are underway.Data availability
Data are available upon request from the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (U23A2084), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0610000), and the Science and Technology Commission of Shanghai Municipality (23JC1404500).References
- T. C. M. Chung, Functional Polyolefins for Energy Applications, Macromolecules, 2013, 46, 6671–6698 CrossRef CAS.
- T. Rünzi and S. Mecking, Saturated Polar-Substituted Polyethylene Elastomers from Insertion Polymerization, Adv. Funct. Mater., 2014, 24, 387–395 CrossRef.
- J. Chen, Y. Gao and T. J. Marks, Early Transition Metal Catalysis for Olefin–Polar Monomer Copolymerization, Angew. Chem., Int. Ed., 2020, 59, 14726–14735 CrossRef CAS PubMed.
- C. Zou and C. Chen, Polar-Functionalized, Crosslinkable, Self-Healing, and Photoresponsive Polyolefins, Angew. Chem., Int. Ed., 2020, 59, 395–402 CrossRef CAS PubMed.
- P. D. Goring, C. Morton and P. Scott, End-Functional Polyolefins for Block Copolymer Synthesis, Dalton Trans., 2019, 48, 3521–3530 RSC.
- A. Keyes, H. E. B. Alhan, E. Ordonez, U. Ha, D. B. Beezer, H. Dau, Y.-S. Liu, E. Tsogtgerel, G. R. Jones and E. Harth, Olefins and Vinyl Polar Monomers: Bridging the Gap for Next Generation Materials, Angew. Chem., Int. Ed., 2019, 58, 12370–12391 CrossRef CAS PubMed.
- D. J. Walsh, M. G. Hyatt, S. A. Miller and D. Guironnet, Recent Trends in Catalytic Polymerizations, ACS Catal., 2019, 9, 11153–11188 CrossRef CAS.
- T. Yan and D. Guironnet, Polyethylene Containing Triblock Copolymers Synthesized by Post-polymerization Functionalization, Macromolecules, 2020, 53, 4338–4344 CrossRef CAS.
- C. A. Tretbar, J. A. Neal and Z. Guan, Direct Silyl Ether Metathesis for Vitrimers with Exceptional Thermal Stability, J. Am. Chem. Soc., 2019, 141, 16595–16599 CrossRef CAS PubMed.
- G. Zhang, X. Zhou, K. Liang, B. Guo, X. Li, Z. Wang and L. Zhang, Mechanically Robust and Recyclable EPDM Rubber Composites by a Green Cross-Linking Strategy, ACS Sustainable Chem. Eng., 2019, 7, 11712–11720 CrossRef CAS.
- J. Tellers, R. Pinalli, M. Soliman, J. Vachon and E. Dalcanale, Reprocessable Vinylogous Urethane Cross-Linked Polyethylene via Reactive Extrusion, Polym. Chem., 2019, 10, 5534–5542 RSC.
- A. Zych, R. Pinalli, M. Soliman, J. Vachon and E. Dalcanale, Polyethylene Vitrimers via Silyl Ether Exchange Reaction, Polymer, 2020, 199, 122567 CrossRef CAS.
- M. Ahmadi, A. Hanifpour, S. Ghiassinejad and E. van Ruymbeke, Polyolefins Vitrimers: Design Principles and Applications, Chem. Mater., 2022, 34, 10249–10271 CrossRef CAS.
- X.-Y. Wang, Y. Gao and Y. Tang, Sustainable Developments in Polyolefin Chemistry: Progress, Challenges, and Outlook, Prog. Polym. Sci., 2023, 143, 101713 CrossRef CAS.
- Y. Kondo, D. García-Cuadrado, J. F. Hartwig, N. K. Boaen, N. L. Wagner and M. A. Hillmyer, Rhodium-Catalyzed, Regiospecific Functionalization of Polyolefins in the Melt, J. Am. Chem. Soc., 2002, 124, 1164–1165 CrossRef CAS PubMed.
- N. K. Boaen and M. A. Hillmyer, Selective and Mild Oxyfunctionalization of Model Polyolefins, Macromolecules, 2003, 36, 7027–7034 CrossRef CAS.
- C. Bae, J. F. Hartwig, N. K. B. Harris, R. O. Long, K. S. Anderson and M. A. Hillmyer, Catalytic Hydroxylation of Polypropylenes, J. Am. Chem. Soc., 2005, 127, 767–776 CrossRef CAS PubMed.
- A. Bunescu, S. Lee, Q. Li and J. F. Hartwig, Catalytic Hydroxylation of Polyethylenes, ACS Cent. Sci., 2017, 3, 895–903 CrossRef CAS PubMed.
- L. Chen, K. G. Malollari, A. Uliana, D. Sanchez, P. B. Messersmith and J. F. Hartwig, Selective, Catalytic Oxidations of C–H Bonds in Polyethylenes Produce Functional Materials with Enhanced Adhesion, Chem, 2021, 7, 137–145 CAS.
- K. Wang, L. Gan, Y. Wu, M.-J. Zhou, G. Liu and Z. Huang, Selective Dehydrogenation of Small and Large Molecules by a Chloroiridium Catalyst, Sci. Adv., 2022, 8, eabo6586 CrossRef CAS PubMed.
- J. X. Shi, N. R. Ciccia, S. Pal, D. D. Kim, J. N. Brunn, C. Lizandara-Pueyo, M. Ernst, A. M. Haydl, P. B. Messersmith, B. A. Helms and J. F. Hartwig, Chemical Modification of Oxidized Polyethylene Enables Access to Functional Polyethylenes with Greater Reuse, J. Am. Chem. Soc., 2023, 145, 21527–21537 CrossRef CAS PubMed.
- C. Tan, M. Chen, C. Zou and C. Chen, Potentially Practical Catalytic Systems for Olefin-Polar Monomer Coordination Copolymerization, CCS Chem., 2024, 6, 882–897 CrossRef CAS.
- K. Hakala, T. Helaja and B. Löfgren, Metallocene/Methylaluminoxane-Catalyzed Copolymerizations of Oxygen-Functionalized Long-Chain Olefins with Ethylene, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 1966–1971 CrossRef CAS.
- I. Jun-ichi, T. Yoshihisa and K. Norio, New Metallocene Catalyst Having an Indenyl Group and a Fluorenyl Group for Ethylene–Polar Monomer Copolymerization, Chem. Lett., 2001, 30, 710–711 CrossRef.
- N. Kashiwa, T. Matsugi, S.-I. Kojoh, H. Kaneko, N. Kawahara, S. Matsuo, T. Nobori and J.-I. Imuta, Functionalization of Polyethylene Based on Metallocene Catalysis and Its Application to Syntheses of New Graft Copolymers Possessing Polar Polymer Segments, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 3657–3666 CrossRef CAS.
- H. Hagihara, T. Ishihara, H. T. Ban and T. Shiono, Precise Control of Microstructure of Functionalized Polypropylene Synthesized by the ansa-Zirconocene/MAO Catalysts, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 1738–1748 CrossRef CAS.
- X.-H. Yang, C.-R. Liu, C. Wang, X.-L. Sun, Y.-H. Guo, X.-K. Wang, Z. Wang, Z. Xie and Y. Tang, [O-NSR]TiCl3-Catalyzed Copolymerization of Ethylene with Functionalized Olefins, Angew. Chem., Int. Ed., 2009, 48, 8099–8102 CrossRef CAS PubMed.
- Z. Chen, J.-F. Li, W.-J. Tao, X.-L. Sun, X.-H. Yang and Y. Tang, Copolymerization of Ethylene with Functionalized Olefins by [ONX] Titanium Complexes, Macromolecules, 2013, 46, 2870–2875 CrossRef CAS.
- M. Bouyahyi, Y. Turki, A. Tanwar, L. Jasinska-Walc and R. Duchateau, Randomly Functionalized Polyethylenes: In Quest of Avoiding Catalyst Deactivation, ACS Catal., 2019, 9, 7779–7790 CrossRef CAS.
- S. Kitphaitun, Q. Yan and K. Nomura, The Effect of SiMe3 and SiEt3 Para Substituents for High Activity and Introduction of a Hydroxy Group in Ethylene Copolymerization Catalyzed by Phenoxide-Modified Half-Titanocenes, Angew. Chem., Int. Ed., 2020, 59, 23072–23076 CrossRef CAS PubMed.
- R. Mundil, C. Bravo, N. Merle and P. Zinck, Coordinative Chain Transfer and Chain Shuttling Polymerization, Chem. Rev., 2024, 124, 210–244 CrossRef CAS PubMed.
- S. G. Correia, M. M. Marques, J. R. Ascenso, A. F. G. Ribeiro, P. T. Comes, A. R. Dias, M. Blais, M. D. Rausch and J. C. W. Chien, Polymerization with TMA-Protected Polar Vinyl Comonomers. II. Catalyzed by Nickel Complexes Containing α-Diimine-Type Ligands, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 2471–2480 CrossRef CAS.
- C. L. P. Carone, G. L. Crossetti, N. R. S. Basso, Á.GO. Moraes, J. H. Z. dos Santos, R. Bisatto and G. B. Galland, Ethylene Polymerization and Copolymerization with 10-Undecen-1-ol Using the Catalyst System DADNi(NCS)2/MAO, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 5199–5208 CrossRef CAS.
- D. Zhang and C. Chen, Influence of Polyethylene Glycol Unit on Palladium- and Nickel-Catalyzed Ethylene Polymerization and Copolymerization, Angew. Chem., Int. Ed., 2017, 56, 14672–14676 CrossRef CAS PubMed.
- Y. Na, S. Dai and C. Chen, Direct Synthesis of Polar-Functionalized Linear Low-Density Polyethylene (LLDPE) and Low-Density Polyethylene (LDPE), Macromolecules, 2018, 51, 4040–4048 CrossRef CAS.
- C. Tan, M. Qasim, W. Pang and C. Chen, Ligand–Metal Secondary Interactions in Phosphine–Sulfonate Palladium and Nickel Catalyzed Ethylene (co)Polymerization, Polym. Chem., 2020, 11, 411–416 RSC.
- X.-W. Han, O. Daugulis and M. Brookhart, Unsaturated Alcohols as Chain-Transfer Agents in Olefin Polymerization: Synthesis of Aldehyde End-Capped Oligomers and Polymers, J. Am. Chem. Soc., 2020, 142, 15431–15437 CrossRef CAS PubMed.
- F. Li, J. He, T. Song, W. Gao, X. Mu and Y. Mu, Zirconium Complexes with Bulkier Amine Bis(phenolate) Ligands and Their Catalytic Properties for Ethylene (Co)polymerization, Inorg. Chem., 2022, 61, 6469–6479 CrossRef CAS PubMed.
- D. Liang, Y. Jiang, S.-H. Li and D.-M. Cui, Copolymerization of Ethylene and Polar Vinyl Monomers by Rare-earth Metal Catalysts, Acta Polym. Sin., 2024, 55, 1487–1494 CAS.
- J.-I. Imuta, N. Kashiwa and Y. Toda, Catalytic Regioselective Introduction of Allyl Alcohol into the Nonpolar Polyolefins: Development of One-Pot Synthesis of Hydroxyl-Capped Polyolefins Mediated by a New Metallocene IF Catalyst, J. Am. Chem. Soc., 2002, 124, 1176–1177 CrossRef CAS PubMed.
- S. Ito, M. Kanazawa, K. Munakata, J.-I. Kuroda, Y. Okumura and K. Nozaki, Coordination−Insertion Copolymerization of Allyl Monomers with Ethylene, J. Am. Chem. Soc., 2011, 133, 1232–1235 CrossRef CAS PubMed.
- M. Li, X. Wang, Y. Luo and C. Chen, A Second-Coordination-Sphere Strategy to Modulate Nickel- and Palladium-Catalyzed Olefin Polymerization and Copolymerization, Angew. Chem., Int. Ed., 2017, 56, 11604–11609 CrossRef CAS PubMed.
- G. Ji, Z. Chen, X.-Y. Wang, X.-S. Ning, C.-J. Xu, X.-M. Zhang, W.-J. Tao, J.-F. Li, Y. Gao, Q. Shen, X.-L. Sun, H.-Y. Wang, J.-B. Zhao, B. Zhang, Y.-L. Guo, Y. Zhao, J. Sun, Y. Luo and Y. Tang, Direct Copolymerization of Ethylene with Protic Comonomers Enabled by Multinuclear Ni Catalysts, Nat. Commun., 2021, 12, 6283 CrossRef CAS PubMed.
- Z. Wang, M. Chen and C. Chen, Catalytic Synthesis of Polyolefin Elastomer Using Unsymmetrical α-Diimine Nickel Catalyst, Acta Chim. Sin., 2023, 81, 559–564 CrossRef CAS.
- J. Barluenga, M. Yus and P. Bernad, Convenient ‘One-flask’ Synthesis of Olefins. Reaction of α-Chloroketones with Grignard Reagents and Lithium, J. Chem. Soc., Chem. Commun., 1978, 19, 847a–847a RSC.
- G. Brieger, S. W. Watson, D. G. Barar and A. L. Shene, Thermal Decomposition of Aluminum Alkoxides, J. Org. Chem., 1979, 44, 1340–1342 CrossRef CAS.
- J. Barluenga, M. Yus, J. M. Concellon and P. Bernad, Direct and Regioselective Transformation of α-Chlorocarbonyl Compounds into Alkenes and Deuteroalkenes, J. Org. Chem., 1981, 46, 2721–2726 CrossRef CAS.
- R. R. Kostikov, A. F. Khlebnikov and V. V. Sokolov, Synthesis by Elimination Reactions, in Category 6, Compounds with All-Carbon Functions, Science of Synthesis, Georg Thieme Verlag KG, 1st edn, 2010, vol. 47b. DOI:10.1055/sos-SD-047-00322.
- T. Wiedemann, G. Voit, A. Tchernook, P. Roesle, I. Gottker-Schnetmann and S. Mecking, Monofunctional Hyperbranched Ethylene Oligomers, J. Am. Chem. Soc., 2014, 136, 2078–2085 CrossRef CAS PubMed.
- Y. Gao, J. Chen, Y. Wang, D. Pickens, A. Motta, Q. J. Wang, Y.-W. Chung, T. L. Lohr and T. J. Marks, Highly Branched Polyethylene Oligomers via Single-Site Polymerization in Very Nonpolar Media, Nat. Catal., 2019, 2, 236–242 CrossRef CAS.
- C. Obuah, B. Omondi, K. Nozaki and J. Darkwa, Solvent and Co-catalyst Dependent Pyrazolylpyridinamine and Pyrazolylpyrroleamine Nickel(II) Catalyzed Oligomerization and Polymerization of Ethylene, J. Mol. Catal. A: Chem., 2014, 382, 31–40 CrossRef CAS.
- G. Si, Y. Na and C. Chen, Ethylene (co)Oligomerization by Phosphine-Pyridine Based Palladium and Nickel Catalysts, ChemCatChem, 2018, 10, 5135–5140 CrossRef CAS.
- X. Ma, X. Hu, Y. Zhang, H. Mu, L. Cui and Z. Jian, Preparation and In Situ Chain-End-Functionalization of Branched Ethylene Oligomers by Monosubstituted α-Diimine Nickel Catalysts, Polym. Chem., 2019, 10, 2596–2607 RSC.
- S. V. Zubkevich, V. A. Tuskaev, S. C. Gagieva, A. A. Pavlov, V. N. Khrustalev, F. Wang, L. Pan, Y. Li, D. Saracheno, A. A. Vikhrov, D. N. Zarubin and B. M. Bulychev, Trapping the Short-Chain Odd Carbon Number Olefins Using Nickel(II)-Catalyzed Tandem Ethylene Oligomerization and Friedel-Crafts Alkylation of Toluene, Chin. J. Chem., 2023, 41, 2855–2865 CrossRef CAS.
- C. Tan and C. Chen, Emerging Palladium and Nickel Catalysts for Copolymerization of Olefins with Polar Monomers, Angew. Chem., Int. Ed., 2019, 58, 7192–7200 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2368595 and 2368596. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4py01480d |
| This journal is © The Royal Society of Chemistry 2025 |