Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Vascular benefit of the use of mepivacaine as an anaesthetic in resilient hyaluronic acid® injectables
Jimmy
Faivre†
 *a,
Romain
Brusini†
a,
Jing
Jing†
a,
Sabrina
Walley†
b,
Lukas
Roubenne
c,
François
Bourdon
a,
Lee
Walker
d,
Bruno
Le Grand
c and
Conor J.
Gallagher
b
*a,
Romain
Brusini†
a,
Jing
Jing†
a,
Sabrina
Walley†
b,
Lukas
Roubenne
c,
François
Bourdon
a,
Lee
Walker
d,
Bruno
Le Grand
c and
Conor J.
Gallagher
b
aResearch and Development Department, Teoxane SA, Rue de Lyon 105, 1203 Geneva, Switzerland. E-mail: j.faivre@teoxane.com
bRevance Therapeutics, Inc., 1222 Demonbreun St, Nashville, TN 94560, USA
cPhysioStim, 10 rue Henri Regnault, 81100 Castres, France
dB City Clinic, 88 Rodney Street, Liverpool L1 9AR, UK.
First published on 11th June 2025
Abstract
The use of lidocaine (0.3% w/w) for pain management in hyaluronic acid-based soft-tissue injectables has been standard for two decades. Given lidocaine's well-known vasodilatory activity it may contribute to the incidence of post-treatment adverse events including bruising in patients. This study seeks to compare these vasodilatory properties of lidocaine with that of another anaesthetic candidate, mepivacaine. Rat aortic rings and human skin resistance arteries (diameter between 200–400 μm) were mounted on an isolated organ bath or myograph, respectively, and exposed to progressively increasing concentrations of lidocaine or mepivacaine from a solution or released from a gel. The concentration-dependent vascular response and kinetics were systematically compared in tissue originating from 3 biological donors. Additionally, tissue perfusion changes induced by 0.3% w/w anaesthetic solutions were assessed using laser Doppler imaging in rabbit ears. Systematically, lidocaine exhibited a greater vasodilatory activity than mepivacaine in clinically relevant concentration ranges in both animal and human models. In contrast to lidocaine, mepivacaine did not have a significant impact on blood vessel vasodilation. In clinical practice, formulation of hyaluronic acid (HA) injectables with mepivacaine may potentially reduce the risk of common adverse events. This characteristic highlights its potential advantages in the practice of hydrogel injections.
Introduction
Injectable hydrogels have garnered significant attention in medical practice due to their versatility and minimal invasiveness. Adopted in various applications including drug delivery, tissue engineering, and wound healing,1 these hydrogels can be injected directly into the desired site, providing a scaffold for cell growth,2,3 a controlled release system for therapeutic agents4,5 or a mechanical correction for the prevention of the sign of skin aging.6,7 In the latest category, the use of hyaluronic acid (HA)-based soft-tissue injectables, in particular, has grown substantially over the past two decades due to their efficiency, reversibility, and natural-looking outcomes.8 Although considered a relatively painless, minimally invasive procedure, soft-tissue injectables commonly incorporate local anaesthetics to improve patient comfort.9,10 Lidocaine, an amino amide–type local anaesthetic, has been the standard option in most soft-tissue injectables. Incorporated at 0.3% w/w into the products, lidocaine was initially chosen for its rapid onset of action, appropriate duration and effective pain relief during and after the procedure. In medical practice, however, lidocaine's known vasodilatory properties require that it generally be used in combination with epinephrine to offset the vasodilatory effects.11,12 In the context of soft tissue injectables, due to its vasodilatory effects, lidocaine can increase blood flow in and around the injected site, potentially inducing undesirable local side effects such as transient inflammation, and bruising, although these effects are generally mild. Mepivacaine is another amino-amide local anaesthetic with a long history of use in non-aesthetic fields like dentistry.13 In contrast to lidocaine, mepivacaine has been reported to have lower vasodilatory properties14,15 and in clinical practice, mepivacaine does not generally require coadministration with epinephrine. This suggests that mepivacaine may appear as a potential valuable candidate for improvement of classical lidocaine-containing HA-based soft tissue injectable formulations, for reduced post-procedural bruising. In a recent preclinical study, we demonstrated that the use of mepivacaine at 0.3% w/w in HA-based soft tissue injectables did not impair the hydrogel characteristics and properties in terms of mechanical performance, preclinical safety and stability when compared to the same products formulated with lidocaine.16 Furthermore, in head to head studies in nasolabial folds, HA gels formulated with mepivacaine were demonstrated to have similar filling effectiveness as those formulated with lidocaine, and mepivacaine was at least as effective at reducing pain and equally safe as lidocaine.10Thus, aiming to advance current scientific knowledge on the positive outcomes of injectable formulations containing anaesthetic agents, the present study seeks to provide a comprehensive evaluation of the comparative biological impacts of mepivacaine and lidocaine, particularly focusing on their vasodilatory properties, with different complex models. We first analysed the vasodilatory effects of different anaesthetic concentrations on rat large vessel (aortas) ex vivo. We then translated our efforts to evaluate their vasodilatory effects on human small vessels (subcutaneous resistance arteries) ex vivo. On these arteries, we additionally investigated the vasodilatory kinetics of anaesthetic concentrations released from a commercial soft tissue injectable. Finally, we conducted an in vivo experiment to assess the artery tissue perfusion after a subcutaneous injection of 0.3% w/w anaesthetic solutions in rabbits’ ears.
Through this study on the differential effects of lidocaine and mepivacaine on local tissue responses, we aim at providing a better understanding of the less potent vasodilatory activity of mepivacaine in hydrogel injectables and help clarify its potential clinical benefits, particularly in terms of minimizing undesirable local effects, including bruising, and improving patient experience for a wide range of injection-based treatments.
Materials and methods
In this study, three models were used to assess the comparative vasodilatory activity of lidocaine and mepivacaine: an ex vivo rat aortic ring model, an ex vivo human subcutaneous resistance artery model (diameter between 200 and 400 μm), and an in vivo rabbit ear model.Rat aortic rings for ex vivo evaluation
Male rat's aorta ring model was selected because of its relevance for the evaluation of the possible vasodilatory effects. 8 weeks-old Male Wistar Han rats were supplied by Charles River, UK (accreditation number for the housing and experimental use of animals for scientific purposes: A 81 065 002). After acclimation period, rats were heparinized (0.5 mL, Heparine Choay® 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U.I. per 5 mL) and euthanized by an intraperitoneal injection of sodium pentobarbital (0.9 to 1.4 mL kg−1, dolethal, vétoquinol). The heart with the aorta was rapidly excised, placed into fresh physiological solution (containing [in mM]: 118 NaCl, 4.7 KCl, 1.2 MgSO4, 25 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2 and 11.1 D-glucose) previously equilibrated for 30 minutes with a 95% O2–5% CO2 gas mixture. The rat thoracic aorta was quickly dissected, cleared of connective tissue and cut into 2 to 4 cylindrical segments. When necessary, the aortic ring was denuded by gently rolling the aorta and rubbing the inner surface, in order to study the involvement of endothelium in anaesthetics-induced vasorelaxation. Endothelium denudation was previously validated with the absence of relaxation induced by acetylcholine.
000 U.I. per 5 mL) and euthanized by an intraperitoneal injection of sodium pentobarbital (0.9 to 1.4 mL kg−1, dolethal, vétoquinol). The heart with the aorta was rapidly excised, placed into fresh physiological solution (containing [in mM]: 118 NaCl, 4.7 KCl, 1.2 MgSO4, 25 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2 and 11.1 D-glucose) previously equilibrated for 30 minutes with a 95% O2–5% CO2 gas mixture. The rat thoracic aorta was quickly dissected, cleared of connective tissue and cut into 2 to 4 cylindrical segments. When necessary, the aortic ring was denuded by gently rolling the aorta and rubbing the inner surface, in order to study the involvement of endothelium in anaesthetics-induced vasorelaxation. Endothelium denudation was previously validated with the absence of relaxation induced by acetylcholine.
Human subcutaneous resistance arteries for ex vivo evaluation
Human subcutaneous resistance arteries were selected as this translational model is relevant for the evaluation of functional vascular effects. Resistance arteries are greatly involved in the regulation of organ-specific perfusion, peripheral resistance and blood pressure and represent an ideal vascular model for evaluation of subcutaneously injected devices. Human subcutaneous samples were obtained from abdominal or thigh skin surgery, then stored at +2–8 °C in a preservative solution until experimentation (authorization to retain human body elements (CODECOH) DC-2023-5686). The day of the experiment, resistance arteries from human subcutaneous sample were carefully isolated and cleared of connective and adipose tissues in fresh physiological solution (containing [in mM]: 118 NaCl, 4.7 KCl, 1.2 MgSO4, 25 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2 and 11.1 D-glucose) previously equilibrated for 30 minutes with a 95% O2–5% CO2 gas mixture. The arteries were cut into 2 mm length rings.Rabbit for in vivo hemodynamics evaluation
6 to 8-week old female New Zealand White rabbits were used in this study. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of the Biologic Resources Laboratory of the University of Illinois-Chicago and approved by the Animal Care and Use Committee of the University of Illinois-Chicago (Approval no. 22-013). Briefly, after the acclimation period, animals were anaesthetized using ketamine/xylazine. Once consciousness was lost the animals were intubated and ventilated, and anaesthesia was maintained via isoflurane during the whole experiment. Rabbits were additionally maintained on a heating pad and body temperature was monitored throughout the experiment.Test articles
Solutions of lidocaine and mepivacaine were freshly prepared in distilled water before each assessment. For ex vivo rat aortic rings and human subcutaneous resistance arteries concentration-response evaluation, the concentrations (final cumulative in myograph bath) of both lidocaine and mepivacaine were 0.1, 0.5, 1, 2.5, 5, 7.5 and 10 mM. For the 2nd experimental phase with human subcutaneous resistance arteries, the investigated concentrations were extracted from their release kinetics from Teosyal® RHA4 (Teoxane SA, Switzerland) as previously described.16 For in vivo rabbit hemodynamics evaluation, 0.3% w/w mepivacaine and 0.3% w/w lidocaine in saline were used as test articles.Ex vivo vascular tension measurement of rat aortic rings
To measure vascular tension after treatment with anaesthetics, isometric tension of isolated rat aortic rings was recorded through a force transducer connected in an isolated bath organ (set-up used to study large vessels, EMKA Technologies, Paris, France). The protocol used follows the ICH S7A. The solution in the isolated organ baths was continuously oxygenated with 95% O2–5% CO2 and maintained at 37.0 ± 0.2 °C. After isolation and mounting in organ baths, rat aortic rings were stretched to an identical length before isometric tension measurements.Ex vivo vascular tension measurement of human resistance arteries
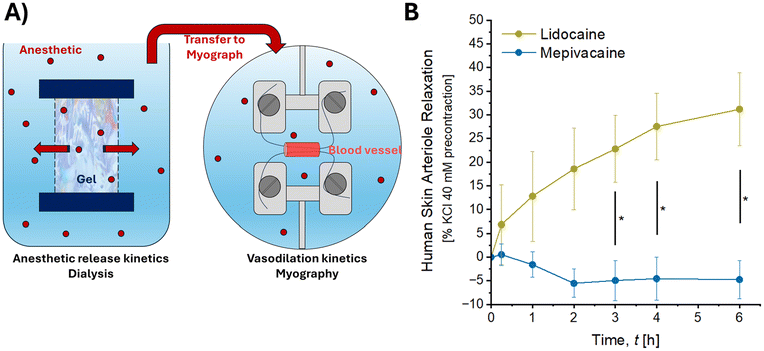
Isometric tensions of human subcutaneous resistance arteries were recorded using a force transducer connected to a wire myograph (set-up used to study small vessels, EMKA Technologies). Photographs of the wire myography system are presented in Fig. 1. The solution contained in the myograph organ baths was continuously gassed with 95% O2–5% CO2 and warmed at +37.0 ± 0.2 °C. For isometric tension measurements, under microscope control, resistance artery rings isolated from human skin samples obtained during abdominoplasty procedures were mounted on two 25 μm tungsten wires in myograph bath (Fig. 1), with one wire attached to a force transducer and the other to a displacement device. Each resistance artery ring was progressively stretched (in 100 to 150 μm steps) to construct passive length-tension curves and determine the tension equivalent to that required to produce 90% of their internal circumference when exposed to a transmural pressure of 100 mmHg. This value was obtained for each vascular ring and allow experimental standardization, vascular responses optimization and reproduction of physiological conditions.17Ex vivo experimental procedure
After mounting (i.e. rat aorta or human resistance artery), the saline solution was replaced every 15 minutes over a stabilization period of at least 1 h before successive challenges with KCl 60 mM to produce a reference contraction, separated by two washes (see Fig. 2). The saline solution was then replaced every 15 minutes between each step during a stabilization period of at least 30 minutes. Endothelial function was tested using acetylcholine (1 μM) to induce endothelial-dependent relaxation following a precontraction with phenylephrine (Sigma-Aldrich, Germany; see Fig. 2). The first and the second experimental phases were carried out successively, separated by a stabilization period of at least 30 minutes with a wash every 10 minutes (Fig. 2). For the first experimental phase (from 0.1 to 10 mM of lidocaine or mepivacaine) and the second experimental phase (from 0.239 to 1.026 mM of lidocaine or mepivacaine), vessels were precontracted with KCl 40 mM until a stable contraction was obtained (Fig. 2). High concentrations of KCl precontraction increase vascular tone, allowing the study of the vasorelaxant potential of anaesthetic agents. In addition, both lidocaine and mepivacaine have been shown not to influence the vascular basal tone.18,19 Each concentration of lidocaine and mepivacaine solutions were added for at least 5 minutes (until a stable effect was obtained) using a cumulative concentration strategy (Fig. 2). Isometric tension was continuously recorded (expressed in grams), at baseline and after each concentration of test compounds, and analysed by a computer. An interactive software program (IOX, version 2.9.5) provided acquisition of data and on-line measurement of analysed parameters. Each experiment (for lidocaine and mepivacaine) was carried out on 5 rat aortic rings and 3 resistance artery rings from 3 different human donors. Baseline step was defined as the values obtained before the injection of the first test article concentration in the bath (when KCl 40 mM precontraction is stable). Test article-induced vascular relaxation was expressed in % of KCl 40 mM precontraction (baseline). These values were measured on the maximal effect observed. All results were expressed as mean ± SEM (standard error of the mean).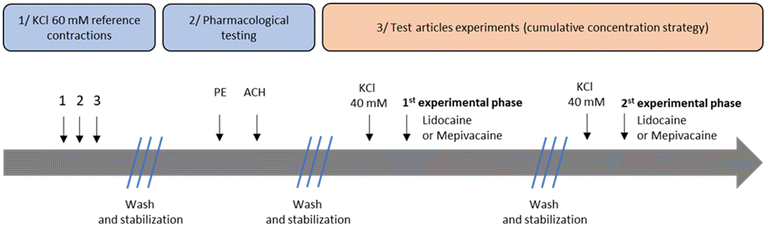 | ||
| Fig. 2 Graphical representation of experimental procedures. KCl: potassium chloride; ACH: acetylcholine; PE: phenylephrine. | ||
In vivo rabbit hemodynamic evaluation
Tissue perfusion was measured in 2 female New Zealand White rabbit ears using a laser Doppler probe (ABLPHI, Transonic Systems, Inc., Ithaca, NY). Following confirmation of anaesthesia, and after a 15-minute baseline period, the ear was shaved, and the probe was attached with adhesive tape lateral to the central ear artery. Test articles (0.1 mL of 0.3% mepivacaine or 0.3% lidocaine in saline) were injected subcutaneously distal to the probe (Fig. 6A). Data were collected for 45 minutes following injection of the anaesthetic. Regional blood flow was measured and reported as Tissue Perfusion Units (TPUs). Changes in TPU values were calculated by averaging into 5-minute bins and normalizing data to baseline values.Statistical analysis
Two-sample t-test was performed for statistical analysis in OriginPro 2023 (OriginLab, USA). The differences were considered statistically significant at p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***) in all studies. Values were expressed as mean ± SE.Results
Ex vivo vasodilatory activity of mepivacaine and lidocaine in contact of rat aorta
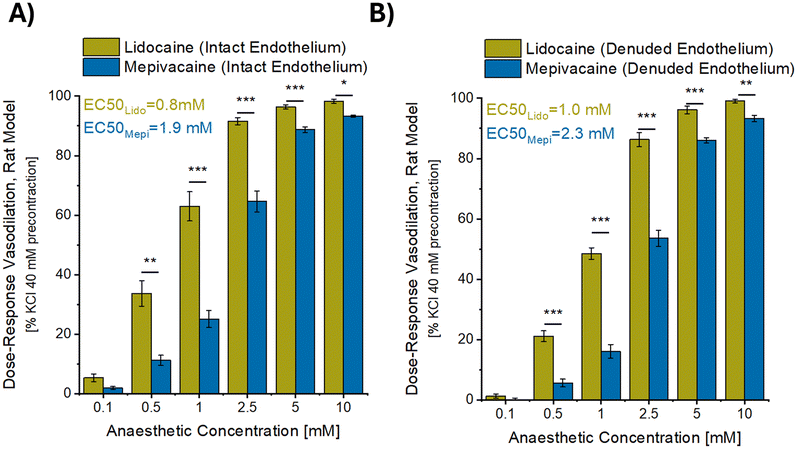
Fig. 3 shows the concentration-dependent vasorelaxant effect of increasing concentrations of the anaesthetics on aortic rings, for both the intact rings (Fig. 3A) and the rings in which the endothelium had been denuded (Fig. 3B). Lidocaine was shown to exhibit a greater vasodilatory effect than mepivacaine through all the tested concentrations, covering a large range of concentration from 0.1 to 10 mM (with significantly different results from 0.5 to 10 mM as compared to mepivacaine). In addition, endothelium denudation appeared to have no influence on lidocaine or mepivacaine-induced relaxation (Fig. 3A and B). Irrespective of the presence or absence of the endothelium, the EC50 (half maximal effective concentration) for maximal vascular relaxation was half that for the lidocaine treated vessels (0.8 mM and 1.0 mM for intact and denuded respectively) in comparison to the mepivacaine treated vessels (1.9 mM and 2.3 mM for intact and denuded respectively).Ex vivo vasodilatory activity of mepivacaine and lidocaine in contact with human skin resistance arteries
A similar range of concentrations of mepivacaine and lidocaine was applied to human skin resistance arteries. Fig. 4A–C present vasodilatory data for each donor showcasing the interindividual variability. The superior vasodilatory activity of lidocaine remained consistent among the three human donors and presented a similar trend as in rat aorta experiments. Pooling all the donors together, mepivacaine did not induce vasodilation until the concentration of 5 mM (24.47% of relaxation) whereas lidocaine already exhibited vasodilation from 0.5 mM (14.55% of relaxation) as depicted in Fig. 4D. Consistently with the prior experiment, the EC50 for maximal vascular relaxation was halved with mepivacaine, being 2.5 mM and 5.4 mM for vessels treated by lidocaine and mepivacaine respectively.Beyond the concentration-response experiment, human resistance arteries were assessed in the presence of increasing amounts of anaesthetics released from RHA4.16,20 Lidocaine and mepivacaine were released through a 12–14 kDa regenerated cellulose membrane as previously described16 and transferred in contact with human subcutaneous resistance arteries of 3 different human donors (Fig. 5A). The complete release of anaesthetics from RHA4 took about 6 hours.16 The kinetics of vasodilation activity for both anaesthetics are presented in Fig. 5B. Over the course of anaesthetics release and from the earliest timepoints (a few minutes after release), lidocaine readily exhibited a vasodilatory activity whereas mepivacaine did not show any impact on vessels behaviour. Notably, vessel relaxation was significantly greater from the 3-hour time point onward in the presence of lidocaine compared to mepivacaine. After 6 hours of anaesthetic release from RHA4, at ∼1 mM concentration for both anaesthetics, lidocaine caused a 31.18 ± 7.74% vasodilation increase compared to mepivacaine which remains at the baseline (−4.74 ± 4.07%).
In vivo vasodilatory activity of mepivacaine versus lidocaine injected in rabbit ears
In the in vivo rabbit ears model, after an initial slight transient increase in tissue perfusion after administration of either anaesthetic in solution, a clear separation occurred in tissue perfusion after the 10-minute mark (Fig. 6B). In the mepivacaine-treated ear, perfusion returned to baseline levels at 20 minutes and continued to decline, stabilizing at a decrease of −22 ± 45% in tissue perfusion by 35 minutes. Conversely in lidocaine treated ears, the tissue perfusion increased steadily up to 117 ± 6% of the baseline value at 30 minutes before progressively declining over the subsequent 15 minutes.Discussion
Pain management associated with use of HA injectables is an essential aspect of patient safety and comfort.21 The addition of lidocaine in soft-tissue injectables is now deemed essential due to the significant pain relief they provide, thus improving patient satisfaction. Lidocaine is widely regarded as the reference standard among commonly used local anaesthetics due to its well-established efficacy, safety profile, and versatility across various clinical applications.22 It serves as a benchmark for comparing the pharmacologic properties of other anaesthetic agents. A recently published update on the use of lidocaine in soft-tissue injectables demonstrated its statistically significant role in pain reduction of the nasolabial folds, while maintaining a similar effectiveness and safety profile as anaesthetic-free products.23 Mepivacaine, another well-known amide-type local anaesthetic, shares many similarities with lidocaine but differs in key aspects.15,24,25 Notably, mepivacaine exhibits lower vasodilatory activity than lidocaine, which contributes to its longer duration of action without the need for vasoconstrictors such as epinephrine. This property makes mepivacaine particularly useful in situations where vasoconstrictors are contraindicated or undesirable. The first hyaluronic acid soft-tissue injectable to be formulated with mepivacaine was approved by the US FDA in 2023. This approval (for the RHA collection, Teoxane SA, Geneva, Switzerland) was based on 2 randomized, double-blinded, split-face clinical trials involving both 30 patients.10 In one, the safety and effectiveness of RHA Redensity with mepivacaine 0.3% was compared to RHA Redensity with lidocaine 0.3% in perioral lines. In the other RHA4 containing mepivacaine 0.3% was compared to RHA4 formulated with 0.3% lidocaine in nasolabial folds. In each study, pain relief was the primary endpoint, and in both cases, RHA-Mepivacaine (RHA-M) and RHA-Lidocaine (RHA-L) were similarly effective in reducing procedural and post-procedural pain. Furthermore, no statistically significant differences were observed in their ability to successfully treat facial wrinkles. A previous comparative preclinical study of these hydrogel injectables incorporating lidocaine or mepivacaine showed that there was no significant difference in gels’ physicochemical properties, preclinical stability, and in vitro degradation profile.16 The in vitro release profiles of the anaesthetic agents were similarly achieved within 6 hours, while the kinetics of vasodilation differed significantly within 3 hours for both anaesthetics in the present study (Fig. 5B). Previously, the efficacy of mepivacaine released has shown that it has a short onset of anaesthesia with a slightly longer duration than lidocaine (T½ 1.9 vs. 1.4 hours) when administered subcutaneously at comparable concentrations (0.3% w/w).16 The same trend has been observed in humans after intravenous (IV) administration of mepivacaine versus lidocaine.14 The plasma concentrations were comparable after 5 minutes with mepivacaine providing a higher degree of analgesia over the 60-minute study period requiring significantly less supplementary analgesia and presenting less adverse events than lidocaine. In the present study, we demonstrated that in all the variety of tested models, ex vivo rat aorta, ex vivo human skin resistance arteries and in vivo rabbit ears, mepivacaine had less vasodilatory effects than lidocaine, showing a lower relaxation amplitude or lower tissue perfusion at each evaluated anaesthetic concentration (from 0.1 to 10 mM) (Fig. 3 and 4). It is noteworthy that the maximal investigated concentration (10 mM) corresponds to the final formulated concentration of the anaesthetics in HA injectables (0.3% w/w), and this would be the maximal concentration of anaesthetic that could be expected in tissue, with a gradient of concentration extending from the site of gel placement. The contrast between anaesthetic agents was particularly marked in resistance arteries from human skin donors in their released concentration ranges.16 Particularly, mepivacaine progressively released from the hydrogel, did not induce any vasorelaxation as opposed to lidocaine (Fig. 5). Lidocaine also showed significantly greater vasorelaxation than mepivacaine in vivo, 30 minutes after injection of the anaesthetic solutions into rabbit arteries (Fig. 6). The delayed timeline relative to Fig. 5B with respect to Fig. 6B reflects the release kinetics from the gel, which required time for the anaesthetic to be released before impacting vessel relaxation. In practice, lidocaine's greater vasodilation capacity than mepivacaine is well-established14 and underlies the frequent concomitant use of epinephrine12,26 with lidocaine. Besides, although both anaesthetics have a long history of use as local anaesthetics in many branches of medicine and are regarded as highly effective and generally safe – allergic reactions to these classes of anaesthetic have been reported, albeit very rare9,27 and cross-reactivity has been shown28,29 – supporting the potential interest to have soft tissue injectables with mepivacaine. The current understanding of the mechanism underlying the vasodilatory activity of lidocaine is that it is mediated via the vascular endothelium, and through the production of vasorelaxation factors such as nitric oxide, endothelium-derived hyperpolarizing factor and prostacyclin.30 Thus, endothelium denudation was used to investigate the potential involvement of this vascular layer in anaesthetics-induced vasorelaxation in rat aorta (Fig. 3B). Interestingly, neither the vasorelaxation induced by lidocaine nor that induced by mepivacaine was significantly affected by removal of the endothelium. These results suggest that the endothelium is not responsible for this vasorelaxation process. This aligns with previous work showing that the relaxation induced by lidocaine and mepivacaine are not reduced by endothelium removal or L-NAME and indomethacin treatments.31–33 Therefore, we can hypothesize that arterial smooth muscle cells might be the target of the action mechanism of both anaesthetic agents. Blockade of voltage-gated L-type calcium channels on these cells has been proposed as a possible vasorelaxation mechanism induced by lidocaine,34 suggesting that lidocaine could inhibit calcium entry in smooth muscle cells to trigger relaxation. Although not yet demonstrated in the context of mepivacaine-induced relaxation, blocking the entry of extracellular calcium into smooth muscle cells could conceivably account for the vasorelaxation seen after exposure to high concentrations of this anaesthetic.35 The diversity of results described in the literature on vascular effects and mechanisms of action of lidocaine and mepivacaine can be explained by the heterogeneity of vascular models (animal or human based), vascular bed (aorta, coronary arteries etc.), methods of pre-contraction (KCl, PE or 5-hydroxytryptamine) and concentrations tested.36 Nonetheless, taken together, these results demonstrate that mepivacaine induces less vasorelaxation than lidocaine (through an endothelium-independent mechanism), hypothesizing that the use of mepivacaine in clinical injection conditions could reduce the extent of bleeding into tissues, thereby reduce the incidence of bruising. Future head-to-head clinical investigations are required with larger groups to confirm the observations statistically. Despite this, the use of various biological donors and models (animal, human, ex vivo/in vivo, large and small arteries) consistently demonstrated greater vasodilatory activity of lidocaine compared to mepivacaine starting at concentrations as low as a tenth of the initial anaesthetic content in soft-tissue injectables. Rates of specific adverse events, including bruising, are being analysed in practice to compare the clinical effects of lidocaine and mepivacaine.Conclusions
This study explores the contrasting vasodilatory profiles of lidocaine and mepivacaine, two structurally related local anaesthetics, in the context of their application in hydrogel-based injectables. Mepivacaine consistently induced a significant lower vasodilation across ex vivo and in vivo models, including animal and human tissues, and both small and large vessels. This finding suggests a potential clinical benefit of using mepivacaine in hydrogel injectables to reduce common injection-related adverse events while enhancing overall patient outcomes. Future research will focus on validating the reduced vasodilatory activity of mepivacaine through in vivo human studies. Given that mepivacaine does not compromise the rheological properties of soft-tissue injectables, its incorporation into the RHA® collection—HA injectables already FDA-approved—presents a promising option for clinical practice.Author contributions
Conceptualization and supervision: J. F., F. B., and C. G.; methodology: J. F., R. B., J. J., S. W., L. R., B. L.-G., and C. G.; investigation: R. B., J. J., S. W., and L. R.; formal analysis: J. F., R. B., J. J., S. W., L. R., L. W., and C. G.; writing: J. F., R. B., J. J., S. W., L. R., F. B., B. L.-G., and C. G.Data availability
Data are available upon reasonable request to the corresponding author.Conflicts of interest
J. F., R. B, J. J., and F. B. are employees of Teoxane SA, Geneva, Switzerland, L. R. is an employee of Physiostim, Castres, Franc, L. W. is a KOL and trainer for Teoxane SA, S. W., and C. G. are employees of Revance Therapeutics, Nashville, TN, USA.There are no conflicts to declare.
References
- S. Almawash, S. K. Osman, G. Mustafa and M. A. El Hamd, Current and Future Prospective of Injectable Hydrogels—Design Challenges and Limitations, Pharmaceuticals, 2022, 15(3), 371 CrossRef CAS PubMed
.
- P. Zarrintaj, M. Khodadadi Yazdi and M. Youssefi Azarfam,
et al., Injectable Cell-Laden Hydrogels for Tissue Engineering: Recent Advances and Future Opportunities, Tissue Eng., Part A, 2021, 27(11–12), 821–843, DOI:10.1089/ten.TEA.2020.0341
.
- A. Kumar Podder, M. A. Mohamed and R. A. Seidman,
et al., Injectable shear-thinning hydrogels promote oligodendrocyte progenitor cell survival and remyelination in the central nervous system, Sci. Adv., 2024, 10(28), eadk9918, DOI:10.1126/sciadv.adk9918
.
- E. S. Nealy, S. J. Reed and S. M. Adelmund,
et al., Versatile tissue-injectable hydrogels capable of the extended hydrolytic release of bioactive protein therapeutics, Bioeng. Transl. Med., 2024, 9(5), e10668, DOI:10.1002/btm2.10668
.
- A. Atwal, T. P. Dale, M. Snow, N. R. Forsyth and P. Davoodi, Injectable hydrogels: An emerging therapeutic strategy for cartilage regeneration, Adv. Colloid Interface Sci., 2023, 321, 103030, DOI:10.1016/j.cis.2023.103030
.
- B. L. Eppley and B. Dadvand, Injectable soft-tissue fillers: clinical overview, Plast. Reconstr. Surg., 2006, 118(4), 98e–106e CrossRef PubMed
.
- D. Cassuto, G. Bellia and C. Schiraldi, An Overview of Soft Tissue Fillers for Cosmetic Dermatology: From Filling to Regenerative Medicine, Clin., Cosmet. Invest. Dermatol., 2021, 14, 1857–1866, DOI:10.2147/CCID.S276676
.
- A. Fallacara, S. Manfredini, E. Durini and S. Vertuani, Hyaluronic acid fillers in soft tissue regeneration, Facial Plast. Surg., 2017, 33(01), 087–096 CrossRef CAS PubMed
.
- L. Smith and K. Cockerham, Hyaluronic acid dermal fillers: can adjunctive lidocaine improve patient satisfaction without decreasing efficacy or duration?, Patient Prefer. Adherence, 2011, 5, 133–139, DOI:10.2147/PPA.S11251
.
- J. Kaufman-Janette, J. H. Joseph, S. H. Dayan, S. Smith, L. Eaton and P. Maffert, Patient Comfort, Safety, and Effectiveness of Resilient Hyaluronic Acid Fillers Formulated With Different Local Anesthetics, Dermatol. Surg., 2022, 48(10), 1065–1070, DOI:10.1097/DSS.0000000000003541
.
- D. Newton, G. McLeod, F. Khan and J. Belch, Mechanisms influencing the vasoactive effects of lidocaine in human skin, Anaesthesia, 2007, 62(2), 146–150 CrossRef CAS PubMed
.
- N. S. Perlmutter, R. A. Wilson, S. W. Edgar, W. Sanders, B. H. Greenberg and R. Tanz, Vasodilatory effects of lidocaine on epicardial porcine coronary arteries, Pharmacology, 1990, 41(5), 280–285 CrossRef CAS PubMed
.
- N. Badr and J. Aps, Efficacy of dental local anesthetics: A review, J Dent Anesth Pain Med, 2018, 18(6), 319–332 CrossRef PubMed
.
- P. Prieto-Álvarez, A. Calas-Guerra, J. Fuentes-Bellido, E. Martínez-Verdera, A. Benet-Català and J. P. Lorenzo-Foz, Comparison of mepivacaine and lidocaine for intravenous regional anaesthesia: pharmacokinetic study and clinical correlation, Br. J. Anaesth., 2002, 88(4), 516–519, DOI:10.1093/bja/88.4.516
.
- N. Su, Y. Liu, X. Yang, Z. Shi and Y. Huang, Efficacy and safety of mepivacaine compared with lidocaine in local anaesthesia in dentistry: a meta-analysis of randomised controlled trials, Int. Dent. J., 2014, 64(2), 96–107, DOI:10.1111/idj.12087
.
- R. Brusini, J. Iehl, E. Clerc, M. Gallet, F. Bourdon and J. Faivre, Comparative Preclinical Study of Lidocaine and Mepivacaine in Resilient Hyaluronic Acid Fillers, Pharmaceutics, 2022, 14(8), 1553, DOI:10.3390/pharmaceutics14081553
.
- M. J. Mulvany and W. Halpern, Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats, Circ. Res., 1977, 41(1), 19–26, DOI:10.1161/01.res.41.1.19
.
- A. Arsyad and G. P. Dobson, Lidocaine relaxation in isolated rat aortic rings is enhanced by endothelial removal: possible role of K(v), K(ATP) channels and A(2a) receptor crosstalk, BMC Anesthesiol., 2016, 16(1), 121, DOI:10.1186/s12871-016-0286-y
.
- P. Meyer, J. Flammer and T. F. Lüscher, Local anesthetic drugs reduce endothelium-dependent relaxations of porcine ciliary arteries, Invest. Ophthalmol. Visual Sci., 1993, 34(9), 2730–2736 CAS
.
- K. Flégeau, S. Ballarini and R. Brusini,
et al., Safety and Performance of RHA4 in the Midface Using the Multilayering Technique: Preclinical and Clinical Evidence, Plast. Reconstr. Surg. Glob. Open, 2025, 13(2), e6560, DOI:10.1097/GOX.0000000000006560
.
- L. Shi, J. Zhang and G. Wang,
et al., A Cross-Sectional Survey on Pain Management in Dermal Filler Injections from Physicians’ and Patients’ Perspectives, Aesthetic Plast. Surg., 2024, 48(7), 1417–1425, DOI:10.1007/s00266-023-03843-9
.
- A. Taylor and G. McLeod, Basic pharmacology of local anaesthetics, BJA Educ, 2020, 20(2), 34–41, DOI:10.1016/j.bjae.2019.10.002
.
- H. Liu, G. Shang, T. Zhu and Q. Shan, Efficacy and Safety of Hyaluronic Acid Fillers With or Without Lidocaine in the Treatment of Nasolabial Folds: An Updated Systematic Review and Meta-Analysis, Aesthetic Plast. Surg., 2024, 48, 4466–4484, DOI:10.1007/s00266-024-04233-5
.
- W. G. Brockmann, Mepivacaine: a closer look at its properties and current utility, Gen. Dent., 2014, 62(6), 70–75 Search PubMed
.
- R. P. Visconti, I. P. Tortamano and I. A. Buscariolo, Comparison of the Anesthetic Efficacy of Mepivacaine and Lidocaine in Patients with Irreversible Pulpitis: A Double-blind Randomized Clinical Trial, J. Endod., 2016, 42(9), 1314–1319, DOI:10.1016/j.joen.2016.06.015
.
- C. J. Sinnott, L. P. Cogswell III, A. Johnson and G. R. Strichartz, On the Mechanism by Which Epinephrine Potentiates Lidocaine’s Peripheral Nerve Block, Anesthesiology, 2003, 98(1), 181–188, DOI:10.1097/00000542-200301000-00028
.
- S. Jiang and M. Tang, Allergy to Local Anesthetics is a Rarity: Review of Diagnostics and Strategies for Clinical Management, Clin. Rev. Allergy Immunol., 2023, 64(2), 193–205, DOI:10.1007/s12016-022-08937-x
.
- R. Fuzier, M. Lapeyre-Mestre and P. M. Mertes,
et al., Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing, Pharmacoepidemiol. Drug Saf., 2009, 18(7), 595–601, DOI:10.1002/pds.1758
.
- I. Koca Kalkan, G. Koycu Buhari and H. Ates,
et al., Identification of Risk Factors and Cross-Reactivity of Local Anesthetics Hypersensitivity: Analysis of 14-Years’ Experience, J. Asthma Allergy, 2021, 14, 47–58, DOI:10.2147/jaa.S292442
.
- P. M. Vanhoutte, C. M. Boulanger and J. V. Mombouli, Endothelium-derived relaxing factors and converting enzyme inhibition, Am. J. Cardiol., 1995, 76(15), 3E–12E, DOI:10.1016/S0002-9149(99)80496-2
.
- A. Arsyad and G. P. Dobson, Lidocaine relaxation in isolated rat aortic rings is enhanced by endothelial removal: possible role of Kv, KATP channels and A2a receptor crosstalk, BMC Anesthesiol., 2016, 16(1), 121, DOI:10.1186/s12871-016-0286-y
.
- Q. X. Shan, D. S. Lin, H. F. Jin, Q. Gao, Y. Lu and Q. Xia, Endothelium-independent vasorelaxant effect of lidocaine in rat aortic rings, Conf. Proc. IEEE Eng. Med. Biol. Soc., 2004, 2004, 3753–3756, DOI:10.1109/iembs.2004.1404053
.
- K. Satoh, M. Chikuda, A. Ohashi, M. Kumagai, M. Sato and S. Joh, The effect of mepivacaine on swine lingual, pulmonary and coronary arteries, BMC Anesthesiol., 2015, 15(1), 101, DOI:10.1186/s12871-015-0085-x
.
- Y. Tanaka, M. Kamibayashi and Y. Yamashita,
et al., Evidence for the possible involvement of Ca2+ entry blockade in the relaxation by class I antiarrhythmic drugs in the isolated pig coronary smooth muscle, Naunyn-Schmiedeberg's Arch. Pharmacol., 2002, 365(1), 56–66, DOI:10.1007/s00210-001-0495-9
.
- S. Fukuda, T. Tsuji, T. Murakawa, H. Takeshita and N. Toda, Effects of Mepivacaine on adrenergic neuroeffector junction of the isolated rabbit aorta, Anesth. Analg., 1982, 61(9), 756–762 CrossRef CAS
.
- F. A. Wali, Effects of local anaesthetics on responses of human saphenous vein and bovine coronary artery to neurotransmitters, acetylcholine, noradrenaline and 5-hydroxytryptamine, Gen. Pharmacol., 1986, 17(4), 405–411, DOI:10.1016/0306-3623(86)90182-5
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |