Open Access Article
Open Access ArticleGlivec to generic imatinib switch: in vitro comparative dissolution assessment, bioequivalence, safety, and tolerability of 400 mg imatinib tablets in healthy volunteers
Samir
Das
ab,
Saurav
Sarkar
 a,
Ranabir
Sahu
a,
Tarun Kumar
Dua
a,
Paramita
Paul
a,
Ranabir
Sahu
a,
Tarun Kumar
Dua
a,
Paramita
Paul
 a and
Gouranga
Nandi
a and
Gouranga
Nandi
 *a
*a
aDepartment of Pharmaceutical Technology, University of North Bengal, Darjeeling 734013, West Bengal, India. E-mail: nandi_gouranga@yahoo.co.in
bDBL Pharmaceuticals, Dhaka-1212, Bangladesh
First published on 30th May 2025
Abstract
Imatinib is currently considered the “gold standard” pharmacotherapy for chronic myelogenous leukemia (CML) at all stages and is most commonly used in the form of tablets taken orally. The aim of the present study was to perform a quality assessment, bioequivalence study, and safety and tolerability assessment of an investigational test product, imatinib tablets (400 mg), and its comparability with a reference product (Glivec tablets, 400 mg). In vitro dissolution studies of the test and reference products were conducted in three different buffer media (pH 1.2, pH 4.5, and pH 6.8) using Apparatus II (paddle), and the results were compared. The similarity (f2) factor was calculated to assess in vitro bioequivalence requirements. An open-label, balanced, randomized, two-treatment, two-sequence, two-period, single oral dose, crossover, bioequivalence study was conducted in normal, healthy, adult human subjects under fed conditions. The pharmacokinetic parameters Tmax, Cmax, AUC0–t, and AUC0–∞ were calculated through a non-compartmental model using Phoenix WinNonlin Version 8.3 (Certara L.P.) software. Statistical evaluation and comparison of the two formulations were carried out using PROC GLM in SAS version 9.4 (SAS Institute Inc., USA). The safety profile of the investigational product was monitored during the study by applying a clinical process for recording observed untoward effects post-administration of the investigational product. The investigational test product met USP and BP pharmacopoeial quality standards for in vitro dissolution. Very rapid dissolution (>85% release in 15 minutes) was obtained for the reference and test products in all three buffered dissolution media (pH 1.2, pH 4.5, and pH 6.8) in in vitro dissolution studies. The dissolution profile of the investigational test product (imatinib tablets, 400 mg) was comparable to that of the reference product (Glivec tablets, 400 mg). Furthermore, pharmacokinetic values (Cmax, Tmax, and AUC) of the test and reference forms of imatinib were similar. Geometric mean ratios (test/reference) for the AUC0–∞, AUC0–t, and Cmax were 95.2, 0.95.2, and 98.4, respectively. Confidence limits for each of these parameters were in the interval (80.00, 125.00), as were the unadjusted confidence limits. The test and reference formulations of imatinib met the criteria for bioequivalence based on the rate and extent of absorption. Based on the findings of this study, it can be concluded that the test product is bioequivalent and safe, thus suggesting the clinical application of the test product as an alternative to Glivec 400 mg film-coated tablets.
Introduction
Imatinib (Glivec), a phenylamino pyrimidine derivative, is a tyrosine kinase inhibitor that targets BCR-ABL, platelet-derived growth factor receptors (PDGF-Rs), and c-KIT receptors for the stem cell factor (SCF).1 Constitutive activation of tyrosine kinases is crucial for the pathogenesis of certain tumors and myeloproliferative disorders.1 Imatinib is currently considered the “gold standard” pharmacotherapy for chronic myelogenous leukemia (CML) at all stages (400 or 600 mg day−1 for advanced stages).2 Imatinib is also approved at doses of 400 or 600 mg day−1 for malignant, unresectable, or metastatic gastrointestinal stromal tumors (GISTs).Imatinib is commercially available in hard gelatin capsules (100 mg) or tablets (100 mg or 400 mg) in the United States and Europe. Imatinib tablets 400 mg allow for a reduced dosing frequency compared to the 100 mg capsules, which can improve patient compliance, as fewer doses are required per day, meaning managing the medication becomes more convenient for patients. The use of scored tablets provides additional flexibility for dose adjustment and adverse event management. To ensure that the majority of patients achieve drug levels at or above the therapeutic threshold (300 mg day−1), a dose of 400 mg day−1 has been selected for use in a number of phase II clinical studies.3,4 It has been reported that doses higher than 400 mg day−1 may yield improved responses.4 Currently, approximately 70% of patients are initially prescribed 400 mg day−1. Adherence to prescribed regimens is an increasingly important issue in oncology since exposure to less than the required doses can lead to disease recurrence.5 A meta-analysis confirmed there is a slight 2-year progression-free survival (PFS) advantage of high-dose imatinib, but imatinib dose escalation of 800 mg day−1 may not lead to any other major clinical benefits compared to a dose of 400 mg day−1, and may bring about more toxic effects for GIST patients.6
Two new dose forms of imatinib, film-coated 400 mg and 100 mg scored tablets, were recently approved by the US Food and Drug Administration (FDA) and in Europe. Since the tablets are also much smaller than the capsules, patients now have the convenience of being able to take a once daily, single, easy-to-swallow tablet of imatinib. The imatinib tablets are highly soluble and dissolve rapidly in aqueous buffer solutions over a pH range of 1–6.8. The dissolution profiles of the investigational 100 and 400 mg tablets were similar to those of the reference product, as demonstrated by the release of more than 85% of the labeled amount of the drug within 15 min.
Pharmacokinetic studies on the generic imatinib tablet form have indicated that imatinib is rapidly absorbed after oral administration, with Cmax achieved within 2–4 h.7 A mean absolute bioavailability of 98%, and elimination half-life of imatinib and its major metabolite (CGP74588) of approximately 18 and 40 h were reported, respectively. Also, the pharmacokinetics of imatinib were reported to be similar in CML and GIST patients.7 In the present study, the bioequivalence, clinical tolerability, and safety of the test product (Imatinib 400 mg tablets) were compared with the reference innovator product (Glivec, imatinib mesylate 400 mg tablets). The aim of the study was to develop a generic imatinib tablet of 400 mg and determine its in vitro comparability with a branded product (the reference product). Specifically, the study aimed to investigate the bioavailability of a generic product of 400 mg imatinib film-coated tablets (the test product) compared to that of a branded product (the reference product, Glivec tablets 400 mg) at the same strength to determine their bioequivalence. The secondary objective of the study was to evaluate the clinical tolerability and safety of Imatinib 400 mg tablets compared to the innovator product (Glivec, imatinib mesylate 400 mg tablets).
Experimental section
Materials
Hydrochloric acid (37% v/v) was purchased from Rankem Laboratory Chemicals, India. Sodium acetate trihydrate, glacial acetic acid, potassium dihydrogen phosphate, and disodium hydrogen phosphate were sourced from Merck, Germany. Glivec tablets 400 mg were manufactured by Novartis Pharma and were purchased from Malaysia.Methods
Regulatory approval. All experiments were performed in accordance with the Indian Council Medical Research “Guidelines for Bioequivalence Research on Human Subjects” and the ICH “Guidance on Good Clinical Practice” and Declaration of Helsinki (2013), New Drugs and Clinical Trials Rules 2019 G.S.R 227(E) by the Central Drug Standard Control Organization (CDSCO) and Indian Good Clinical Practise Guidelines released by the CDSCO, India. The bioequivalence study protocols were approved by the ethics committee, Drug Controller General (India) and a No Objection Certificate (NOC) was issued. Informed consent was obtained from all human participants in this study.
Subjects, diagnosis, and inclusion criteria. A group of 28 non-smoking, normal, healthy, adult, human subjects aged between 18 and 45 years old (both inclusive), having a body mass index (BMI) between 18.5 and 30.0 kg m−2 (both inclusive), who were able to understand and comply with the study procedures and who gave their written informed consent were enrolled in the study. Following reported methodology for determining the necessary number of study participants,12 it was found that 24 subjects would be sufficient for our bioequivalence study, considering the reported maximum intrasubject variability (%CV) of 15% for Glivec tablets (for Cmax),13 expected maximum difference (test vs. reference product) of 10%, significance level (α value) of 0.05, and desired power (1 − β value) of 80%. Also, considering the possibility of 3–4 subjects dropouts, 28 subjects were finally included in the study. The participants did not have any significant diseases or clinically significant abnormal findings during screening, medical history, clinical examination, laboratory evaluations, 12-lead ECG, or chest X-ray (posterior-anterior view) recordings. Screening was carried out within 28 days prior to the drug administration in Period I. Urine scans for the drugs of abuse and breath tests for alcohol consumption were carried out prior to check-in in each period. Laboratory tests for hematology and liver function tests (Bilirubin Total, SGOT, and SGPT) were performed prior to the check-in in Period II. Laboratory tests for hematology and biochemistry (except random glucose, sodium, potassium, chloride, and alkaline phosphate) were also done at the end of the study (at the time of check-out at the end of Period II) to assess the post-study safety. Axillary body temperature measurements were performed at regular intervals for all the housed subjects in each period. Volunteers who complied with all the inclusion criteria were included in the study.
Restrictions during the study. All the subjects were instructed to abstain from any xanthine-containing food or beverages (tea, coffee, chocolates, or cola drinks, or any other), tobacco, and tobacco-containing products (gutkha, pan/pan masala) for 24 h prior to tablet administration in each period till the last pharmacokinetic sample was collected in each period. All the subjects were instructed to abstain from grapefruit and grapefruit products within 72 h prior to the drug administration in Period I till the last pharmacokinetic sample collection in Period II.
All the subjects were instructed to abstain from alcohol, alcoholic products, and recreational drugs within 48 h prior to the drug administration in Period I till the last pharmacokinetic sample collection in Period II. All the subjects were also instructed to abstain from any unusual diets, for whatever reason (e.g., fasting, high potassium, or low sodium), for four weeks prior to drug administration in Period I and throughout their participation in the study.
Study design. This was an open-label, balanced, randomized, two-treatment, two-sequence, two-period, single oral dose, crossover, bioequivalence study performed in normal, healthy, adult human subjects under fed conditions, with a screening period of 28 days prior to drug administration in Period I. Subjects were selected according to the inclusion and exclusion criteria in order to obtain a low individual variability within the subject group. Two periods were separated by a washout period of 10 days.
Dose and mode of administration. After overnight fasting for at least 10 h, the subjects were served a high-fat, high-calorie vegetarian breakfast, which they consumed completely within 30 min prior to the scheduled drug dosing time on day 1 (Period I) and day 10 (Period II). A single oral dose (400 mg) of either the test product or the reference product was administered 30 min after serving breakfast. The tablet was swallowed as a whole without chewing or crushing. The administration was as per the randomization schedule and under open-label conditions. The tablet was administered to the subjects with 240 ± 2 mL of drinking water at ambient temperature in a sitting posture. The subjects maintained a sitting posture or ambulatory position for the first 4 h after dosing in each period unless medically necessary due to adverse events or procedurally required or for a natural exigency. Subjects refrained from drinking water from 1 h before till 1 h after dosing in each period. The subjects received lunch at least 5 h after dosing in each period. The two periods were separated by a washout period of 10 days.
Blood sampling. In each study period, 23 blood samples, including one pre-dose blood sample, were collected from each subject, except for the withdrawn subjects, to analyze the pharmacokinetic profile of the test as well as the reference product. To determine the plasma concentration of imatinib and its primary metabolite N-desmethyl CGP74588, 5.5 ml of whole blood was drawn from each of the subjects. The time points at which blood was collected in each case were before dosing and 0.5, 1, 1.5, 2, 2.5, 4, 6, 8, 12, 24, 36, 48, 72, and 96 h after dosing. These samples were stored at −18 °C for subsequent evaluation.
Sample processing and bioanalysis. The parameters related to the bioanalytical procedure are presented in Table 1. The blood samples were centrifuged at 3000 rpm for 5 min at 3 °C to separate the plasma. For precautionary purposes, the blood samples were kept in an ice-cold water bath before centrifugation and during separation. The separated plasma was transferred to pre-labeled polypropylene tubes. The samples were stored upright in a freezer at a temperature maintained at −55 °C or below for interim storage till bioanalysis. The plasma samples of the subjects were analyzed using a single validated LC-MS/MS method for imatinib. Calibration curves using a 9-point calibration curve standard, with concentrations ranging from 10.172 to 7001.704 ng mL−1, were used to determine the concentrations of imatinib in the samples of all the analyzed subjects.
| Parameters | Details |
|---|---|
| Samples to be analyzed | Plasma |
| Analyte to be measured | Imatinib |
| Analytical technique | HPLC and LC-MS/MS data systems |
| Equipment | Waters Quattro Premier XE |
| Software | Acquisition and quantification – MassLynx Software Version 4.1 |
| Review of chromatographic data – Biolyte Software Version 1.4 and above | |
| Reporting of the data for statistical analysis | Data transferred through Biolyte software for pharmacokinetic and statistical analysis. |
Pharmacokinetic evaluation and statistical methods. For the pharmacokinetic evaluations, a total of 23 blood samples were collected from each subject in each period at the time points specified in the protocol. The pharmacokinetic parameters for imatinib were determined using a standard non-compartmental model in Phoenix® WinNonlin® Version 8.3 software (Certara L.P.).
Descriptive statistics were calculated and reported for the pharmacokinetic parameters of imatinib. ANOVA, the power, intrasubject variability, and ratio analyses for the ln-transformed pharmacokinetic parameters Cmax, AUC0–t, and AUC0–∞ were calculated and reported for imatinib. The 90% confidence intervals (90% CIs) for the ratio of the geometric least-squares means between the drug formulations were calculated for the ln-transformed pharmacokinetic parameters Cmax, AUC0–t, and AUC0–∞ for imatinib. The 90% CIs for the difference between the least-squares means on the log scale were calculated using Dunnett's test.8 The non-parametric Wilcoxon signed rank test was performed to assess the pharmacokinetic parameter Tmax for imatinib.
The bioequivalence of the Imatinib 400 mg film-coated tablets (Test Product-T) vs. Glivec 400 mg film-coated tablets (Reference Product-R) was concluded if the 90% confidence interval for the ratio of the geometric least-squares means fell within the acceptance range as defined below for the ln-transformed pharmacokinetic parameters for imatinib. According to current FDA guidelines, the bioequivalence criterion for the two dose forms could be considered met if the 90% confidence interval around the ratio of the pharmacokinetic parameters AUC and Cmax was entirely contained in the interval (0.80, 1.25).9
As per regulatory guidelines, data were presented up to three decimal, two decimal, one decimal, and five decimal points, for the concentration, CI interval data, ratios and power, and ANOVA table related to p-values, respectively.
All the statistical analyses for imatinib were performed using the PROC GLM procedure in SAS® Version 9.4 software (SAS Institute Inc., USA).
Safety assessment. Safety was assessed from the screening period to the end of the study, through clinical examination, vital signs assessment, body temperature, 12-lead electrocardiogram (ECG), chest X-ray (posterior-anterior view), clinical laboratory parameters (e.g., hematology, biochemistry, urine analysis, and immunological tests), subjective symptomatology, and monitoring adverse events.
Results and discussion
Formulation details
The composition details of the generic imatinib tablets are presented in Table 2.| Ingredients | % w/w |
|---|---|
| Imatinib mesylate, Ph Eur | 63.717 |
| Microcrystalline cellulose, NF | 18.603 |
| Hypromellose, USP 6 mPa s | 1.333 |
| Colloidal silicon dioxide, NF (Aerosil 200 Pharma) | 0.667 |
| Crospovidone, NF (Type A) | 14.933 |
| Magnesium stearate, NF | 0.747 |
| Opadry Complete Film Coating System 03B665018, Brown, IH | 4.000 |
A comparison of the physical parameters of the test product with the reference product is presented in Table 3.
| Product name | Glivec® tablets, 400 mg | Imatinib tablets, 400 mg |
|---|---|---|
| Shape | Biconvex | Biconvex |
| Color of tablet | Dark yellow to brownish orange | Dark yellow to brownish orange |
| Tablet length and width | 19.0 × 7.7 mm | 19.4 × 7.7 mm |
| Tablet thickness | 6.4 mm | 6.6 mm |
| Tablet weight | Approximately 780.0 mg | Approximately 780.8 mg |
| Score line | Yes | Yes |
In vitro dissolution study
The results from the comparative dissolution studies conducted in all three dissolution media are presented in Fig. 1, 2, and 3. | ||
| Fig. 1 Comparative dissolution profile of imatinib tablets 400 mg and Glivec® 400 mg tablets (imatinib mesylate) in 1000 mL pH 1.2 HCl, at 50 rpm, and at 37 °C using Apparatus II (paddle). | ||
 | ||
| Fig. 2 Comparative dissolution profile of Imatinib tablets 400 mg and Glivec® 400 mg tablets (imatinib mesylate) in 1000 mL pH 4.5 acetate buffer, at 50 rpm, and at 37 °C using Apparatus II (paddle). | ||
From Fig. 3, it is evident that the dissolution profiles of all the generic Imatinib tablets 400 mg were comparable to that of the Glivec tablets 400 mg. Rapid dissolution (>85% release in 15 min) was obtained in both the reference and test products in all three dissolution media; hence, similarity factor calculation was not necessary, and the test product was considered to be similar to the reference product.
Pharmacokinetic study
The pharmacokinetic parameters of imatinib for Test Product-T and Reference Product-R are summarized in Table 4.| Parameters (units) | Mean ± SD (untransformed data) | |
|---|---|---|
| T | R | |
| a T max is represented as the median (min–max) value. | ||
T
max![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (h) a (h) |
3.500 (1.500–6.000) | 3.500 (1.500–6.000) |
| C max (ng mL−1) | 2913.040 ± 1085.039 | 2963.451 ± 1042.450 |
| AUC0–t (ng h mL−1) | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 304.727 ± 18 304.727 ± 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 136.1711 136.1711 |
55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 291.496 ± 20 291.496 ± 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 321.9504 321.9504 |
| AUC0–∞ (ng h mL−1) | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 629.503 ± 19 629.503 ± 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 225.7273 225.7273 |
58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 789.463 ± 21 789.463 ± 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416.7000 416.7000 |
| λz (1 h−1) | 0.038 ± 0.0062 | 0.039 ± 0.0048 |
| t½ (h) | 18.523 ± 3.1690 | 18.095 ± 2.3511 |
The relative bioavailability analyses (i.e., geometric least-squares means, ratio, 90% confidence interval, intrasubject CV, and power) of Test Product-T vs. Reference Product-R for imatinib are summarized in Table 5.
| Parameters | Results | ||
|---|---|---|---|
| Geometric least-squares means | ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cmax Cmax |
T | 2748.484 |
| R | 2792.605 | ||
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AUC0–t AUC0–t |
T | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325.345 325.345 |
|
| R | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 786.201 786.201 |
||
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AUC0–∞ AUC0–∞ |
T | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 467.588 467.588 |
|
| R | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 088.090 088.090 |
||
| Geometric least-squares means ratios (T/R) % | ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cmax Cmax |
98.4 | |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AUC0–t AUC0–t |
95.2 | ||
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AUC0–∞ AUC0–∞ |
95.2 | ||
| 90% confidence interval | ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cmax Cmax |
94.56–102.44 | |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AUC0–t AUC0–t |
91.13–99.56 | ||
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AUC0–∞ AUC0–∞ |
91.31–99.34 | ||
| Intrasubject CV (%) | ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cmax Cmax |
8.3 | |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AUC0–t AUC0–t |
9.1 | ||
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AUC0–∞ AUC0–∞ |
8.7 | ||
| Power (%) | ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cmax Cmax |
100.0 | |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AUC0–t AUC0–t |
100.0 | ||
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AUC0–∞ AUC0–∞ |
100.0 | ||
| Bioequivalence conclusion | ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cmax Cmax |
YES | |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AUC0–t AUC0–t |
YES | ||
As per the approach specified in the protocol, the data of the 25 subjects were analyzed using the ANOVA model with the terms Sequence, Subject (Sequence), Formulation, and Period as fixed effects. The ANOVA p-values for imatinib are summarized in Table 6.
| Parameters | ANOVA (p-value) | ||
|---|---|---|---|
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cmax Cmax |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AUC0–t AUC0–t |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AUC0–∞ AUC0–∞ |
|
| Formulation | 0.5024 | 0.5024 | 0.0596 |
| Sequence | <0.0001 | <0.0001 | <0.0001 |
| Period | 0.5414 | 0.3225 | 0.3395 |
| Subject (sequence) | <0.0001 | <0.0001 | <0.0001 |
| Parameters (units) | Mean | |
|---|---|---|
| N = 28 (dosed subjects) | N = 25 (subjects included in the BE evaluation) | |
| Age (years) | 34.10 ± 5.53 | 34.70 ± 5.31 |
| Height (cm) | 165.64 ± 5.61 | 165.96 ± 5.73 |
| Weight (kg) | 66.51 ± 10.50 | 68.46 ± 9.32 |
| BMI (kg m−2) | 24.24 ± 3.68 | 24.88 ± 3.35 |
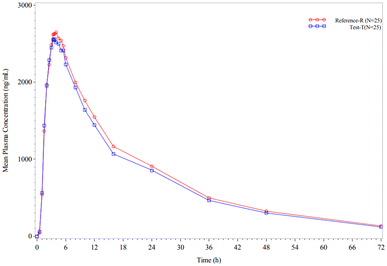
The absorption of imatinib was rapid. The maximum absorption (Cmax) was found to be comparable between the generic imatinib tablets and Glivec tablets 400 mg and was achieved in approximately 3.5 h for both tablets. Comparable values were also obtained for the AUC measurements of imatinib at different time points for Test Product-T and Reference Product-R. The coefficient of variation for Cmax and the AUCs showed considerable intersubject variability. The mean plasma concentration–time profile of Test Product-T (Fig. 4) demonstrated similar kinetics for the appearance of imatinib in plasma after oral administration. The pharmacokinetic values (Cmax, Tmax, and AUC) were similar for both the test and reference dose forms of imatinib. The test/reference ratios obtained for the AUC0–∞, AUC0–t, and Cmax were 95.2, 95.2, and 98.4 (Table 5). The confidence limits for each of these parameters were in the interval (80.00, 125.00). The test/reference ratios of the pharmacokinetic parameters obtained indicated that the generic imatinib tablets were bioequivalent to the Glivec tablets 400 mg.
Safety and tolerability
All the subjects were followed up until the resolution of their AEs. The causality assessment was judged as possible for four AEs and as unlikely for one AE. There were no deaths or serious AEs reported during the conducting of the study. However, out of the total reported five AEs, one AE was significant. The subject was withdrawn from the study on medical grounds, and was treated appropriately and followed up until the resolution of the AE. The causality assessment was judged as unlikely for the AE.
The dissolution profile of all the generic Imatinib 400 mg tablets was comparable to the Glivec tablets 400 mg. More than 85% release was obtained in 15 min for both formulations in multimedia (pH 1.2 to pH 6.8); hence, F2 calculation was not required, and the dissolution profile of the Imatinib 400 mg tablets was similar to the Glivec tablets 400 mg. The current study demonstrated comparable bioavailability of the imatinib 400 mg tablets and generic imatinib 400 mg tablets. The pharmacokinetic values (Cmax, Tmax, and AUC) were also found to be nearly identical for both the test and reference dose forms of imatinib. The in vitro dissolution tests indicated that the tablet forms of imatinib could dissolve easily over the same pH range (1.2–6.8). This study indicated the rapid absorption of the 400 mg reference tablet (median Tmax 3.5 h) after oral administration, which was comparable with that of the generic tablet. Taken together, these results demonstrate that imatinib is as highly soluble and as rapidly absorbed in tablet form. The coefficient of variation for Cmax and the AUCs showed considerable intersubject variability. Although the cause of this was not clear, it may be attributed to intersubject differences in plasma proteins binding to the parent compound or to variations in CYP3A4, the major CYP isoenzyme involved in the microsomal metabolism of imatinib. The variability in CYP3A activity between individuals was large, and may in part have contributed to the large intersubject variability.10 In this study, the adverse effects of imatinib administration were closely monitored and found to have a similar distribution between the test product and reference product. One of these effects was clinically significant. However, the causality assessment was judged as unlikely for the AE. Overall, five adverse events were reported in 4 out of 28 subjects, and only four were considered to be related to the administration of imatinib. These drug-related adverse events were either mild or moderate in severity and included low-grade headache, nausea, and vomiting. The types of adverse events observed in this study are consistent with those reported in other studies.11 The data presented in this study clearly demonstrate the safety and tolerability of the 400 mg formulation of Test Product-T.
Conclusion
The dissolution study results indicated that the in vitro dissolution profile of generic Imatinib tablets 400 mg was comparable to that of Glivec tablets 400 mg. The results of this single-dose study in healthy adult subjects indicated that Imatinib 400 mg film-coated tablets (Test Product-T) are bioequivalent to Glivec 400 mg film-coated tablets manufactured by Novartis Pharma GmbH (Reference Product-R). Test Product-T, compared with Reference Product-R, met the bioequivalence criteria with respect to Cmax and AUC0–t for imatinib under the fed conditions. The data from this study also demonstrated that the test and the reference products were well tolerated. Five adverse events (AEs) were reported by 4 subjects during the conduct of the study, out of which one AE was significant. There were no deaths or serious AEs reported during the conducting of this study. There were no clinically significant findings in the vital sign assessment or the laboratory tests in any of the subjects in the study, except for one subject, who had abnormal laboratory values during the post-study safety assessment. An adverse event was recorded for the same, and the subject was followed up until the resolution of the AE. In conclusion, the test and reference formulations of imatinib met the regulatory criteria for bioequivalence based on their rate and extent of absorption. Based on the findings of this study, it can be concluded that the product is bioequivalent and safe, thus suggesting the possible clinical application of the test product as an alternative to Glivec 400 mg film-coated tablets.Data availability
All the relevant data have been presented in the article, and the raw data will be available from the authors upon request.Conflicts of interest
The authors declare that there is no financial or non-financial conflict of interest with this research article.Acknowledgements
We thank Lambda Therapeutic Research for the clinical study and bioanalysis of the blood of volunteers and the raw data analysis.References
- E. Buchdunger, C. L. Cioffi, N. Law, D. Stover, S. Ohno-Jones, B. J. Druker and N. B. Lydon, J. Pharmacol. Exp. Ther., 2000, 295, 139–145 CrossRef CAS.
- K. Peggs and S. Mackinnon, N. Engl. J. Med., 2003, 348, 1048–1050 CrossRef PubMed.
- B. J. Druker, M. Talpaz, D. J. Resta, B. Peng, E. Buchdunger, J. M. Ford, N. B. Lydon, H. Kantarjian, R. Capdeville and S. Ohno-Jones, N. Engl. J. Med., 2001, 344, 1031–1037 CrossRef CAS PubMed.
- M. W. Deininger, S. G. O′brien, J. M. Ford and B. J. Druker, J. Clin. Oncol., 2003, 21, 1637–1647 CrossRef CAS PubMed.
- A. H. Partridge, J. Avorn, P. S. Wang and E. P. Winer, J. Natl. Cancer Inst., 2002, 94, 652–661 CrossRef PubMed.
- P. Yang, B. Chen, Y. Zhou and X.-T. Wu, Clin. Res. Hepatol. Gastroenterol., 2012, 36, 484–490 CrossRef CAS PubMed.
- P. Traxler, G. Bold, E. Buchdunger, G. Caravatti, P. Furet, P. Manley, T. O'Reilly, J. Wood and J. Zimmermann, Med. Res. Rev., 2001, 21, 499–512 CrossRef CAS PubMed.
- C. W. Dunnett, J. Am. Stat. Assoc., 1955, 50, 1096–1121 CrossRef.
- F. Guidance, Center for Drug Evaluation and Research, 2001 Search PubMed.
- G. R. Wilkinson, J. Pharmacokinet. Biopharm., 1996, 24, 475–490 CrossRef CAS PubMed.
- G. D. Demetri, M. Von Mehren, C. D. Blanke, A. D. Van den Abbeele, B. Eisenberg, P. J. Roberts, M. C. Heinrich, D. A. Tuveson, S. Singer and M. Janicek, N. Engl. J. Med., 2002, 347, 472–480 CrossRef CAS PubMed.
- S. C. Chow and H. Wang, J. Pharmacokinet. Pharmacodyn., 2001, 28, 154–169 Search PubMed.
- R. Arora, M. Sharma, T. Monif and S. Iyer, Cancer Res. Treat., 2016, 48, 1120–1129 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |