Open Access Article
Open Access ArticleBabassu oil-based microemulsion promotes uniform in vitro release of diclofenac sodium and donepezil hydrochloride†
Felipe Schlichta de
Gouveia
 ab,
Gabriela
Spingolon
ac and
Tanira Alessandra Silveira
Aguirre
ab,
Gabriela
Spingolon
ac and
Tanira Alessandra Silveira
Aguirre
 *ab
*ab
aGrupo de Pesquisa em Nanomedicina – TANANO, Universidade Federal de Ciências da Saúde de Porto Alegre, Porto Alegre, RS, Brasil. E-mail: tanira@ufcspa.edu.br; Tel: +55 51 3303.8821
bPrograma de Pós-Graduação em Biociências, Universidade Federal de Ciências da Saúde de Porto Alegre, Porto Alegre, RS, Brasil
cPrograma de Pós-Graduação em Química, Instituto de Química – Campus Vale, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brasil
First published on 12th June 2025
Abstract
Microemulsions are nanostructured and thermodynamically stable systems with a reduced droplet size. They can improve drug absorption and distribution. Babassu oil has been investigated for various therapeutic properties reported by popular use. This work aimed to develop, optimize, and characterize babassu oil-based water-in-oil microemulsions to promote a controlled and uniform release of drugs with different physicochemical properties. The optimal microemulsion composition was investigated through pseudoternary diagrams, with water, surfactant mixture, and oil mixture as vertices. The formulations were characterized based on pH, conductivity, size, polydispersity index, and drug content. In vitro drug release was carried out using multidimensional and unidimensional techniques. An optimum microemulsion contains (w/w): 10% water, 17.8% babassu oil, 26.2% medium-chain triglycerides, 29.7% Span™ 83, 7.1% Tween® 80, and 9.2% Transcutol® HP. Diclofenac sodium (DS), donepezil hydrochloride (DH), and insulin were associated with the dispersed phase of the microemulsion. These formulations presented a droplet size of 26.9 ± 1.9, 22.6 ± 0.4, and 35.5 ± 0.7 nm, respectively. The polydispersity index was <0.1 for all formulations. Microemulsions controlled the outflow of drugs, showing a uniform release compared to the respective controls. It was evidenced that these profiles depend on the features of the molecule associated. According to the selection criteria, most experimental DS and DH release kinetics in water or pH 7.2 fit well with the Gompertz model. In conclusion, a babassu oil-based water-in-oil microemulsion was developed for the first time, optimized, and characterized, supporting further investigation of the formulation as a drug delivery system.
1. Introduction
Potency and efficacy are important characteristics of drugs. However, due to intrinsic properties, many substances have low bioavailability when administered by non-parenteral routes. These drugs present particular difficulties in crossing some of the body's barriers; for example, for transdermal delivery, the skin is a major biological obstacle for the drug intended to reach the circulatory system. Incorporating drugs into different carriers can optimize the delivery and improve drug efficacy. Nanotechnology applied to drug delivery aims to develop solutions for delivering drugs using nanocapsules,1 nanoparticles,2 nanoemulsions,3 microemulsions,4 and other carriers. These nanosystems can increase efficacy, stability, and bioactivity and decrease the side effects and toxicity of the drugs.Microemulsions are nanostructured systems consisting of two immiscible liquids (oil and water) and are stabilized by a layer of surfactants and, generally, a co-surfactant or electrolyte.5 They are thermodynamically stable, self-organizing nanostructures and isotropic systems.6,7 These systems are optically translucent due to their reduced droplet size, less than 100 nm,8 so the light can cross through without diffraction.9 The reduced droplet size of the microemulsion promotes better absorption and distribution of a drug compared to traditional formulations, which improves efficiency. There are several microemulsion formulations that require low-energy methods to prepare them, and industrial production can be easily scaled-up. Microemulsions can be applied through many administration routes, such as ocular,10 nasal,11 oral,12 topical,13 transdermal,14 parenteral,15 vaginal,16 and rectal.17 Neoral® (Novartis, UK) is an example of a commercially available microemulsion, an oral formulation of cyclosporin A encapsulated into a microemulsion system.18 Another example is Topicaine® (ESBA Labs, USA), a microemulsion loaded with 4% lidocaine for transdermal applications.19
Babassu oil (BBS, Attalea speciosa Mart ex. Spreng) is a vegetable extract from almonds belonging to the Arecaceae family and the Orbignya and Attalea genera.20,21 It is a product of Brazilian origin, obtained from a renewable source and generating a sustainable economy for a significant portion of families in the state of Maranhão.22 BBS has been investigated as having anti-inflammatory, antitumor, antimicrobial, and healing pharmacological effects.23,24 Some applications are scientifically proven, while others are known by traditional knowledge of people and communities. The anti-inflammatory activity of BBS was previously reported by Reis et al., and they demonstrated that it was improved by a microemulsion formulation.25 The use and study of this oil as a component in pharmaceutical formulations are justified by its composition based on different small fatty acids: lauric (dodecanoic) and myristic (tetradecanoic) acids are present in higher proportions.26 The fatty acids confer characteristics, such as resistance to non-enzymatic oxidation, low melting point, low molecular weight, and amphiphilic properties, to babassu oil.27,28 Lauric acid, for example, has proven to have absorption-promoting effects in the permeation of opioids and, consequently, the ability to increase drug permeability topically.27 More than 50% of BBS is lauric and myristic acids;29,30 therefore, this oil presents the required elevated hydrophilic–lipophilic balance, which helps in its interaction with polar regions of drugs.26 Furthermore, the use of BBS oil in formulations means to incorporate a natural product obtained from renewable sources to afford sustainable technologies. This is in accordance with the 2030 Agenda for Sustainable Development agreed upon by the member states of the United Nations, and it meets several of its proposed goals (3, 8, 9, and 15).31
There are three main different types of microemulsions: the oil-in-water (O/W), in which droplets of oil are dispersed in an aqueous phase; water-in-oil (W/O), in which droplets of water are dispersed in a lipophilic continuous phase; and the bicontinuous also called middle-phase microemulsions.32 Pessoa et al. and Reis et al., respectively, presented biological activity in vitro and in vivo of oil-in-water microemulsions containing ≤12% of BBS as a dispersed phase. They demonstrated increased release of superoxides from human blood phagocytes by babassu oil ME, and increased anti-inflammatory activity in mouse ear edema by microemulsified babassu oil. Microemulsions can be formed using components that play more than one role within the system (multifunctional component). Typically, the primary function of a multifunctional component in a microemulsion is to contribute to the system's structure, while its secondary role is to impart an additional activity to the formulation.3,33 Olive oil can be used as a multifunctional component in a W/O microemulsion, for example. These formulations have many applications: skin hydration, active ingredient release, encapsulation matrix, and drug stability.34–36 Besides its use as a therapeutic agent of BBS, this substance has been demonstrated to have action as a permeation enhancer.30 Therefore, the presence of babassu oil as the external phase may be an interesting feature of a drug delivery system in which small drugs and biomolecules can be encapsulated within the aqueous internal phase of the system, and the oil is available to exert its therapeutic effect. To our knowledge, this is the first report of a water-in-oil microemulsion containing babassu oil.
Despite the widespread clinical use of diclofenac sodium (DS), donepezil hydrochloride (DH), and insulin, their delivery methods still present limitations that impact patient adherence. DS is a non-steroidal anti-inflammatory drug (NSAID) commonly used to treat inflammatory conditions. It has a short half-life and typically requires frequent administration. This regimen can lead to gastrointestinal irritation in some patients.37 Although transdermal delivery is a potential alternative for DS, the plasma concentration after topical application remains typically under 10% of oral dosing.38–40 DH is prescribed for the treatment of Alzheimer's disease, and is most commonly administered orally. However, due to the long duration of therapy, gastrointestinal side effects are frequently reported.41 Additionally, its rapid absorption after oral administration may exacerbate these adverse effects.42 While transdermal formulations of donepezil are available on the market, their pharmacokinetic profiles differ from oral forms and exhibit high interindividual variability.43 Insulin is a macromolecular drug, requiring subcutaneous injections due to enzymatic degradation in the gastrointestinal tract and poor membrane permeability, complicating adherence and patient compliance.44
Water-in-oil (W/O) microemulsions represent a promising strategy for developing delivery systems with sustained and uniform drug release. Their nanometric size, thermodynamic stability, and biphasic structure enable efficient encapsulation, protection, and controlled release of both hydrophilic45 and lipophilic46 drugs. These characteristics can enhance therapeutic efficacy, minimize side effects, and ultimately improve patient adherence across a range of pharmacological treatments.
Therefore, this work aims to develop and characterize babassu oil-based water-in-oil microemulsions to promote a controlled and uniform release of drugs. Additionaly, to demonstrate the versatility of the formulation in incorporating drugs with different hydrophilicity and sizes.
2. Materials and methods
2.1. Materials
The materials that compose the formulations are Babassu oil (Attalea speciosa Mart ex. Spreng, Mundo dos Óleos, Brazil), ultra-pure water (MilliQ®), medium-chain triglycerides (MCT) (caprylic and capric acids, Delaware, Brazil), sorbitan sesquioleate (Span™ 83, Sigma-Aldrich, Brazil), sorbitan monooleate (Span™ 80 – Sigma-Aldrich/MKCK7756), polysorbate 80 (Tween® 80, Sigma-Aldrich, Brazil), 2-(2-ethoxyethoxy) ethanol (Transcutol® HP, Gatefossé, France), diclofenac sodium (Infinity pharma, China), donepezil hydrochloride (Ambeed, USA), and insulin (Sigma-Aldrich, Switzerland). Babassu oil study was registered at SisGen (identification ABCECDB).2.2. Experimental methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2.95
1 to 2.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio or Tween® 80 + Span™ 83, ranging from 0.41
1 ratio or Tween® 80 + Span™ 83, ranging from 0.41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3.17
1 to 3.17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio), water, and BBS. The final volume of the emulsions was set at 10 mL, with 85% (w/w) consisting of water, 5% (w/w) babassu oil, and 10% (w/w) a blend of two surfactants. The blend's individual portions of the surfactants determine the oil affinity by exploring their proportions. For this purpose, quantities of Tween® 80 were dissolved in the aqueous portion of the formulations, while Span™ 80 or Span™ 83 was solubilized in the oil. Both phases were dissolved and maintained at 60 °C under magnetic stirring at 750 rpm. After solubilization, the aqueous phase was injected into the oily phase, and the obtained formulation was homogenized using an Ultra Turrax® disperser (8000 rpm) for 5 min.23 The stability of the formulations was evaluated by observing the absence of phase separation for 7 days.
1 ratio), water, and BBS. The final volume of the emulsions was set at 10 mL, with 85% (w/w) consisting of water, 5% (w/w) babassu oil, and 10% (w/w) a blend of two surfactants. The blend's individual portions of the surfactants determine the oil affinity by exploring their proportions. For this purpose, quantities of Tween® 80 were dissolved in the aqueous portion of the formulations, while Span™ 80 or Span™ 83 was solubilized in the oil. Both phases were dissolved and maintained at 60 °C under magnetic stirring at 750 rpm. After solubilization, the aqueous phase was injected into the oily phase, and the obtained formulation was homogenized using an Ultra Turrax® disperser (8000 rpm) for 5 min.23 The stability of the formulations was evaluated by observing the absence of phase separation for 7 days.
Once the first formulation was deemed stable, the remaining ones were designed considering their surfactant proportions in the blend. Thus, the hydrophilic or lipophilic tendency of the oil is determined by the relationship between the sum of the products of the mass of surfactants by their corresponding calculated rHLB values over the total mass of the surfactant mixture, according to eqn (1).
 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Transcutol®
Transcutol®![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tween® 80–6.5
Tween® 80–6.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) and an oil mixture (MCT
1.5) and an oil mixture (MCT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BBS – 6
BBS – 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), both having an HLB close to 6. The sample composition with longer visual stability (ME-blank) was used for the incorporation of the drugs: diclofenac sodium (ME-DS), donepezil hydrochloride (ME-DH), and insulin (ME-IN).
4), both having an HLB close to 6. The sample composition with longer visual stability (ME-blank) was used for the incorporation of the drugs: diclofenac sodium (ME-DS), donepezil hydrochloride (ME-DH), and insulin (ME-IN).
Aqueous solutions of the drugs diclofenac sodium, and donepezil hydrochloride, were prepared at a concentration of 5 mg mL−1. Insulin was solubilized in HCl 0.001 mol L−1 at a concentration of 1 mg mL−1. These solutions were used to prepare drug-loaded microemulsions (Fig. 2) at diclofenac sodium, donepezil hydrochloride, and insulin concentrations of 0.5, 0.5, and 0.1 mg mL−1, respectively.
Each microemulsion sample (1 g) was produced using the simple combination of elements method, which consists of quantitatively adding and mixing different concentrations of each component.
2.2.3.1. Conductivity and apparent pH. The apparent pH of blank and drug-loaded formulations was measured directly in the samples (n = 3, for each formulation). The instrument (pHmeter model 86505, AZ Instrument Corp.) was calibrated using standard solutions of pH 4.0, 7.0, and 10.0.
The conductivity of the formulations was measured directly in the samples (n = 3, for each formulation) using the same equipment calibrated for conductivity. The range used for the reading was from 0 to 199.9 μS cm−1.
2.2.3.2. Droplet size and polydispersity index. The droplet size diameter and polydispersity index were determined by dynamic light scattering (DLS) (ZetaSizer® ZS, Malvern Instruments, UK) with a laser of 633 nm, and the detectors were placed at 173° angle to detect backscattering. The sample was stabilized at 32 °C for 5 min, using a refractive index of 1.445 (external phase) and 1.330 (internal phase). The analyses were carried out in triplicate for each formulation.
2.2.3.3. Diclofenac sodium and donepezil hydrochloride quantification. A high-performance liquid chromatography (HPLC) methodology based on the Brazilian Pharmacopeia was employed for diclofenac sodium analysis.47 The mobile phase was composed of methanol and phosphate buffer pH 3 (70
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30), C-18 column (150 × 3.9 mm × 4 μm, Waters NovaPak) as the stationary phase, a flow rate of 1 mL min−1, 40 °C oven temperature, injection volume of 20 μL, retention time of 3.1 min, run time of 6 min and the analysis wavelength of 276 nm. For the drug content assay, the formulation samples (40 μL) were previously treated with ultrapure water (4960 mL), vortexed for 3 min, filtered with a syringe filter (PVDF, 0.45 μm), transferred to a vial, and injected.
30), C-18 column (150 × 3.9 mm × 4 μm, Waters NovaPak) as the stationary phase, a flow rate of 1 mL min−1, 40 °C oven temperature, injection volume of 20 μL, retention time of 3.1 min, run time of 6 min and the analysis wavelength of 276 nm. For the drug content assay, the formulation samples (40 μL) were previously treated with ultrapure water (4960 mL), vortexed for 3 min, filtered with a syringe filter (PVDF, 0.45 μm), transferred to a vial, and injected.
For donepezil hydrochloride analysis, an isocratic HPLC methodology was developed using a mobile phase composed of phosphate buffer (pH 2) and acetonitrile (71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29), a C-18 column (150 × 4.6 mm × 5 μm) as the stationary phase, a flow rate of 1 mL min−1, 30 °C oven temperature, an injection volume of 20 μL, a retention time of 4.57 min, a run time of 8 min and an analysis wavelength of 268 nm. For the drug content assay, 40 μL of formulation samples were previously treated with 4960 mL of ultrapure water
29), a C-18 column (150 × 4.6 mm × 5 μm) as the stationary phase, a flow rate of 1 mL min−1, 30 °C oven temperature, an injection volume of 20 μL, a retention time of 4.57 min, a run time of 8 min and an analysis wavelength of 268 nm. For the drug content assay, 40 μL of formulation samples were previously treated with 4960 mL of ultrapure water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (1
methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and vortexed for 2 min. Then, the samples were filtered with a syringe filter (PVDF, 0.45 μm), transferred to a vial, and injected.
1) and vortexed for 2 min. Then, the samples were filtered with a syringe filter (PVDF, 0.45 μm), transferred to a vial, and injected.
Both methods were validated in terms of specificity, linearity, precision, and accuracy.
2.2.3.4. Attenuated total reflectance Fourier-transform infrared (ATR-FTIR) spectroscopy. Fourier transform infrared (FT-IR) was used to assess the existence of any incompatibility between drugs and excipients. This technique was carried out using IR Prestige-21 (Shimadzu, Japan), and the sample absorbance was determined in the region of 4000 to 600 cm−1 at room temperature. The KBr pellet was prepared at a ratio of 99
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of KBr
1 of KBr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sample, using a force of 80 kN for the pure drugs diclofenac sodium, donepezil hydrochloride, and insulin. The ATR-8200HA (Shimadzu, Japan) accessory was used to measure the infrared spectrum of the liquid samples (ME-blank, ME-DS, ME-DH, and ME-IN) using 1–2 mL for each assay, through the attenuated total reflectance (ATR) technique.
sample, using a force of 80 kN for the pure drugs diclofenac sodium, donepezil hydrochloride, and insulin. The ATR-8200HA (Shimadzu, Japan) accessory was used to measure the infrared spectrum of the liquid samples (ME-blank, ME-DS, ME-DH, and ME-IN) using 1–2 mL for each assay, through the attenuated total reflectance (ATR) technique.
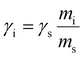 | (2) |
2.2.5.1. Multidimensional dialysis (MD) assay. This assay used a modified cellulose membrane (cut-off 14 kDa, Sigma-Aldrich). The dialysis bag with the formulation (2 mL) was immersed in a medium (55 mL) under constant magnetic stirring at 37 °C. Successive samples of the medium (1 mL) were collected until a maximum time of 72 h, and the same amount withdrawn from the medium was replenished after each sampling. Ultrapure water and phosphate buffer pH 7.2 were used as a medium for the ME-DS and ME-DH formulations, respectively.
An aqueous solution of respective drugs was prepared in the same microemulsion concentration (control group) for both formulations. Successive samples of the medium (1 mL) were collected until a maximum time of 48 h for DS solution, and 24 h DH solution, and the same amount withdrawn was replenished after each sampling.
2.2.5.2.Unidimensional dialysis (UD) assay. The UD assay was carried out using Franz cells mounted with a modified cellulose membrane (cut-off 14 kDa, Sigma-Aldrich). An aliquot of formulations (0.2 mL) was added to the superior part of the cell. The basolateral side of the cell was filled with 14 mL of media under constant magnetic stirring at 32 °C. Successive samples (1 mL) were collected until a maximum time of 72 h, and the same amount was replenished after each sampling. Ultrapure water and phosphate buffer of pH 7.2 were used as a medium for ME-DS and ME-DH formulations, respectively.
For both formulations, an aqueous solution of respective drugs was prepared in the same concentration of the microemulsion (control group). Successive samples of the medium were collected (1 mL) until a maximum time of 24 h for DS solution and 4 h DH solution, the same amount withdrawn was replenished after each sampling.
3. Results and discussion
3.1. Characterization of babassu oil
In the determination of the rHLB of babassu oil, the employed methodology proposes that the proportions of different surfactants with known HLB values, when adjusted correctly, lead to the stabilization of the emulsion and indicate the hydrophilic or lipophilic tendency of the oil. When the HLB value of the surfactants closely matches the rHLB value of the oil, the surfactant molecules will be packed more tightly at the oil–water interface, leading to a minimum free-energy in the system. Considering that babassu oil comprises small fatty acids acting as amphiphilic molecules, it is worth noting that it can interact with both types of surfactants utilized in this study. The results for rHLB determination of BBS using Tween® 80 + Span™ 80 mixtures are shown in Table 1. With the restriction of the rHLB range of the oil between 11 and 12, three formulations showed stability, with calculated rHLB values of 11.56 (formulation 4), 11.70 (formulation 5), and 11.87 (formulation 6).| Formulation | Tween® 80 (%) | Span™ 80 (%) | Ratio Tween® 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Span™ 80 Span™ 80 |
Calculated HLB |
|---|---|---|---|---|
| a Refer to stable formulations. | ||||
| 1 | 64.99 | 35.01 | 1.86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11.25 |
| 2 | 65.98 | 34.02 | 1.94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11.36 |
| 3 | 67.05 | 32.95 | 2.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11.47 |
| 4a | 67.85 | 32.15 | 2.11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11.56 |
| 5a | 69.12 | 30.88 | 2.24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11.70 |
| 6a | 70.74 | 29.26 | 2.42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11.87 |
| 7 | 71.99 | 28.01 | 2.57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12.00 |
| 8 | 73.10 | 26.90 | 2.72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12.12 |
| 9 | 73.69 | 26.31 | 2.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12.18 |
| 10 | 74.67 | 25.33 | 2.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12.29 |
The region for stable formulations (over 7 days) found in this study is consistent with the data reported in the literature for the same methodology using the same pair of surfactants,3 which determines the rHLB of BBS between 11 and 12.
Tween® 80 and Span™ 80 are nonionic surfactants both containing the same fatty acid tail formed of oleic acid. This leads to well packed interfacial film formation, reducing surface tension and improving emulsion stability. While Span™ 80 stabilizes the oil phase of the emulsion, Tween® 80 stabilizes the aqueous phase. Therefore, this pair is often used for rHLB determination of oils. However, in order to form a water-in-oil microemulsion, Span™ 83 was used in this work, because it has a lower HLB (3.7 vs. 4.3 for Span™ 80).
The rHLB of babassu oil was experimentally determined using mixtures of Tween® 80 and Span™ 83 (Table 2). Formulations with HLB values ranging from 6.98 to 12.28 were prepared; the samples with rHLB > 9 destabilize just after preparation. The most stable formulations were 1, 2, and 3 (with rHLB values between 6.98 and 7.54), which suggests that the rHLB of BBS in the microemulsion system is 7–7.5.
| Formulation | Tween® 80 (%) | Span™ 83 (%) | Ratio Tween® 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Span™ 83 Span™ 83 |
Calculated HLB |
|---|---|---|---|---|
| a Refer to stable formulations. | ||||
| 1a | 29.0 | 71.0 | 0.41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6.98 |
| 2a | 33.0 | 67.0 | 0.49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.43 |
| 3a | 44.0 | 56.0 | 0.79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.54 |
| 4 | 36.5 | 63.5 | 0.57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.82 |
| 5 | 38.0 | 62.0 | 0.61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.99 |
| 6 | 40.0 | 60.0 | 0.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8.22 |
| 7 | 42.0 | 58.0 | 0.72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8.45 |
| 8 | 47.0 | 53.0 | 0.89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9.01 |
| 9 | 52.0 | 48.0 | 1.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9.58 |
| 10 | 56.0 | 44.0 | 1.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10.03 |
| 11 | 60.0 | 40.0 | 1.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10.48 |
| 12 | 67.0 | 33.0 | 2.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11.27 |
| 13 | 69.5 | 30.5 | 2.28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11.55 |
| 14 | 71.0 | 29.0 | 2.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11.72 |
| 15 | 72.5 | 27.5 | 2.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11.89 |
| 16 | 76.0 | 24.0 | 3.17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12.28 |
The results showed that the rHLB of BBS oil varies depending on the surfactant pair used: 11–12 for Span™ 80 and 7–7.5 for Span™ 83. Both surfactants are sorbitan esters derived from sorbitol (sugar) and fatty acids, but they differ in the type of fatty acid and consequently in their properties. Span™ 80 (span monooleate) contains oleic acid, while Span™ 83 (span sesquioleate) contains a mixture of sorbitan esters, with a combination of mono and di-oleate structures, the sesquioleate indicates that the sorbitan molecule is esterified with 1.5 oleic acid molecules. For systems formed of surfactant–water–oil as the emulsions prepared for rHLB determination, the oil and water domains are separated by an interfacial surfactant monolayer. This film divides the space into volume fractions required by the three constituents while optimizing the packing of the amphiphilic molecules within the film. The packing parameter of the interfacial film mainly depends on the chemical structure of the surfactant and the ability of oil molecules to penetrate more or less deeply into the surfactant layer.49 Therefore, the rHLB value for BBS depends on the surfactants used, and an experimental approach for the determination of its rHLB value would be recommended for each formulation.
With a defined rHLB range using Tween® 80 and Span™ 83, it was possible to support the development of stable microemulsions. Our group previously characterized the same oil used here regarding fatty acid composition.26 Lauric and myristic acids are the major unsaturated components of this oil, accounting for 61.4% (w/w), and this composition probably leads to an elevated rHLB value due to short apolar chains of these molecules.
3.2. Development of microemulsions
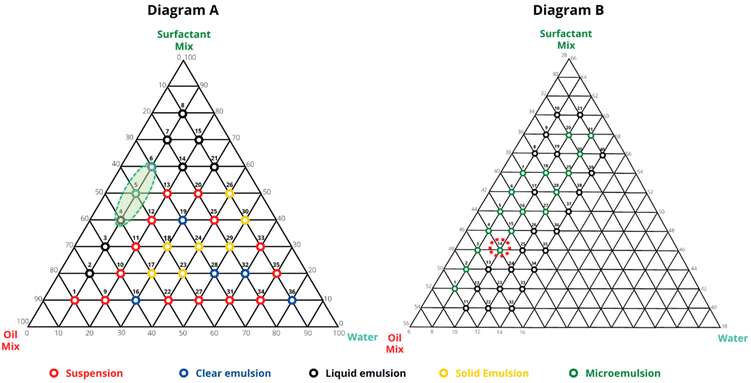
The first diagram was used to make a general scan of all the proportions, so 36 samples were prepared, varying the concentration of the vertices from 10 to 10% (Fig. 1A and Table S1†). They were classified by visual appearance into six emulsified systems: phase separation, clear emulsion, liquid emulsion, solid emulsion, and microemulsion. Through these samples, a microregion located around the 5A sample was identified (region demarcated in Diagram A of Fig. 1), that seemed promising to form microemulsions. Then the second diagram (Fig. 1B and Table S2†) aimed at amplifying the investigation of this delimited region, whereby more 40 samples were prepared between 8 to 14% water, 30 to 50% oil mixture, and 40 to 60% surfactant mixture. Among these samples, two stood out as promising microemulsions, 3B (8% water 46% oil mixture, and 46% surfactant mixture) and 14B (10% water, 44% oil mixture, and 46% surfactant mixture), so they were subjected to a visual stability test over 10 days. As a result, the 14B formulation (Fig. 2) proved to present longer stability, therefore, it was selected for further studies. The microemulsions were prepared by a low energy method, in which the components were mixed together in a specific order, and the system was spontaneously organized. This is an advantage over other methods, such as high pressure homogenization, which requires more energy and are expensive in terms of scale up production.
3.3. Physicochemical characterization of microemulsions
The aim of this study was to develop water-in-oil (W/O) microemulsions using babassu oil as the external phase, with the goal of demonstrating the formulation's versatility in incorporating drugs with varying hydrophilicity and molecular sizes. Diclofenac sodium, donepezil hydrochloride, and insulin were selected as model compounds due to their distinct physicochemical properties, representing a spectrum of drug types that challenge conventional delivery systems.Diclofenac sodium, which exhibits moderate to high aqueous solubility, particularly at neutral to basic pH, was chosen as a representative small hydrophilic molecule. Therefore, it was incorporated into the internal aqueous phase of the microemulsion (ME-DS) to assess its influence, mainly, on droplet size and release profile. To further explore the system's versatility, donepezil hydrochloride was selected as the second small molecule with differing characteristics. Although not classically categorized as a model lipophilic drug (it is a BCS Class I drug), it has a comparable molecular weight (∼379.5 g mol−1vs. 318.1 g mol−1 for diclofenac sodium) and a higher predicted log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D value (2.48 vs. 1.10 for diclofenac sodium),52 indicating significant affinity for nonpolar environments. However, it presents moderate water solubility at the tested concentration. Therefore, donepezil hydrochloride was also incorporated into the internal phase of the microemulsion (ME-DH) to enable direct comparison without altering the phase of drug incorporation. Insulin, a hydrophilic macromolecule with high aqueous solubility, was used to evaluate the system's capacity to encapsulate large biomolecules (5778 g mol−1). Like the other two compounds, insulin was included in the internal phase (ME-IN), facilitating a comparative analysis of the physicochemical characteristics of the microemulsion across a range of drug types.
D value (2.48 vs. 1.10 for diclofenac sodium),52 indicating significant affinity for nonpolar environments. However, it presents moderate water solubility at the tested concentration. Therefore, donepezil hydrochloride was also incorporated into the internal phase of the microemulsion (ME-DH) to enable direct comparison without altering the phase of drug incorporation. Insulin, a hydrophilic macromolecule with high aqueous solubility, was used to evaluate the system's capacity to encapsulate large biomolecules (5778 g mol−1). Like the other two compounds, insulin was included in the internal phase (ME-IN), facilitating a comparative analysis of the physicochemical characteristics of the microemulsion across a range of drug types.
The formulations ME-blank (sample 14B), ME-DS, and ME-DH were subjected to physicochemical characterization studies regarding conductivity, pH, droplet size, polydispersity index (PDI), and drug content. ME-IN was characterized for conductivity, pH, droplet size and PDI. All results are summarized in Table 3 and triplicates are shown in Table S3.†
| Apparent pH | Conductivity (μS cm−1) | Drug content (mg mL−1) | Droplet size (nm) | Polydispersity index | |
|---|---|---|---|---|---|
| Differences between means were analyzed by ANOVA one way and test t-Student. a – different from ME-blank at p < 0.05 in the same table column; b – different from ME-blank at p < 0.001 in the same table column; c – different from ME-DS at p < 0.01 in the same table column; d – different from ME-DS at p < 0.001 in the same table column; e – different form ME-DH at p < 0.001 in the same table column; f – statistically equal at p > 0.05 in the same table column. | |||||
| ME-blank | 7.49 ± 0.01 | 1.8 ± 0.1 | — | 27.9 ± 0.7 | 0.06 ± 0.04f |
| ME-DS | 7.72 ± 0.07 | 1.2 ± 0.2b | 0.46 ± 0.04f | 26.9 ± 1.9 | 0.09 ± 0.05f |
| ME-DH | 7.80 ± 0.19a | 1.0 ± 0.1b | 0.53 ± 0.04f | 22.6 ± 0.4b,c | 0.09 ± 0.04f |
| ME-IN | 5.10 ± 0.10b,d,e | 0.2 ± 0.1b,d,e | — | 35.5 ± 0.7b,d,e | 0.04 ± 0.02f |
As the microemulsions are water-in-oil systems, the pH cannot be measured accurately, therefore, an apparent pH will be reported for comparison. The formulation ME-blank showed a neutral apparent pH (7.49 ± 0.01), ME-DS showed no statistical difference compared to ME-blank. Donepezil hydrochloride contains a tertiary amine in its structure, therefore for ME-DH the drug incorporation increased the apparent pH compared to the blank formulation (p < 0.05). Insulin was solubilized under acidic conditions, leading to a decrease in the apparent pH in ME-IN formulation (p < 0.001). Conductivity values for formulations ME-blank, ME-DS, ME-DH, and ME-IN were 1.8 ± 0.1, 1.2 ± 0.2 1.0 ± 0.1, and 0.2 ± 0.1 μS cm−1 respectively. The ME-blank was statistically different (p < 0.001) from ME-DS, ME-DH, and ME-IN. But there was no statistical difference (p > 0.05) for comparing ME-DS and ME-DH. ME-IN is also statistically different (p < 0.001) from ME-DS, and ME-DH. This property is important for microemulsion to indicate how the system is organized. Low conductivity values, in the range of μS cm−1, suggest a W/O microemulsion.8 So, the conductivity values for ME-blank, ME-DS, ME-DH, and ME-IN, indicate that these formulations are indeed W/O microemulsions.
Microemulsions have the feature of possessing droplet size in the range of 10 to 100 nm,8 lower than nanoemulsions (>100 nm), which is misleading in the literature because of the prefix name of these systems. The droplet size was measured by a dynamic light scattering methodology using Mie scattering theory and the cumulant method for data fitting. Average droplet sizes were 27.9 ± 0.7 nm, 26.9 ± 1.9 nm, 22.6 ± 0.4 nm, and 35.5 ± 0.7 nm, respectively, for the formulations ME-blank, ME-DS, ME-DH, and ME-IN. Diclofenac sodium does not cause changes in droplet size compared to blank microemulsions (p > 0.05), but it is statistically different in size for donepezil hydrochloride and insulin. For ME-DH, a decrease in droplet size was observed. The ME employed in this study comprises 10% water, 44% oil mixture, and 46% surfactant mixture. This composition suggests the formation of a water-in-oil (W/O) or bicontinuous microemulsion. Although combined techniques are necessary to affirm that the droplets in the analyzed microemulsions are spherical, the size results were consistent among different batches, and the data fitting was good quality. In theory, DLS assumes spherical particles/droplet. The translational diffusion coefficient is calculated through the autocorrelation functions obtained by data fitting. After that, the hydrodynamic radius of solid spherical particles can be derived using Stokes–Einstein equation.53 Therefore, even though a combined system organization can exist, all developed formulations are in the adequate size range and structure for W/O microemulsion, but it was evident from the results that the droplet size is influenced by the drug loaded into the system.
To investigate why the change in droplet size happened in different formulations, the superficial tension of the aqueous solution of DS and DH was measured by the pendant drop method. We assumed 72 mN m−1 as the superficial tension of the water, as documented in the literature, for eqn (2). As a result, it was observed that the drugs reduced the superficial tension of water, but DS (46 mN m−1) reduced more than DH (59 mN m−1), so the superficial tension of drug aqueous solutions was not the primary factor that influenced the size of the microemulsion droplet, as the size of ME-DS > ME-DH.
DH is a drug with apolar affinity, having a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D value of 2.48, so in the microemulsion, this drug may be moving from the droplet toward the oil phase of the formulation. The lipophilic affinity of DH and the fact that DH aqueous solution in an ionic solution has free ions in solution (H+ and Cl−), which can interact with the polar heads of the surfactants by attractive forces, may be the features responsible for the decrease in the droplet size in the ME-DH formulation. Also, For the ME-IN formulation, an increase of almost 8 nm was observed in the droplet size compared to the ME-blank; this can be explained by the size of insulin, which is a large biomolecule, and its often agglomerated into dimers, tetramers, and hexamers, with larger sizes of 3.94, 4.56 and 5.39 nm, respectively.54 The PDI was <0.1 showing that all developed formulations have good homogeneity, with no statistical difference between them (p > 0.05). Furthermore, this low PDI corroborates the hypothesis that spherical water droplets are dispersed into oil phase in these microemulsions.
D value of 2.48, so in the microemulsion, this drug may be moving from the droplet toward the oil phase of the formulation. The lipophilic affinity of DH and the fact that DH aqueous solution in an ionic solution has free ions in solution (H+ and Cl−), which can interact with the polar heads of the surfactants by attractive forces, may be the features responsible for the decrease in the droplet size in the ME-DH formulation. Also, For the ME-IN formulation, an increase of almost 8 nm was observed in the droplet size compared to the ME-blank; this can be explained by the size of insulin, which is a large biomolecule, and its often agglomerated into dimers, tetramers, and hexamers, with larger sizes of 3.94, 4.56 and 5.39 nm, respectively.54 The PDI was <0.1 showing that all developed formulations have good homogeneity, with no statistical difference between them (p > 0.05). Furthermore, this low PDI corroborates the hypothesis that spherical water droplets are dispersed into oil phase in these microemulsions.
The FTIR spectra of all drugs were consistent with the previously reported literature (Fig. 3). For diclofenac sodium (DS), the characteristic absorption bands include C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching observed from 1550–1600 cm−1, C
O stretching observed from 1550–1600 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching bands at 1500 cm−1, and a large band from 3200 to 3500 cm−1, which is associated with a NHx stretching of secondary amine.55 In the case of donepezil hydrochloride (DH), the principal bands include a strong and well-defined C
C stretching bands at 1500 cm−1, and a large band from 3200 to 3500 cm−1, which is associated with a NHx stretching of secondary amine.55 In the case of donepezil hydrochloride (DH), the principal bands include a strong and well-defined C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of ketone strong and defined at 1700 cm−1; stretching bands of N–C and C–O–C from 1000 to 1300 cm−1.56 Insulin exhibits characteristic peptide bands, specifically strong amide I and amide II bands at 1650 and 1550 cm−1, respectively, due to the C
O of ketone strong and defined at 1700 cm−1; stretching bands of N–C and C–O–C from 1000 to 1300 cm−1.56 Insulin exhibits characteristic peptide bands, specifically strong amide I and amide II bands at 1650 and 1550 cm−1, respectively, due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the structure.57 The FTIR spectra of the developed microemulsions, recorded using the ATR technique, are also presented in Fig. 3. All three drug-loaded microemulsions exhibited spectra that closely resembled that of the ME-blank, with no additional or unexpected peaks observed. This indicates compatibility between the drugs and the formulation, and these results suggest that the drugs are encapsulated within the formulation.
O stretching of the structure.57 The FTIR spectra of the developed microemulsions, recorded using the ATR technique, are also presented in Fig. 3. All three drug-loaded microemulsions exhibited spectra that closely resembled that of the ME-blank, with no additional or unexpected peaks observed. This indicates compatibility between the drugs and the formulation, and these results suggest that the drugs are encapsulated within the formulation.
 | ||
| Fig. 3 The FT-IR spectrum for diclofenac sodium, donepezil hydrochloride, and insulin, and for the developed microemulsions, ME-Blank, ME-DS, ME-DH, and ME-IN. | ||
3.4. In vitro drug release
Both dialysis methodologies employed in the study of drug release kinetic maintained the microemulsion intact at the end of the experiment as inspected visually. The formulation is separated from the medium by a modified cellulose membrane, and there is no apparent water transport into the microemulsion compartments. ME-DS showed 34.8 ± 1.9% drug release after 24 h and 39.9 ± 3.4% at 72 h in the multidimensional dialysis (MD) assay (Fig. 4A and Table S4†). For the control group, 70.2 ± 2.8% of the drug was released from formulation after 6 h, reaching 87.5 ± 1.1% at the 24 h time point. Therefore, a significant difference is observed between the release of DS from the control and ME (p < 0.05). For ME-DS, the release kinetic is faster in the first hours of the experiment, and as time progresses, the release speed reduces.ME-DH displayed a controlled release (Fig. 4B) of the drug provided by the formulation differently from the control (p < 0.001). After 1 h, the control group released 92.0 ± 15.4%. The microemulsion released 19.6 ± 2.7% of the encapsulated drug after 72 h, showing a slow, but constant increase over time. This result suggests that a complete release of DH may happen at a prolonged time, which is an attractive feature for drug delivery, extending the interval between doses.
The results of the unidimensional dialysis (UD) assay are shown in Fig. 5, Tables S5 and S6.† ME-DS showed a distinct drug release profile from the respective control group, evidencing that microemulsion controls the outflow of the drug. After 24 h of experimentation, 48.6 ± 6.5% of diclofenac sodium was released from ME-DS, while after 2 h the control group reached 39.8 ± 28.8% drug released. ME-DH showed a similar profile to ME-DS, in which the amount of drug released from the microemulsion (8.2 ± 1.6% at 24 h) is inferior to the control group (43.5 ± 25.1% at 4 h). For both formulations (ME-DS and ME-DH), it was observed that the microemulsion system reduces standard deviation compared to respective controls. A similar feature was observed in clinical trials58 of a commercial microemulsion Neoral®, where an improvement in the linearity of dosing and pharmacokinetic profile of cyclosporine A across patients was observed. When the drug was encapsulated in microemulsion (Neoral®), compared to the original Sandimmune® formulation, the intra-patient variability was reduced by 35% and inter-patient variability by more than 12%.59
Another feature observed with in vitro tests was that the release profile depends on the drug that is encapsulated by the microemulsion. As is shown in Fig. 4 (MD assay) and Fig. 5 (UD assay), diclofenac sodium flows out of the microemulsion exhibiting an accelerated kinetic profile compared to donepezil hydrochloride which was also encapsulated. Some intrinsic physicochemical characteristics of these drugs may be responsible for this behavior. DH has a higher pKa (8.8) than DS (4.0), so in a neutral pH like the medium assays DH is more ionized than DS. This ionization directly impacts the release profile of the drugs in MD and UD assays, which can be observed by the data of the control groups for both drugs. In this case, control-DH presents a maximum release between 2 and 4 h, while for control-DS the maximum occurs at 24 h.
Nevertheless, for drug-loaded microemulsions, the drug release kinetics is primarily governed by the partitioning of the active compound between the different ME domains (aqueous phase, interfacial layer, and oil phase), and the diffusional resistance posed by the microemulsion interface and dialysis membrane. DH (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D 2.48) is more lipophilic than DS (log
D 2.48) is more lipophilic than DS (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D 1.10), therefore it may be moving from the internal aqueous phase to the lipophilic phase (babassu oil and MCT) or the surfactant-rich interfacial region. Moreover, the inclusion of Span™ 83 (a non-ionic surfactant with low HLB) further stabilizes the lipophilic domains and enhances DH retention within the ME structure. Thus, the release of DH may be lower than that of DS because the mass transport through the lipophilic matrix of the formulation is hampered due to the high affinity of the drug and the external microemulsion phase. Additionally, Transcutol® HP may act to increase solubilization of both drugs within the interfacial region, but its effect would be more pronounced for more hydrophilic DS. It could interact with the water phase and improve the mobility of DS through the lipophilic external phase of ME, thereby facilitating the release.60 Different tendency was observed by Yew and Misran to W/O ME prepared using olive oil. They showed faster release for lidocaine (log
D 1.10), therefore it may be moving from the internal aqueous phase to the lipophilic phase (babassu oil and MCT) or the surfactant-rich interfacial region. Moreover, the inclusion of Span™ 83 (a non-ionic surfactant with low HLB) further stabilizes the lipophilic domains and enhances DH retention within the ME structure. Thus, the release of DH may be lower than that of DS because the mass transport through the lipophilic matrix of the formulation is hampered due to the high affinity of the drug and the external microemulsion phase. Additionally, Transcutol® HP may act to increase solubilization of both drugs within the interfacial region, but its effect would be more pronounced for more hydrophilic DS. It could interact with the water phase and improve the mobility of DS through the lipophilic external phase of ME, thereby facilitating the release.60 Different tendency was observed by Yew and Misran to W/O ME prepared using olive oil. They showed faster release for lidocaine (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 2.40) compared to ascorbic acid (log
P 2.40) compared to ascorbic acid (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P −1.85) in the UD assay at pH 7.4;35 emphasizing the influence of both ME composition and drug physicochemical profiles on modulating release kinetics.
P −1.85) in the UD assay at pH 7.4;35 emphasizing the influence of both ME composition and drug physicochemical profiles on modulating release kinetics.
Mathematical modeling of in vitro release data was carried out, testing several different models, including models that are the most physically relevant. For ME-DS the three-parameter Gompertz model presented the highest model selection criteria evaluated (R2adjusted and Model Selection Criterion – MSC) for UD and MD (Table 4). This model is used mainly for comparing the release profiles of good solubility drugs and intermediate release rates.61 The solubility of diclofenac sodium in purified water is 14 mg mL−1.62 The Gompertz equation parameters can be interpreted in terms of release kinetics.63 Therefore, according to the Gompertz model, the maximum diclofenac sodium release was 43.5 ± 5.0% and 58.1 ± 6.1% for MD and UD dialysis, respectively. The flux toward the media for ME-DS was favored for UD (k = 0.084 ± 0.003 h−1) compared to MD (k = 0.072 ± 0.005 h−1) probably because the layer thickness of microemulsion was smaller in UD compared to MD, which impacted the mass transport of DS. According to the model, the microemulsion containing diclofenac sodium delivered 50% of the total possible release amount after 4.9 ± 3.2 h for MD and 5.6 ± 2.4 h for UD. This longer time observed for UD may be related to the higher maximum drug release observed despite the release rate. For microemulsion containing donepezil hydrochloride and assayed by UD, the same Gompertz model was the most adequate to describe the drug release according to the selection criteria, even though DH is less soluble in aqueous-based media than DS. The maximum DH release was 12.5 ± 1.8%, the release rate was 0.070 ± 0.020 h−1, and the time to release 50% of the maximum amount of drug was 11.5 ± 2.7 h. However, although the Gompertz function presented elevated model selection criteria values for DH-MD, the Higuchi and Baker–Lonsdale equations displayed the best results. The Higuchi model applies to low-soluble drugs incorporated into a uniform matrix, and assumes that there are no changes in the matrix due to water.64 The Baker–Lonsdale equation is based on the Higuchi model for a homogeneous spherical matrix.
| Model | DS-MD | DS-UD | DH-MD | DH-UD | ||||
|---|---|---|---|---|---|---|---|---|
| R 2adjusted | MSC | R 2adjusted | MSC | R 2adjusted | MSC | R 2adjusted | MSC | |
| Baker–Lonsdale | 0.8466 | 1.8559 | 0.9247 | 2.4270 | 0.9815 | 3.8364 | 0.9300 | 2.5817 |
| Fisrt order | 0.1543 | 0.0572 | 0.5505 | 0.6429 | 0.8013 | 1.4228 | 0.7017 | 1.0681 |
| Gompertz | 0.8779 | 1.8816 | 0.9401 | 2.5201 | 0.9720 | 3.2973 | 0.9664 | 3.0521 |
| Higuchi | 0.8102 | 1.6459 | 0.8737 | 1.9631 | 0.9820 | 3.8676 | 0.9359 | 2.6503 |
| Hixson–Crowell | 0.0874 | 0.0233 | 0.4191 | 0.3841 | 0.7884 | 1.3596 | 0.6924 | 1.0352 |
| Korsmeyer–Peppas | 0.8616 | 1.8587 | 0.7965 | 1.3533 | 0.9743 | 3.4699 | 0.9270 | 2.4202 |
| Logistic | 0.8158 | 1.4164 | 0.8608 | 1.6565 | 0.9208 | 2.2111 | 0.9189 | 2.2079 |
| Quadratic | 0.5928 | 0.7611 | 0.7974 | 1.4271 | 0.9546 | 2.8130 | 0.9246 | 2.3878 |
| Zero order | 0.0772 | 0.1976 | 0.1562 | 0.0250 | 0.7597 | 1.2315 | 0.6724 | 0.9684 |
Nevertheless, for DH both model criteria selections used were elevated (R2adjusted > 0.95 and MSC > 3.0), however, for DS it did not happen (R2adjusted < 0.95 and MSC < 3.0). Gompertz and Higuchi models assume Fickian diffusion, which occurs due to a gradient in chemical potential caused by the difference in the amount of drug loaded into the microemulsion and the amount present in the release media. Perhaps more than one release mechanism may be involved in the drug release of diclofenac sodium from microemulsions. To investigate this hypothesis, the data from DS-MD and DS-UD were fitted to the Peppas–Sahlin equation. For DS-MD the Peppas–Sahlin parameters were 0.9533 and 2.7801 for R2adjusted and MSC, respectively; while for DS-UD these parameters were 0.9502 and 2.7255, in the same order. This model combines diffusional and relaxation mechanisms of drug release from polymeric devices.65 The diffusion also follows Fick's law, while the relaxation process happens because of stresses and state transition in a hydrophilic polymer matrix that can swell when in contact with aqueous media. Considering that the microemulsions are not a polymeric matrix and did not have contact with the external media or displayed any visual change during the analysis, the Peppas–Shalin model lacks physical meaning for these formulations. However, the higher values obtained for model criteria selection compared to the Gompertz model indicate that a combined drug release mechanism may be involved for DS release from the microemulsion.
Therefore, all these features are responsible for the results of the in vitro assays, where DS has a major percentual release compared to DH. Microemulsions containing both drugs remarkably improved the uniformity of release compared to the respective controls. The UD may relate better than the MD to a possible in vivo administration by different routes since the drug flux occurs in one direction.66 For example, Franz cells are routinely used as an in vitro model to assess formulations for topical and transdermal delivery.67,68
Although further studies are necessary to evaluate the in vitro permeation and retention profiles of the incorporated drugs, this work reports, for the first time, the development of a versatile babassu oil-based water-in-oil microemulsion capable of uniformly delivering drugs with diverse physicochemical properties. Notably, the formulation can be prepared using a low-energy process and contains a moderate surfactant concentration (46% w/w) alongside a high oil content (44% w/w) compared to oil-in-water microemulsions. Babassu oil serves as a multifunctional component, contributing not only to the structural integrity of the lipophilic phase but also potentially offering therapeutic benefits. The high concentration of babassu oil, particularly in the external phase of the microemulsion, may enhance the formulation's efficacy when delivered by specific routes; however, dedicated studies are required to substantiate these hypotheses and fully elucidate its role.
4. Conclusion
In this work, a novel water-in-oil babassu oil-based microemulsion was produced by a low-energy method, optimized, and characterized. The required hydrophilic–lipophilic balance value for babassu oil was determined to support microemulsion production. Three different drugs (diclofenac sodium, donepezil hydrochloride, and insulin) were associated with the developed formulation, and it was observed that each drug altered the physicochemical properties of the microemulsion. Through the in vitro assays, it was found that the formulations present a slow, homogeneous, sustained, and controlled release, which may be suitable for the delivery of different drugs by varied routes of administration. Additional research is needed to confirm whether the formulation can be effectively scaled up.Author contributions
FSG: investigation, methodology, data curation, and writing – original draft; GS: investigation, methodology, and writing – original draft; TASA: conceptualization, funding acquisition, project administration, resources, supervision, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the ESI.†Acknowledgements
The authors thank Universidade Federal de Ciências da Saúde de Porto Alegre for supporting this research work. F. S. G. and G. S. received a scholarship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).References
- B. Yingngam, A. Chiangsom, P. Pharikarn, K. Vonganakasame, V. Kanoknitthiran, W. Rungseevijitprapa and C. Prasitpuriprecha, Optimization of menthol-loaded nanocapsules for skin application using the response surface methodology, J. Drug Delivery Sci. Technol., 2019, 53, 101138 CrossRef CAS.
- M. K. Miller, F. A. Chapa-Villarreal, H. F. Oldenkamp, M. G. Elder, A. K. Venkataraman and N. A. Peppas, Stimuli-responsive self-assembled polymer nanoparticles for the oral delivery of antibodies, J. Controlled Release, 2023, 361, 246–259 CrossRef CAS.
- E. da C. R. Rodrigues, A. M. Ferreira, J. C. E. Vilhena, F. B. de Almeida, R. A. S. Cruz, J. R. Rodríguez Amado, A. C. Florentino, J. C. T. Carvalho and C. P. Fernandes, Development of babassu oil based nanoemulsions, Lat. Am. J. Pharm., 2015, 34, 338–343 CAS.
- M. S. Erdal, A. Gürbüz, S. Birteksöz Tan, S. Güngör and Y. Özsoy, In Vitro Skin Permeation and Antifungal Activity of Naftifine Microemulsions, Turk. J. Pharm. Sci., 2020, 17, 43–48 CrossRef CAS.
- B. P. G. L. Damasceno, J. A. Silva, E. E. Oliveira, W. L. L. Silveira, I. B. Araújo, A. G. Oliveira and E. S. T. Egito, Microemulsão: Um promissor carreador para moléculas insolúveis, Rev. Cienc. Farm. Basica Apl., 2011, 32, 9–18 CAS.
- H. Y. Karasulu, Microemulsions as novel drug carriers: The formation, stability, applications and toxicity, Expert Opin. Drug Delivery, 2008, 5, 119–135 CrossRef CAS.
- Z. Ait-Touchente, N. Zine, N. Jaffrezic-Renault, A. Errachid, N. Lebaz, H. Fessi and A. Elaissari, Exploring the Versatility of Microemulsions in Cutaneous Drug Delivery: Opportunities and Challenges, Nanomaterials, 2023, 13, 1688 CrossRef CAS.
- G. Tartaro, H. Mateos, D. Schirone, R. Angelico and G. Palazzo, Microemulsion Microstructure(s): A Tutorial Review, Nanomaterials, 2020, 10, 1657 CrossRef CAS.
- T. P. Formariz, M. C. Cocenza Urban, A. A. Da Silva, M. P. Daflon Gremião and A. G. De Oliveira, Microemulsões e fases líquidas cristalinas como sistemas de liberação de fármacos, Braz. J. Pharm. Sci., 2005, 41, 301–313 CAS.
- T. Naz, S. Nazir, M. A. Rashid, M. N. Akhtar, M. Usman, M. Abbas and G. Abbas, The Study of Stability and Location of Chloramphenicol in Newly Formed Microemulsion Based Ocular Drug Delivery System, Pharm. Chem. J., 2020, 53, 1047–1052 CrossRef CAS.
- A. Katdare, D. Khunt, S. Thakkar, S. N. Polaka and M. Misra, Comparative evaluation of fish oil and butter oil in modulating delivery of galantamine hydrobromide to brain via intranasal route: pharmacokinetic and oxidative stress studies, Drug Delivery Transl. Res., 2020, 10, 1136–1146 CrossRef CAS.
- F. Yin, S. Meng, X. Zhao, H. Wang, Y. Ning, Y. Li and Z. Chen, Development and In vitro and In vivo Evaluations of a Microemulsion Formulation for the Oral Delivery of Oxaprozin, Curr. Drug Delivery, 2022, 19, 347–356 CrossRef CAS.
- N. Aggarwal, S. Goindi and R. Khurana, Formulation, Characterization and evaluation of an optimized microemulsion formulation of griseofulvin for topical application, Colloids Surf., B, 2013, 105, 158–166 CrossRef CAS PubMed.
- R. Mishra, K. S. Prabhavalkar and L. K. Bhatt, Preparation, optimization, and evaluation of Zaltoprofen-loaded microemulsion and microemulsion-based gel for transdermal delivery, J. Liposome Res., 2016, 26, 297–306 CrossRef CAS PubMed.
- M. H. Aboumanei, A. A. Abdelbary, I. T. Ibrahim, M. I. Tadros and M. T. El-Kolaly, Design and development of microemulsion systems of a new antineoplaston A10 analog for enhanced intravenous antitumor activity: In vitro characterization, molecular docking, 125I-radiolabeling and in vivo biodistribution studies, Int. J. Pharm., 2018, 545, 240–253 CrossRef CAS.
- Y. G. Bachhav and V. B. Patravale, Microemulsion based vaginal gel of fluconazole: Formulation, in vitro and in vivo evaluation, Int. J. Pharm., 2009, 365, 175–179 CrossRef CAS.
- L. L. Wang, S. Huang, H. H. Guo, Y. X. Han, W. S. Zheng and J. D. Jiang, In situ delivery of thermosensitive gel-mediated 5-fluorouracil microemulsion for the treatment of colorectal cancer, Drug Des., Dev. Ther., 2016, 10, 2855–2867 CrossRef CAS.
- W.A Ritschel, Microemulsion technology in the reformulation of cyclosporine: the reason behind the pharmacokinetic properties of Neoral, Clin. Transplant., 1996, 10, 364–373 CrossRef CAS.
- V. B. Patravale and A. A. Date, Microemulsions: Applications in Transdermal and Dermal Delivery, Crit. Rev. Ther. Drug Carrier Syst., 2007, 24, 547–596 CrossRef.
- V. C. Gumiero and P. A. da Rocha Filho, Babassu Nanoemulsions Have Physical and Chemical Stability, J. Dispersion Sci. Technol., 2012, 33, 1569–1573 CrossRef CAS.
- M. M. Cavallari and M. M. Toledo, What is the name of the babassu? A note on the confusing use of scientific names for this important palm tree, Rodriguesia, 2016, 67, 533–538 CrossRef.
- A. Francisca, S. Saraiva, N. Marques De Oliveira, M. Xavier, P. Filho and W. S. Lopes, Cadeia Produtiva do babaçu em cidelândia-MA: uma análise a partir da abordagem de cadeia global de valor, Rev. Bras. de Gestão e Desenvolvimento Reg., 2019, 15, 13–23 Search PubMed.
- E. Amorim, J. E. F. Matias, J. C. U. Coelho, A. C. L. Campos, H. J. Stahlke Jr, J. R. R. Timi, L. C. de A. Rocha, A. T. R. Moreira, D. Z. Rispoli and L. M. Ferreira, Efeito do uso tópico do extrato aquoso de Orbignya phalerata (babaçu) na cicatrização de feridas cutâneas: estudo controlado em ratos, Acta Cirúrgica Brasileira, 2006, 21, 67–76 CrossRef PubMed.
- M. H. S. L. Souza, C. A. Monteiro, P. M. S. Figueredo, F. R. F. Nascimento and R. N. M. Guerra, Ethnopharmacological use of babassu (Orbignya phalerata Mart) in communities of babassu nut breakers in Maranhão, Brazil, J. Ethnopharmacol., 2011, 133, 1–5 CrossRef.
- M. Y. F. A. Reis, S. M. D. Santos, D. R. Silva, M. V. Silva, M. T. S. Correia, D. M. A. F. Navarro, G. K. N. Santos, F. Hallwass, O. Bianchi, A. G. Silva, J. V. Melo, A. B. Mattos, R. M. Ximenes, G. Machado and K. L. A. Saraiva, Anti-Inflammatory Activity of Babassu Oil and Development of a Microemulsion System for Topical Delivery, Evidence-Based Complementary Altern. Med., 2017, 2017, 3647801 CrossRef PubMed.
- J. V. R. de Oliveira, P. L. Silveira, G. Spingolon, G. A. L. Alves, F. P. Peña and T. A. S. Aguirre, Polymeric nanoparticles containing babassu oil: A proposed drug delivery system for controlled release of hydrophilic compounds, Chem. Phys. Lipids, 2023, 253, 105304 CrossRef CAS.
- V. C. Gumiero, Desenvolvimento e avaliação de nanoemulsões à base de óleo de babaçu (Orbignya oleífera) e extratos vegetais (Areca catechu, Glycyrrhiza glabra e Portulaca oleracea) para uso póssol, Universidade de São Paulo, 2011.
- L. Bajerski, L. R. Michels, L. M. Colomé, E. A. Bender, R. J. Freddo, F. Bruxel and S. E. Haas, The use of Brazilian vegetable oils in nanoemulsions: An update on preparation and biological applications, Braz. J. Pharm. Sci., 2016, 52, 347–363 CrossRef CAS.
- ANVISA , Agência Nacional de Vigilância Sanitária. Resolução RDC no482, de 23 de setembro de 1999. Regulamento Técnico para Fixação de Identidade e Qualidade de Óleos e Gorduras Vegetais, 1999.
- D. J. Brayden and J. M. Dee, US Pat., 6423334B1, 2002 Search PubMed.
- United Nations , 70/1. Transforming our world: the 2030 Agenda for Sustainable Development, 2015.
- M. J. Lawrence, Microemulsions as drug delivery vehicles, Curr. Opin. Colloid Interface Sci., 1996, 1, 826–832 CrossRef CAS.
- R. S. Pessoa, E. L. França, E. B. Ribeiro, P. K. D. Lanes, N. G. A. Chaud, L. C. A. Moraes and A. C. Honorio-França, Microemulsion of babassu oil as a natural product to improve human immune system function, Drug Des., Dev. Ther., 2014, 9, 21–31 Search PubMed.
- M. Changez, M. F. Anwar and H. Alrahbi, Olive Oil-Based Reverse Microemulsion for Stability and Topical Delivery of Methotrexate: In Vitro, ACS Omega, 2024, 9, 7012–7021 CrossRef CAS PubMed.
- H. Yew and M. Bin Misran, Nonionic Mixed Surfactant Stabilized Water–in–Oil Microemulsions for Active Ingredient In Vitro Sustained Release, J. Surfactants Deterg., 2016, 19, 49–56 CrossRef CAS.
- M. D. Chatzidaki, E. Mateos-Diaz, F. Leal-Calderon, A. Xenakis and F. Carrière, Water-in-oil microemulsions versus emulsions as carriers of hydroxytyrosol: an in vitro gastrointestinal lipolysis study using the pHstat technique, Food Funct., 2016, 7, 2258–2269 RSC.
- R. Altman, B. Bosch, K. Brune, P. Patrignani and C. Young, Advances in NSAID Development: Evolution of Diclofenac Products Using Pharmaceutical Technology, Drugs, 2015, 75, 859–877 CrossRef CAS.
- J. Kienzler, M. Gold and F. Nollevaux, Systemic Bioavailability of Topical Diclofenac Sodium Gel 1% Versus Oral Diclofenac Sodium in Healthy Volunteers, J. Clin. Pharmacol., 2010, 50, 50–61 CrossRef CAS.
- FDA , Medicine leaflet - Voltaren® Gel.
- M. Hagen and M. Baker, Skin penetration and tissue permeation after topical administration of diclofenac, Taylor and Francis Ltd, 2017, preprint, DOI:10.1080/03007995.2017.1352497.
- L. Sutthapitaksakul, C. R. Dass and P. Sriamornsak, Donepezil—an updated review of challenges in dosage form design, J. Drug Delivery Sci. Technol., 2021, 63, 102549 CrossRef CAS.
- P. Sozio, L. S. Cerasa, L. Marinelli and A. Di Stefano, Transdermal donepezil on the treatment of Alzheimer's disease, Neuropsychiatr. Dis. Treat., 2012, 8, 361–368 CAS.
- FDA , Adlarity - Clinical Pharmacology Review(S) - Application Number: 212304Orig1s000, 2021.
- D. R. Owens, B. Zinman and G. Bolli, Alternative routes of insulin delivery, Diabetic Med., 2003, 20, 886–898 CrossRef CAS PubMed.
- K. S. Yadav, G. Soni, D. Choudhary, A. Khanduri, A. Bhandari and G. Joshi, Microemulsions for enhancing drug delivery of hydrophilic drugs: Exploring various routes of administration, Med. Drug Discovery, 2023, 20, 100162 CrossRef CAS.
- Y. Cao, H. Gao, H. Xia, X. Zhu, B. Li, X. Zhou and Y. Jin, Development and Evaluation of a Water-in-oil Microemulsion Formulation for the Transdermal Drug Delivery of Teriflunomide (A771726), Chem. Pharm. Bull., 2019, 67, 786–794 CrossRef CAS PubMed.
- Agência Nacional de Vigilância Sanitária, Farmacopeia Brasileira, 6th edn, 2019, vol. 1 Search PubMed.
- Y. Zhang, M. Huo, J. Zhou, A. Zou, W. Li, C. Yao and S. Xie, DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles, AAPS J., 2010, 12, 263–271 CrossRef CAS PubMed.
- J.-M. Aubry, J. F. Ontiveros, J.-L. Salager and V. Nardello-Rataj, Use of the normalized hydrophilic-lipophilic-deviation (HLDN) equation for determining the equivalent alkane carbon number (EACN) of oils and the preferred alkane carbon number (PACN) of nonionic surfactants by the fish-tail method (FTM), Adv. Colloid Interface Sci., 2020, 276, 102099 CrossRef CAS.
- Y. Zheng, M. Zheng, Z. Ma, B. Xin, R. Guo and X. Xu, in Polar Lipids, Elsevier, 2015, pp. 215–243 Search PubMed.
- K. A. Antoni, T. A. S. Aguirre and V. R. Botelho, Development of artificial neural network models to predict the concentration range of formation of microemulsions containing babassu oil, Chem. Phys. Lett., 2024, 843, 141237 CrossRef.
- Chemaxon, Playground 1.2, Chemaxon, 2025, preprint, https://playground.calculators.cxn.io Search PubMed.
- S. Bhattacharjee, DLS and zeta potential – What they are and what they are not?, J. Controlled Release, 2016, 235, 337–351 CrossRef CAS.
- Y. Casamayou-Boucau and A. G. Ryder, Quantitative analysis of weakly bound insulin oligomers in solution using polarized multidimensional fluorescence spectroscopy, Anal. Chim. Acta, 2020, 1138, 18–29 CrossRef CAS.
- R. P. Swain, R. Nagamani and S. Panda, Formulation, in vitro characterization and stability studies of fast dispersing tablets of diclofenac sodium, J. Appl. Pharm. Sci., 2015, 5, 94–102 CrossRef CAS.
- P. V. K. Kumari and Y. S. Rao, Formulation and evaluation of orodispersible tablets of donepezil hydrochloride, Int. J. Curr. Pharm. Res., 2020, 45–51 CrossRef CAS.
- A. K. Prusty and S. K. Sahu, Development and Evaluation of Insulin Incorporated Nanoparticles for Oral Administration, ISRN Nanotechnol., 2013, 2013, 1–6 CrossRef.
- C. J. Dunn, A. J. Wagstaff, C. M. Perry, G. L. Plosker, K. L. Goa, S. Birkeland, A. Jain, G. Mazariegos and A. Olyaei, Cyclosporin An Updated Review of the Pharmacokinetic Properties, Clinical Efficacy and Tolerability of a Microemulsion-Based Formulation (Neoral ®) 1 in Organ Transplantation, Drugs, 2001, 1957–2016 CrossRef CAS PubMed.
- P. Keown, D. Landsberg, P. Halloran, A. Shoker, D. Rush, J. Jeffery, D. Russell, C. Stiller, N. Muirhead, E. Cole, L. Paul, J. Zaltzman, R. Loertscher, P. Daloze, R. Dandavino, A. Boucher, P. Handa, J. Lawen, P. Belitsky and P. Parfrey, A randomized, prospective multicenter pharmacoepidemiologic study of cyclosporine microemulsion in stable renal graft recipients1,2,3, Transplantation, 1996, 62, 1744–1752 CrossRef CAS PubMed.
- D. W. Osborne and J. Musakhanian, Skin Penetration and Permeation Properties of Transcutol®—Neat or Diluted Mixtures, Springer New York LLC, 2018, preprint, DOI:10.1208/s12249-018-1196-8.
- J. A. Fuentes-García, A. C. Alavarse, C. E. de Castro, F. C. Giacomelli, M. R. Ibarra, J.-J. Bonvent and G. F. Goya, Sonochemical route for mesoporous silica-coated magnetic nanoparticles towards pH-triggered drug delivery system, J. Mater. Res. Technol., 2021, 15, 52–67 CrossRef.
- M. Kincl, M. Meleh, M. Veber and F. Vrečer, Study of physicochemical parameters affecting the release of diclofenac sodium from lipophilic matrix tablets, Acta Chim. Slov., 2004, 51, 409–425 CAS.
- C. V. Pabón, P. Frutos, J. L. Lastres and G. Frutos, Matrix Tablets Containing HPMC and Polyamide 12: Comparison of Dissolution Data Using the Gompertz Function, Drug Dev. Ind. Pharm., 1994, 20, 2509–2518 CrossRef.
- P. Costa, J. Manuel and S. Lobô, Modeling and comparison of dissolution profiles, 2001, vol. 13 Search PubMed.
- N. A. Peppas and J. J. Sahlin, A simple equation for the description of solute release. III. Coupling of diffusion and relaxation, 1989, vol. 57 Search PubMed.
- M. Grassi, N. Coceani and L. Magarotto, Mathematical Modeling of Drug Release from Microemulsions: Theory in Comparison with Experiments, J. Colloid Interface Sci., 2000, 228, 141–150 CrossRef CAS PubMed.
- U. A. Shinde, S. H. Modani and K. H. Singh, Design and Development of Repaglinide Microemulsion Gel for Transdermal Delivery, AAPS PharmSciTech, 2018, 19, 315–325 CrossRef CAS.
- T. Shukla, N. Upmanyu, M. Agrawal, S. Saraf, S. Saraf and A. Alexander, Biomedical applications of microemulsion through dermal and transdermal route, Biomed. Pharmacother., 2018, 108, 1477–1494 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5pm00022j |
| This journal is © The Royal Society of Chemistry 2025 |