Open Access Article
Open Access ArticleEfficient and selective N-benzylation of amines using Pd-doped La-BDC MOF†
Amreet
Kaur
a,
Yadvinder
Singh
 b,
Avtar
Singh
b,
Avtar
Singh
 cd,
Sandeep
Kaushal
cd,
Sandeep
Kaushal
 *e and
Rahul
Badru
*e and
Rahul
Badru
 *a
*a
aDepartment of Chemistry, Sri Guru Granth Sahib World University, Fatehgarh Sahib, Punjab, India. E-mail: rahulbadru@gmail.com
bDepartment of Botany, Central University of Punjab, Bathinda-151401, Punjab, India
cDepartment of Chemistry, School of Science, Navajo Technical University, Crownpoint, NM 87313, USA
dDepartment of Chemistry, Sri Guru Teg Bahadur Khalsa College, Anandpur Sahib-140118, Punjab, India
eRegional Institute of Education, NCERT, Ajmer, Rajasthan, India. E-mail: kaushalsandeep33@gmal.com
First published on 27th June 2025
Abstract
Transition metal catalysis has become increasingly important in direct N-alkylation via the hydrogen borrowing mechanism, an environmentally friendly pathway that produces only water as a by-product. However, the application of inner-transition metal catalysts for the alkylation of amines has been explored on a limited scale. Herein, we investigate the potential of a Pd-doped La-BDC (benzene-1,4-dicarboxylate, terephthalic acid) MOF in the N-benzylation of amine substrates. The isolation of imine and benzaldehyde intermediates confirms that the reaction follows a hydrogen auto-transfer pathway. Reaction conditions, including temperature, reaction time, and catalyst loading, were optimized to achieve high conversion and selectivity. Extensive characterization using FTIR, FESEM, HRTEM, EDS, XPS, and nitrogen adsorption–desorption measurements assessed the structural and textural properties of the synthesized Pd@La-BDC MOF. Compared to previous literature, our findings provide valuable insights into the application of La-derived MOFs in sustainable catalysis and offer new possibilities for synthesizing N-benzylated products using benzyl alcohol as an alkylating agent.
1. Introduction
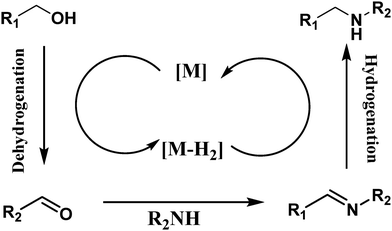
The relentless pursuit to improve and simplify chemical reactions is paramount, especially with the goal of eliminating hazardous chemicals while achieving high yields. This endeavor to develop safer and more sustainable chemical processes is vital for advancing science, biology, and materials science, ultimately benefiting humanity through the principles of green chemistry. Such research and technological advancements are critical for the growth of various scientific domains, fostering the creation of processes that significantly contribute to environmental preservation and human well-being.The usage of dimethyl carbonates and alcohols as alkylating agents has gained attention in the past two decades due to their environmentally friendly nature. These methods align with green chemistry principles by reducing hazardous waste and improving the overall sustainability of chemical processes. For example, the use of dimethyl carbonate and alcohols in N-alkylation reactions has been shown to produce minimal by-products and operate under milder conditions, making it a preferred choice for sustainable synthesis.1–3 Among these two modes, the N-alkylations with dialkylcarbonates generate alcohols as the byproduct, whereas water is the only side product of the reaction among amines and alcohols. Additionally, N-alkylation with dialkylcarbonates has always suffered from selectivity issues as it generates a mixture of alkylated and carboxymethylated products, as reflected in the tremendous work reported with them.4,5 They have also been reported to deliver over-alkylated products due to dialkylation or methylation of the activated CH2 group in addition to the normal amine functionality. N-Alkylation with alcohols thus remains the preferred approach among all other documented methods.6,7N-Alkylation with alcohols follows hydrogen borrowing methodology (Scheme 1), wherein the transition metal catalysts are involved in hydrogen auto-transfer with the amine substrate, generating aldehydes which then condense with an amine to liberate an imine, which is reduced to an alkylated amine by back H-transfer.2,3 This technique has become popular and stands out of all methodologies as it's a quicker way of accessing C–C and C–N bond formulations in a one-pot sustainable mode avoiding the need for separation and purification of any intermediates. Moreover, this technique avoids the need for specialized conditions or pre-functionalized substrates.
Much research has been conducted previously to investigate the use of various transition metal (Rh, Ru, Mn, Co, Pd, Ir)-based catalysts in N-alkylations via hydrogen auto-transfer.8–13 There have also been numerous reports on the use of tandem catalysts in these reactions, involving transition metal combination in the same catalyst.14,15 Aside from these, metal–organic frameworks (MOFs) or supported MOF systems have been used to achieve N-alkylation with alcohols as alkylating agents.15,16
MOFs have served as versatile efficient catalysts in a number of organic transformations, owing to their tunable pore size and high surface area to volume ratio. The MOFs are insoluble in most organic solvents and thus the easy post-process separation adds on to their importance as heterogenous catalysts. Although their catalytic potential has been extensively established in a number of other organic synthesis processes, their utilization in N-alkylation with alcohols is only partially studied.15–18,34 Although a high efficiency and selectivity have been achieved with these protocols, all of these processes suffer from one or more drawbacks, including the use of expensive non-recoverable catalysts, limited substrate scope, need for co-catalysts, ligands or bases and a high time interval.
To the best of our knowledge, reports on inner transition metal-based catalysts for N-alkylation of amines are minimal,17,18 and our comparative analysis with these limited studies highlights the distinctiveness of our results. So, in order to address this and to fulfil our continued interest in developing effective and sustainable methodologies for organic transformations,19–23 we hereby report the application of a Pd-incorporated La-BDC MOF as a catalyst in the N-benzylation of amines, avoiding the use of hydrogen, organic ligands and high-pressure conditions. With the present methodology, it is possible to selectively and efficiently benzylate a variety of aromatic amine substrates with benzyl alcohol without isolating any poly-alkylation products. The reaction conditions are benign in comparison to previous methodologies. The reusability and stability of the MOF catalyst in the executed reaction conditions have also been examined, which adds onto the sustainability of the protocol. Interestingly, mechanistic details have been established based on products and intermediates isolated while assessing the reaction profile on a time scale using gas chromatography. The reaction has been found to follow the hydrogen auto-transfer mechanism.
2. Experimental
2.1. Representative procedure for La-BDC MOF synthesis
The solvothermal method has been employed to synthesize La-BDC MOF, similar to the one reported in the literature.24 In a 30 mL 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water–DMF mixture, 10 mmol each of the salts lanthanum nitrate and BDC were dissolved and the whole mixture was moved to a 150 mL Teflon-lined autoclave and kept at 120 °C in a pre-heated oven for 24 h. The contents were then allowed to cool to room temperature, and the MOF precipitated out. The solid thus obtained was filtered and washed with distilled water. Before being used, the filtered solid was activated for 4 hours by placing it in a vacuum oven maintained at 100 °C.
1 water–DMF mixture, 10 mmol each of the salts lanthanum nitrate and BDC were dissolved and the whole mixture was moved to a 150 mL Teflon-lined autoclave and kept at 120 °C in a pre-heated oven for 24 h. The contents were then allowed to cool to room temperature, and the MOF precipitated out. The solid thus obtained was filtered and washed with distilled water. Before being used, the filtered solid was activated for 4 hours by placing it in a vacuum oven maintained at 100 °C.
2.2. Representative procedure for Pd-supported La-BDC MOF synthesis
To prepare a Pd-loaded MOF, 5 mg of palladium nitrate was loaded into a pre-formed solution containing 100 mg of La-BDC MOF in 40 mL of ethanol. The in situ reduction of palladium ions was done by the addition of a 10% solution of sodium borohydride in water. The mixture was stirred continuously using a magnetic stirrer for 1 h for reduction to complete the reaction. The Pd-loaded MOF thus obtained was filtered off from the solution and washed with 20 mL ethanol and 20 mL water. The isolated solid Pd@La-BDC MOF was dried in a vacuum oven at 100 °C for 24 hours.2.3. Representative procedure for the catalytic N-benzylation reaction
The N-benzylation reactions were carried out under autoclave conditions. The Teflon tube was charged with 10 mmol of amine, 50 mmol benzyl alcohol, 10 mL toluene and 5 mol% of MOF catalyst. The Teflon tube was sealed with Teflon tape before being immersed into a preheated furnace. After commencement of time, the reaction mixture was cooled and the heterogenous catalyst was filtered off. The percentage yields of all the products were ascertained using GC analysis and adding n-hexadecane as the internal standard. After completion of the reaction, 20 mL of n-hexadecane was added to the reaction mixture, which was diluted with ethyl acetate and subjected to filtration over a plug of silica and then analysed with GC. All the products were obtained over a silica gel column eluted with a hexane–ethyl acetate mixture.2.4. Kinetic study for the N-benzylation of aniline
The kinetic studies were carried out in a glass reactor equipped with a magnetic stirrer. To establish the reaction kinetics, varying amounts of benzyl alcohol (0.1–0.4 mol) and aniline (0.1–0.4 mol) in toluene were made to react at 150 °C (while varying only one reactant at a time), with 5 mol% of MOF catalyst. n-Hexadecane (0.2 mmol) was added as an internal standard. At different intervals, about 50 μL aliquots were withdrawn. Each aliquot was diluted with ethyl acetate before being centrifuged, and the supernatant thus isolated was examined on a gas chromatogram.3. Results and discussion
3.1. Pd@Al-BDC MOF characterization
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond stretching appeared at 694 cm−1, 1570 cm−1, 1380 and 1100 cm−1 in the FTIR spectra recorded for the La-BDC MOF. A slight peak shifting of carboxylate stretching and M–O stretching was observed in the FTIR spectra of the Pd@La-BDC MOF. The M–O peak shifted to 680 cm−1 and the new carboxylate stretchings were shifted towards a lower wavenumber of 1515 cm−1.
C bond stretching appeared at 694 cm−1, 1570 cm−1, 1380 and 1100 cm−1 in the FTIR spectra recorded for the La-BDC MOF. A slight peak shifting of carboxylate stretching and M–O stretching was observed in the FTIR spectra of the Pd@La-BDC MOF. The M–O peak shifted to 680 cm−1 and the new carboxylate stretchings were shifted towards a lower wavenumber of 1515 cm−1.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonding, respectively.32
O bonding, respectively.32
3.2. Catalytic evaluation
To start with assessment of the catalytic efficacy of the synthesized MOFs in the N-alkylation of amines, firstly aniline, chosen as a model amine substrate, was treated with benzyl alcohol at 150 °C in toluene as a solvent (Scheme 2). The reaction was carried out both with and without the La-BDC MOF and Pd@La-BDC MOF catalyst and the percentage conversion of the reactant achieved in each case was evaluated using GC-FID experiments using n-hexadecane as an internal standard and the results are reported in Table 1. | ||
| Scheme 2 Reaction of aniline with benzyl alcohol in the catalytic presence of La-BDC and Pd@La-BDC MOFs. | ||
| Entry | Catalyst | Base (equiv.) | Solvent | % conversion |
|---|---|---|---|---|
| General reaction conditions: aniline 10 mmol, benzyl alcohol 50 mmol, catalyst 5 mol%, 150 °C, 6 h; conversion and selectivity percentage were determined by GC-FID using hexadecane as an internal standard. | ||||
| 1 | No catalyst | — | — | Nil |
| 2 | La-BDC MOF | — | Toluene | 61 |
| 3 | Pd@La-BDC MOF | — | Toluene | 94 |
| 4 | Pd@La-BDC MOF | KOH (2) | Toluene | 89 |
| 5 | Pd@La-BDC MOF | KOH (5) | Toluene | 92 |
| 6 | Pd@La-BDC MOF | NaOH (2) | Toluene | 89 |
| 7 | Pd@La-BDC MOF | NaOH (5) | Toluene | 91 |
| 8 | Pd@La-BDC MOF | KOtBu (2) | Toluene | 91 |
| 9 | Pd@La-BDC MOF | KOtBu (5) | Toluene | 95 |
| 10 | Pd@La-BDC MOF | (C2H5)3N (2) | Toluene | 83 |
| 11 | Pd@La-BDC MOF | (C2H5)3N (5) | Toluene | 85 |
| 12 | Pd@La-BDC MOF | — | Hexane | 91 |
| 13 | Pd@La-BDC MOF | — | Xylene | 87 |
| 14 | Pd@La-BDC MOF | — | THF | 77 |
| 15 | Pd@La-BDC MOF | — | DMF | 72 |
Notably, no product formation took place when the reaction was performed in the absence of the MOF catalyst (Table 1, entry 1) even after 6 h, and only 61% substrate conversion took place in an interval of 6 h, when the same reaction was carried out with 5 mol% of La-BDC MOF (Table 1, entry 2). To our surprise, upon replacing the La-BDC MOF with Pd@La-BDC MOF in the same reaction, the % conversion of aniline was found to enhance to 94% (Table 1, entry 4). Thus, integrating Pd into the La-BDC MOF catalyst was proven to improve the yields for the reaction product, possibly due to greater H-borrowing tendency of the La from the substrate as explained in H-borrowing methodology explained in the later section under mechanistic studies.
 | ||
| Scheme 3 H-borrowing reaction pathway for N-benzylation of aniline in the catalytic presence of Pd@La-BDC MOF. | ||
It was observed that at lower temperatures of 90–120 °C, the yields of the alkylated product (2) were low in comparison to the imine product (1) (Fig. 8a). But an increase in temperature beyond 120 °C increased the selectivity towards the amine product (2) and at 150 °C a maximum selectively of 97% towards the amine product was achieved (Fig. 8a).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 or greater, a selectivity of 97% was achieved for N-benzylaniline in an interval of 5 hours at 150 °C. Interestingly, no over-alkylated tertiary amine products were isolated under any conditions. The effect of catalytic dosage on the % conversion of substrate is shown in Fig. 8c. It is clear from Fig. 8c that a maximum conversion of 97% was achieved with 5 mol% of catalytic dosage in 5 h. Thus, a reactant ratio of 1
5 or greater, a selectivity of 97% was achieved for N-benzylaniline in an interval of 5 hours at 150 °C. Interestingly, no over-alkylated tertiary amine products were isolated under any conditions. The effect of catalytic dosage on the % conversion of substrate is shown in Fig. 8c. It is clear from Fig. 8c that a maximum conversion of 97% was achieved with 5 mol% of catalytic dosage in 5 h. Thus, a reactant ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, catalytic dosage of 5 mol%, and temperature of 150 °C were selected for further exploring the substrate scope.
5, catalytic dosage of 5 mol%, and temperature of 150 °C were selected for further exploring the substrate scope.
To determine the overall kinetics of the reaction, the amount of one of the reactants out of aniline and benzyl alcohol was varied (0.2–0.8 mol) while that of the other was kept constant at 0.4 mol and the results are plotted in Fig. 9. The initial reaction rates were determined in both cases. The reaction rates were then plotted against the conc. of the reactant under consideration (Fig. 9c and d). The rate of the reaction varied linearly with conc. of benzaldehyde, while it was found to be independent of the aniline concentration. Thus, the rate of the reaction can be considered first order w.r.t. benzyl alcohol and thus the formation of benzaldehyde is the rate limiting step in the present case, which is in agreement with previous reports.15,33
3.3. Hydrogen borrowing mechanism
The N-alkylation of amines in the catalytic presence of transition metal-based catalysts has been reported to follow the H-borrowing pathway,14 wherein the transition metal is involved in the exchange of hydrogen with the substrate as depicted in Schemes 1 and 4. In order to establish the same in the present case, the reaction mixture was monitored over a period of time and the % conversion for substrate, intermediates and product were plotted against time (Fig. 10). The reaction profile (Fig. 10) showed that benzaldehyde pre-formed in larger quantities declined with the passage of time and the amount of another intermediate imine increased in a slow fashion in the initial phase and began to decline after an interval of 2 h. At the end of the reaction, i.e. after 6 h, the alkylated amine was the major product, with little or no imine and aldehyde substrate. | ||
| Fig. 10 (a) Percentage conversion for the substrate, intermediates and amines during the course of the reaction; (b) hot filtration tests; 10 mmol aniline, 5 mol% Pd@La-BDC, 150 °C. | ||
Additionally, hot filtration tests were carried out to verify that the reaction ceases after the catalyst is removed from the reaction mixture. The hot reaction mixture was filtered after an interval of 2 h to separate the catalyst and the remaining solution was subjected to similar conditions of temperature. No further changes were observed in the percentage conversion (Fig. 10b), showing that the reaction ceased after elimination of the catalyst from the reaction mixture.
Based on the results in Fig. 10a, the plausible reaction mechanism can be outlined as in Scheme 5. The reaction begins with the deprotonation of benzyl alcohol by the metallic cluster on the Pd@La-BDC MOF, resulting in the formation of a benzaldehyde molecule and the generation of Pd-hydride species. The formed benzaldehyde molecule adsorbs onto the Pd site, which increases its electrophilicity and, consequently, its reactivity. This activated benzaldehyde molecule becomes more susceptible to nucleophilic attack by the amine substrate, generating an imine intermediate, and in the process, a water molecule is eliminated. Subsequently, the Pd-hydride species generated in the first step comes into play. It hydrogenates the imine group, reducing it to a secondary amine product. The active site on the Pd@La-BDC MOF is regenerated during the final step, making it available for subsequent catalytic cycles.
 | ||
| Scheme 5 H-borrowing mechanism for the Pd@La-BDC MOF-mediated N-alkylation reaction, illustrating the key steps and intermediate species involved in the catalytic process. | ||
The results in Table 2 showed that all the examined aromatic amines could be converted into their corresponding N-benzylated products during the alkylation reactions, albeit the yields varied. The chloro and bromo derivatives of aniline delivered products in slightly lower yields (Table 2, entries 5 and 6), while the yields of N-benzylation products were higher with electron rich anilines, i.e. o-toluidine, p-toluidine and anisidine. Furthermore, indole and methyl glycinate were also subjected to alkylation under similar experimental conditions, and in both cases, yields of 87% and 85% were recorded for the corresponding N-alkylated products (Table 2, entries 8 and 9). Thus, the present methodology served to benzylate a diverse array of amine substrates under high to excellent yields.
3.4. Catalytic reusability
A crucial component of heterogeneous catalysis is the catalyst's capacity for reuse. To evaluate this, the heterogenous MOF catalyst separated at the end of the reaction was washed with a water–ethanol mixture to get rid of reactants adhered to its surface. Later, it was dried by keeping it in an oven at 150 °C for 2 h, and then used for the subsequent run. Upon re-using the same catalyst five times, only a meagre drop in catalytic action was noticed (Fig. 11a). The XRD patterns of the catalyst retrieved after the fifth run were recorded to look for any modifications that might have happened during the course of the reaction (Fig. 11b). Notably, the XRD pattern showed no additional peaks w.r.t. the XRD patterns of the original MOF catalyst. Thus, it can be generalized that the catalyst remained stable during the reaction and is reusable.3.5. Comparative analysis with existing literature
The outcomes of the present work have been compared with those obtained in the presence of other transition metal-based heterogenous catalysts. Upon comparison with the literature reports provided in Table 3, it can be figured out that the Pd@La-BDC MOF catalyst utilized for N-benzylation in the present investigation performs on par with the results obtained with already reported MOF catalysts. A higher alkylation percentage has been obtained in the present case under less drastic conditions of temperature (entries 1 and 6, Table 3). Moreover, a number of amine substrates could be selectively converted to the corresponding mono N-methylated products under the optimized reaction conditions. The heterogenous catalyst was easily retrieved at the end of the reaction and the reusability of the heterogenous catalyst has also been established (Fig. 11).| Entry | Catalyst | Reaction conditions | Alkylation source | Alkylated product (% yield) | Ref. | Advantages/disadvantages |
|---|---|---|---|---|---|---|
| 1 | Cu-Fe(3)HT-300 | 163 °C, 24 h | Benzyl alcohol | 88 | 14 | Low substrate ratio, longer duration, byproducts |
| 2 | TiOH-80 | 180 °C, 15–96 h | Alcohols | 90–97 | 36 | Wide substrate scope, longer duration, byproduct |
| 3 | MnBr(CO)5 | 130 °C, 20 h | Alcohols | 29–92 | 10 | Wide substrate scope, base requirement, longer duration |
| 4 | Hf-MOF-808 | 120–140 °C, 3–23 h | Benzyl alcohol | 70–90 | 15 | Low temp., low yields, longer duration |
| 5 | UiO66-NH2-[LIr]BF4 | 80–180 °C, 2–24 h | Benzyl alcohol | 90–100 | 37 | High yields, wide substrate scope, longer duration |
| 6 | Pd@La-BDC MOF | 150 °C, 4–6 h | Benzyl alcohol | 79–94 | Present work | Short duration, high selectivity |
4. Conclusion
This study successfully synthesized and utilized a Pd-incorporated La-BDC MOF catalyst for the N-benzylation of amines, employing a solvothermal method and in situ reduction of the Pd precursor with NaBH4. The resulting Pd-doped La-BDC MOF composite demonstrated a high surface area of 368.14 m2 g−1 and pore volume of 0.1256 cm3 g−1 with a microporous structure (micropore volume of 0.00376523 cm3 g−1) that significantly enhanced its catalytic performance. The catalyst achieved a maximum selectivity of 97% under the optimized conditions, efficiently benzylating various amine substrates including electron poor aromatic amines and amino acid esters. The isolation of imine and benzaldehyde intermediates during the time-monitored reaction profile confirmed that the reaction followed a hydrogen auto-transfer pathway. The robustness and practical utility of the Pd@La-BDC MOF catalyst were further validated through reusability and hot filtration tests, underscoring its potential as a reliable heterogeneous catalyst for N-benzylation reactions.Author contributions
Amreet Kaur: experimental and writing original draft; Dr Sandeep Kaushal: conceptualization, characterization and formal analysis; Dr Rahul Badru: conceptualization, methodology, draft revision and supervision; Dr Yadvinder Singh & Dr Avatar Singh: validation, reviewing and editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the ESI.†Acknowledgements
The authors acknowledge SGGSWU, Fatehgarh Sahib for providing the necessary laboratory facilities. They are also thankful to SAIF Lab at Panjab University, Chandigarh, and Punjabi University, Patiala, for spectral analysis.References
- G. Fiorani, A. Perosa and M. Selva, Dimethyl carbonate: a versatile reagent for a sustainable valorization of renewables, Green Chem., 2018, 20, 288–322 RSC.
- A. Corma, J. Navas and M. J. Sabater, Advances in One-Pot Synthesis through Borrowing Hydrogen Catalysis, Chem. Rev., 2018, 118(4), 1410–1459 CrossRef CAS PubMed.
- B. G. Reed-Berendt, D. E. Latham, M. B. Dambatta and L. C. Morrill, Borrowing Hydrogen for Organic Synthesis, ACS Cent. Sci., 2021, 7(4), 570–585 CrossRef CAS PubMed.
- P. Tundo and M. Selva, The Chemistry of Dimethyl Carbonate, Acc. Chem. Res., 2002, 35(9), 706–716 CrossRef CAS PubMed.
- P. Tundo, M. Musolino and F. Arico, The reactions of dimethyl carbonate and its derivatives, Green Chem., 2018, 20, 28–85 RSC.
- S. Bera, L. M. Kabadwal and D. Banerjee, Harnessing alcohols as sustainable reagents for late-stage functionalisation: synthesis of drugs and bio-inspired compounds, Chem. Soc. Rev., 2024, 53, 4607–4647 RSC.
- G. Sivakumar, R. Kumar and V. Yadav, et al., Multi-Functionality of Methanol in Sustainable Catalysis: Beyond Methanol Economy, ACS Catal., 2023, 13(22), 15013–15053 CrossRef CAS.
- S. M. Khake and N. Chatani, Rhodium(III)-catalyzed oxidative C-H alkylation of aniline derivatives with allylic alcohols to produce β-Aryl Ketones, ACS Catal., 2022, 12(8), 4394–4401 CrossRef CAS.
- K. O. Marichev and J. M. Takacs, Ruthenium-catalyzed amination of secondary alcohols using borrowing hydrogen methodology, ACS Catal., 2016, 6, 2205–2210 CrossRef CAS PubMed.
- V. G. Landge, A. Mondal and V. Kumar, et al., Manganese catalyzed N-alkylation of anilines with alcohols: ligand enabled selectivity, Org. Biomol. Chem., 2018, 16, 8175–8180 RSC.
- Z. Ma, B. Zhou and R. G. Kadam, et al., Reusable Co-nanoparticles for general and selective N-alkylation of amines and ammonia with alcohols, Chem. Sci., 2022, 13, 111–117 RSC.
- T. T. Dang, B. Ramalingam, S. P. Shan and A. M. Seayad, An efficient Palladium-catalyzed N-alkylation of amines using primary and secondary alcohols, ACS Catal., 2013, 3, 2536–2540 CrossRef CAS.
- G. S. Lu, Z. L. Ruan and Y. Wang, et al., Catalytic Reductive Amination and Tandem Amination–Alkylation of Esters Enabled by a Cationic Iridium Complex, Angew. Chem., Int. Ed., 2025, 64(12), e202422742 CrossRef CAS PubMed.
- W. S. Putro, T. Hara, N. Ichikuni and S. Shimazu, One-pot synthesis of aniline N-alkylation from benzyl alcohol over Cu-Fe catalyst, Appl. Catal., A, 2020, 602, 117519 CrossRef CAS.
- B. Bohigues, S. Rojas-Buzo, M. Moliner and A. Corma, Coordinatively unsaturated Hf-MOF-808 prepared via hydrothermal synthesis as a bifunctional catalyst for the tandem N-alkylation of amines with benzyl alcohol, ACS Sustainable Chem. Eng., 2021, 9(47), 15793–15806 CrossRef CAS PubMed.
- Y. Lu, H. Chai and K. Yu, et al., A reusable MOF supported single-site nickel-catalyzed direct N-alkylation of anilines with alcohols, Tetrahedron, 2022, 124, 132993 CrossRef CAS.
- X. Wang, T. Li and H. Wang, et al., Identifying active sites at the Cu/Ce interface for hydrogen borrowing reactions, J. Catal., 2023, 418, 163–177 CrossRef CAS.
- T. Tong, M. Douthwaite and L. Chen, et al., Uncovering structure-activity relationships in Pt/CeO2 catalysts for Hydrogen-borrowing amination, ACS Catal., 2023, 13(2), 1207–1220 CrossRef CAS PubMed.
- A. Sharma, S. Bedi and K. Verma, et al., Ce-Zr UiO-66 MOF as recyclable heterogeneous catalyst for selective N-methylation, Polyhedron, 2023, 242, 116517 CrossRef CAS.
- A. Sharma, K. Verma, S. Kaushal and R. Badru, A novel 2-D accordion like Al-BPED MOF as reusable and selective catalyst for N-alkylation of amines with dialkylcarbonates, Appl. Organomet. Chem., 2022, 36(11), e6814 CrossRef CAS.
- A. Sharma, K. Verma, S. Kaushal and R. Badru, Selective N-alkylation of amines with DMC over biogenic Cu-Zr bimetallic nanoparticles, ACS Omega, 2021, 6, 15300–15307 CrossRef CAS PubMed.
- M. Kaur, A. Sharma and Y. Singh, et al., Pd supported Al-BDC MOF for efficient and selective N-methylation of amines under solventless conditions, Emergent Mater., 2024, 7, 1683–1693 CrossRef CAS.
- M. Sharma, K. Verma and A. Kaushik, et al., DBU-MIm coupled ionic liquids as reusable catalysts for the Biginelli reaction, Mol. Catal., 2023, 536, 112906 CrossRef CAS.
- M. Govarthanan, C. Jeon and W. Kim, Synthesis and characterization of Lanthanum-based metal organic framework decorated polyaniline for effective adsorption of lead ions from aqueous solutions, Environ. Pollut., 2022, 303, 119049 CrossRef CAS PubMed.
- Q. Wu, C. Shen and C. Liu, Amino acid (histidine) modified Pd/SiO2 catalyst with high activity for selective hydrogenation of acetylene, Appl. Surf. Sci., 2023, 607, 154976 CrossRef CAS.
- Y. Zhang, K. Nie and L. Yi, et al., Recent Advances in Engineering of 2D Materials-Based Heterostructures for Electrochemical Energy Conversion, Adv. Sci., 2023, 10(31), e2302301 CrossRef PubMed.
- B. Chettiannan, G. Arumugam and M. Selvaraj, R. Rajendran. Exploring the effect of morphology on electrochemical performance of nickel and cobalt metal-organic frameworks and their derivatives for supercapacitor and hydrogen evolution, J. Energy Storage, 2024, 94, 112390 CrossRef.
- W. Hao, C. Sui and G. Cheng, et al., High-Strength Polycrystalline Covalent Organic Framework with Abnormal Thermal Transport Insensitive to Grain Boundary, Nano Lett., 2024, 24(14), 4248–4255 CrossRef CAS PubMed.
- T. Shi, Z. Wu and Z. Wu, et al., Postsynthetic amine modification of porous organic polymers for CO2 capture and separation, J. Polym. Sci., 2024, 62(8), 1554 CrossRef CAS.
- X. Hu, J. Wang and S. Li, et al., Pd-doped HKUST-1 MOFs for enhanced hydrogen storage: effect of hydrogen spillover, RSC Adv., 2023, 13, 14980–14990 RSC.
- C. V. Ramana, R. S. Vemuri and V. V. Kaichev, et al., X-ray Photoelectron Spectroscopy Depth Profiling of La2O3/Si thin films deposited by reactive magnetron sputtering, ACS Appl. Mater. Interfaces, 2011, 3(11), 4370–4373 CrossRef CAS PubMed.
- M. F. Sunding, K. Hadidi and S. Diplas, et al., XPS characterisation of in situ treated lanthanum oxide and hydroxide using tailored charge referencing and peak fitting procedures, J. Electron Spectrosc. Relat. Phenom., 2011, 184(7), 399–409 CrossRef CAS.
- S. Rojas-Buzo, P. Concepcion and A. Corma, et al., In Situ generated active Hf-hydride in Zeolites for the tandem N-alkylation of amines with benzyl alcohol, ACS Catal., 2021, 11(13), 8049–8061 CrossRef CAS.
- N. Hofmann and K. C. Hultzsch, Switching the N-Alkylation of Arylamines with Benzyl Alcohols to Imine Formation Enables the One-Pot Synthesis of Enantioenriched α-N-Alkylaminophosphonates, Eur. J. Org. Chem., 2019, 3105–3111 CrossRef CAS.
- S. Ghaffari and F. Kazemi, Highly Efficient Synthesis of N-Alkyl-α-amino Acid Methyl Esters by Microwave Irradiation, ChemistrySelect, 2021, 6, 2901–2905 CrossRef CAS.
- F. Niu, Q. Wang and Z. Yan, et al., Highly efficient and selective N-alkylation of amines with alcohols catalyzed by in situ rehydrated Titanium hydroxide, ACS Catal., 2020, 10(5), 3404–3414 CrossRef CAS.
- A. M. Rasero-Almansa, A. Corma, M. Iglesias and F. Sanchez, Design of a bifunctional Ir-Zr based metal-organic framework heterogeneous catalyst for the N-alkylation of amines with alcohols, ChemCatChem, 2014, 6(6), 1794–1800 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ma00372e |
| This journal is © The Royal Society of Chemistry 2025 |