Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
External stimuli responsive syneresis of amino acid-based bioactive hydrogels: a sustainable platform for environmental remediation†
Abhinandan
Bera
a,
Ritu Raj
Patel
b,
S. Daisy
Precilla
c,
Meenakshi
Singh
 b,
B.
Agiesh Kumar
c,
Sudipta
Bhowmik
b,
B.
Agiesh Kumar
c,
Sudipta
Bhowmik
 cd,
Mayank
Varshney
e and
Subhasish
Roy
cd,
Mayank
Varshney
e and
Subhasish
Roy
 *a
*a
aDepartment of Chemistry, Birla Institute of Technology and Science-Pilani, K K Birla Goa Campus, NH 17B, Zuarinagar, Sancoale, Goa 403726, India. E-mail: subhasishr@goa.bits-pilani.ac.in
bDepartment of Medicinal Chemistry, Institute of Medicinal Chemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005, UP, India
cMahatma Gandhi Medical Advanced Research Institute (MGMARI), Central Inter-Disciplinary Research Facility (CIDRF), Sri Balaji Vidyapeeth (Deemed to be University), Puducherry, 607402, India
dDepartment of Biophysics, Molecular Biology and Bioinformatics, University of Calcutta, 92, A.P.C. Road, Kolkata, 700009, India
eSenior Application Specialist, Characterization Division, Anton Paar India Pvt. Ltd., Phase V, Udyog Vihar Industrial Area, Gurgaon 122016, Haryana, India
First published on 9th June 2025
Abstract
Syneresis of hydrogels in the presence of external stimuli has potential applications in waste-water treatment and water purification. Fmoc-3-(1-naphthyl)-L-alanine (Fmoc-1Nap-A) and Fmoc-3-(2-naphthyl)-L-alanine (Fmoc-2Nap-A) form stable hydrogels in 50 mM phosphate buffer of pH 7.4 with minimum gelation concentrations (MGCs) 0.0455% (w/v) and 0.0375% (w/v) respectively. This is the first report on hydrogelation of Fmoc-1Nap-A and Fmoc-2Nap-A in phosphate buffer at physiological pH and their external stimuli-responsive syneresis. Fmoc-1Nap-A and Fmoc-2Nap-A are found to be biocompatible and exhibit potent anti-bacterial activity. Fmoc-1Nap-A shows anticancer activity towards the pancreatic cancer cell line, PANC-1 cells. Syneresis of Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels has been studied in the presence of various toxic metal ions and the thioflavin T dye. The hydrogel obtained from Fmoc-1Nap-A shows syneresis in the presence of HgII and NiII ions selectively and also in the presence of the thioflavin T (TT) dye; however, the hydrogel obtained from Fmoc-2Nap-A shows syneresis only in the presence of the thioflavin T (TT) dye. Structural, mechanical, and morphological characterization have been performed by using X-ray powder diffraction, FT-IR, rheology, and FE-SEM analyses. Interestingly, the absorption of the TT dye from water is more efficient for Fmoc-2Nap-A compared to Fmoc-1Nap-A. Various spectroscopic experiments have been carried out to estimate the absorption capacity for toxic metal ions and also the TT dye. Minimum amounts of 3.14 mM of 0.6 mL of HgII ions, 4.21 mM of 0.3 mL NiII ions and 3.14 mM of 0.1 mL of the TT dye for Fmoc-1Nap-A and 3.1361 mM of 0.2 mL of the TT dye for Fmoc-2Nap-A are at least required to be added on top of 2 mL of 0.08% w/v concentration of hydrogels to exhibit syneresis. The absorption capacities for the TT dye are estimated to be 912.51 μg and 981.92 μg from 1000 μg of TT dye solution by 2 mL of Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels respectively at a concentration of 0.08% w/v (1600 μg gelator molecules) each by using UV-Vis spectroscopy. ICP-MS data also reveal that 2 mL of the Fmoc-1Nap-A gel is capable of removing 208.29 μg of HgII ions from a total of 440.61 μg of HgII contaminated water after syneresis. These hydrogels are capable of removing toxic heavy metal ions (HgII) and toxic dye (TT) selectively from waste-water sustainably, showing applicability in the field of water research for environmental remediation.
Introduction
Amino acid-based low-molecular-weight hydrogelators are gaining significant attention globally in the era of modern cutting-edge research due to their versatile applications from bench to bedside.1–12 The presence of the hydrophilic edge (–COOH, C terminus), hydrophobic edge (protected N-terminus), and appropriate side chains makes the molecules efficient hydrogelators by maintaining a judicious balance of hydrophobicity and hydrophilicity. In the three-dimensional network structures, hydrogelator molecules are bound together through various weak non-covalent interactions including hydrogen bonding, pi–pi stacking, and van der Waals interaction13–17 and hold huge amounts of water to form hydrogels. Research on amino acid-based hydrogels extends to the field of tissue engineering,18 drug delivery systems,19–24 toxic metal ions and anion sensing,25–28 dye absorption and toxic metal ion removal,29–31 nanoparticle and nanocluster syntheses,32–34 antibacterial agents,35–38 photo-switching behavior39 and catalysis.40–42 Yang and his co-workers have previously reported hydrogelation of Fmoc-3-(2-naphthyl)-D-alanine in aqueous Na2CO3 solution and its hybrid hydrogelation within the agarose hydrogel that can remove toxic dyes like methyl violet from aqueous solution.43 When a gel expels solvent from its three-dimensional cross-linked matrix system, the volume of the gel matrix decreases from its initial volume, called syneresis of the gel.44 Thus, it is a process of phase-separation from the gel state to a mixture of gel and solution states by excluding solvent from the initial volume of the gel state. Syneresis is a common and natural phenomenon that happens when serum release during blood clotting.45 The food quality has also been determined by syneresis in various foods including jams, surimi, jellies, sauces, dairy products, tomato juice, meat, and soybean products. Various hydrogels show syneresis by themselves upon aging.46,47 A different type of hydrogelator that forms a stable hydrogel in the presence of an aqueous buffer and is stable for a long time, however, undergoes shrinking by releasing some water from the hydrogel matrix upon the addition of specific stimuli through absorption followed by co-assembly are known as external stimuli responsive syneresis. The self-shrinking of the hydrogels is comparatively common; however, the external stimuli-induced shrinkage of hydrogels is not yet explored well. The first thermally responsive syneresis effect of supramolecular polymers based on glycosylated amino acid has been reported by the Hamachi research group.44 They have shown that gels expelled water from the three-dimensional matrix upon application of heat and again upon cooling down the gel swelled to regain its pristine state. In the process of syneresis, hydrogels release water molecules from their three-dimensional network induced by external stimuli, which may be due to the enhanced hydrophobicity at their local network structures by reducing volume compared to the initial stage. Banerjee's group has reported a self-shrinkable low molecular weight, triphenyl alanine-based hydrogel.47 After forming a stable hydrogel at pH 7.4, it shows syneresis upon aging for 7 days. Syneresis has been used for the removal of toxic metal ions and dye including PbII and methylene blue.47 Ulijn and his research group have reported mechanical contact assisted syneresis of dipeptide diphenylalanine (FF) and its amidated derivative (FF-NH2)-based hydrogels.48 Liu and co-workers have reported a peptide-based hydrogelator, OGAc (N-octadecanoyl-1,5-bis(L-glutamic acid)-L-glutamic diamide), which forms a stable hydrogel in the presence of a wide range of metal ions, and concluded that most of the metal ions triggered the syneresis of the gel.49 They have observed that with the use of increasing amounts of metal ions in the gel, the syneresis occurs more rapidly.49 Das and his research group have discovered azobenzene-functionalized short peptide-based stable gel formation in an aqueous medium; however, upon irradiation with UV light (365 nm) the gel exhibited syneresis, and the gel matrix released around 50% of solvent from the matrix.50 Adams and his research group have investigated the change in the hydrophobicity of the system with the addition of different organic salts and the controlled syneresis property of a peptide-based hydrogel.51 External stimuli can be any salt,51 compound,52 metal ions,47 pH,53–55 temperature,56 UV light50,56 and mechanical force.48 As a result, the expelled water may carry hydrogel nanofibers due to leaching. Syneresis of hydrogels is very useful in some research areas, including water purification,47,50 drug release,49 biosensing,55 and microfluidic devices. In addition to amino acid/peptide-based shrinkable hydrogels, some hydrogels are evolved based on other organic molecules and show syneresis upon aging57–59 or in the presence of external stimuli,60 which may be due to the increase of local hydrophobicity.Here, we report stable hydrogel formation by Fmoc-3-(1-naphthyl)-L-alanine (Fmoc-1Nap-A) and Fmoc-3-(2-naphthyl)-L-alanine (Fmoc-2Nap-A) in 50 mM phosphate buffer of pH 7.4. The Fmoc-1Nap-A hydrogel exhibits a very interesting syneresis property in the presence of external stimuli including toxic metal ions (HgII), NiII ions, and thioflavin T (TT) toxic dye; however, Fmoc-2Nap-A shows syneresis only in the presence of external stimulus, thioflavin T (TT) toxic dye. Thus, the isomeric Fmoc-1Nap-A and Fmoc-2Nap-A can be distinguished by simple external stimuli induced syneresis by toxic heavy metal ions HgII in water and NiII ions in water, which is a very unique property. Both Fmoc-1Nap-A and Fmoc-2Nap-A are very selective and have not shown any syneresis in the presence of other metal ions including AgI, BaII, CaII, CdII, CoII, CrVI, CuII, FeII, KI, MgII, NaI, PbII and ZnII. Interestingly, the TT dye absorbing capacity of the Fmoc-2Nap-A hydrogel is much higher than that of Fmoc-1Nap-A. Spectroscopic investigations suggest that waste-water treatment can be done to obtain pure water as the dye-absorbed hydrogels release clean water after syneresis. Thus, this approach is one of the green, clean, cost-effective and sustainable platforms for environmental remediation indeed. As waste-water contains high concentrations of toxic dyes and heavy metal ions, these Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels can be used to treat waste-water samples to get pure water. The syneresis of Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels induced by high concentrations of HgII ions in contaminated waste-water can be used to distinguish between the two isomers of Fmoc-1Nap-A and Fmoc-2Nap-A because Fmoc-1Nap-A only shows syneresis in the presence of the external stimulus of HgII ions; however, Fmoc-2Nap-A does not show syneresis by the external stimulus like HgII ions. In terms of real field applications, it is important for the materials that will be used for water purification to be safe to handle, non-toxic and are capable of killing and removing bacteria. Water born bacteria can also be killed and purified by using such hydrogels. Thus, there is a relation between water purification and biocompatibility and antibacterial activity. Bio-compatible antimicrobial materials attract high interest in terms of environmental remediation for water research. Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels are found to be biocompatible and show potent anti-bacterial, anti-oxidant and anticancer activities. Such kinds of biocompatible hydrogel materials can be used for sustainable water research in the future.
Experimental section
Instrumentation, sample preparation, and method of analysis
Instrumentation techniques, detailed sample preparation procedures, instrumentations and instrumental methods of analysis are discussed in the ESI.†Materials and methods
Fmoc-3-(1-naphthyl)-L-alanine (Fmoc-1Nap-A) and Fmoc-3-(2-naphthyl)-L-alanine (Fmoc-2Nap-A) are purchased from TCI and the thioflavin T dye (molecular weight: 318.87) was purchased from SRL. Dulbecco's modified Eagle's medium (DMEM, catalogue no. AL007S), Trypsin-EDTA solution 10× (catalogue no. TCL070), and phosphate buffered saline (PBS) (catalogue no. TS1006) were purchased from Hi-Media, Mumbai. Fetal Bovine Serum (FBS) (catalogue no. A5256701) was procured from Thermo Fisher Scientific, South America. Penicillin/streptomycin antibiotic solution (catalogue no. A001) was purchased from Hi-Media, Mumbai. All the other chemicals and reagents used in this study were purchased from SRL, Merck, TCI and Thermo Fisher Scientific, South America.Results and discussion
Hydrogelation studies
Amphiphilic Fmoc-3-(1-naphthyl)-L-alanine (Fmoc-1Nap-A) and Fmoc-3-(2-naphthyl)-L-alanine (Fmoc-2Nap-A) undergo self-assembly to form hydrogels in 50 mM phosphate buffer of pH 7.4 separately. Initially, 2 mg of each hydrogelator (Fmoc-1Nap-A and Fmoc-2Nap-A) were transferred into two different 5 mL screw-capped glass vials followed by the addition of 2 mL of 50 mM phosphate buffer solution of pH 7.4. After that, the screw-capped glass vials containing the hydrogelators in phosphate buffer were sonicated for 5 minutes followed by heating strongly on a hot plate (at 150 °C for 10 minutes) to dissolve completely and then kept at rest at room temperature. Both Fmoc-1Nap-A and Fmoc-2Nap-A form stable hydrogels after 10 minutes. To find the minimum gelation concentrations (MGCs), 200 μL of phosphate buffer solutions at each interval were added into these hydrogel vials and heated to dissolve followed by keeping them at rest to check whether the formation of the hydrogels is taking place or not. This way the minimum concentration of these hydrogelators in the phosphate solution has been estimated after repeating each hydrogel in three different vials. The minimum gelation concentration (MGC) of Fmoc-1Nap-A is estimated to be 0.0455 ± 0.0007% (w/v) (1.0400 mM) and for Fmoc-2Nap-A it is estimated to be 0.0375 ± 0.0023% (w/v) (0.8572 mM). The chemical structures and hydrogels in tilted glass vials of Fmoc-1Nap-A and Fmoc-2Nap-A are shown in Fig. 1. | ||
| Fig. 1 Chemical structures of Fmoc-3-(1-naphthyl)-L-alanine (Fmoc-1Nap-A) and Fmoc-3-(2-naphthyl)-L-alanine (Fmoc-2Nap-A) and hydrogels in tilted glass vials. | ||
Gel melting temperature (Tgel) estimation
To know the thermal stability of these hydrogels (Fmoc-1Nap-A and Fmoc-2Nap-A), hydrogels at different concentrations have been prepared for both the hydrogelators. Concentrations of 0.06% (w/v), 0.07% (w/v), 0.08% (w/v), 0.09% (w/v), 0.10% (w/v) and 0.11% (w/v) hydrogels have been used for both Fmoc-1Nap-A and Fmoc-2Nap-A for a comparative study.The melting points of the hydrogels at different concentrations for both Fmoc-1Nap-A and Fmoc-2Nap-A have been measured by submerging in a digitally displayed temperature-controlled water bath. At each concentration, three hydrogels at three different glass vials have been prepared and their melting points have been measured. The average melting points at every concentration have been considered for Tgelvs. concentration profiles (Fig. 2). The standard deviation error for each concentration has been incorporated (Fig. 2). At each temperature, the hydrogel vials have been kept submerged in the water bath for at least 5 minutes to check the gelation melting points. Fig. 2 shows that the Fmoc-2Nap-A hydrogel is thermally more stable than the Fmoc-1Nap-A hydrogel.
Rheological studies
To know the mechanical strength and viscoelastic behavior61 of the Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels at 0.1% (w/v), frequency sweep rheological experiments have been performed. Both the Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels’ storage moduli (G′) are found to be higher than the loss moduli (G′′) (Fig. 3), indicating the stability of the hydrogels within the studied angular frequency region. Fig. 3 indicates the higher mechanical stability of the Fmoc-2Nap-A hydrogel compared to Fmoc-1Nap-A, which may be due to the lower MGC value of Fmoc-2Nap-A than Fmoc-1Nap-A. | ||
| Fig. 3 Rheological frequency sweep profile as a function of storage modulus (G′) and loss modulus (G′′) for Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels. | ||
UV-Vis spectroscopic study
A UV-Vis absorption spectroscopic study of the hydrogels Fmoc-1Nap-A and Fmoc-2Nap-A and their dilute solutions has been performed to understand the aggregation behavior of the hydrogels. Absorption maxima (Fig. S1, ESI†) at 265 nm have been observed for both Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels. A decrease in absorption intensities with no shift in absorption maxima has been observed for gel to sol transition upon dilution, indicating a higher scattering effect and absorption from the higher order aggregates (Fig. S1, ESI†) for both the hydrogels compared to their diluted solution states.62,63Fluorescence spectroscopic study
A fluorescence spectroscopic study of Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels below their MGCs at various dilutions has been performed. Fmoc-1Nap-A shows emission maxima at 337 nm; however, Fmoc-2Nap-A shows fluorescence emission maxima at 336 nm upon excitation at a wavelength of 265 nm (Fig. S2, ESI†). Upon dilution from 918.45 μM hydrogel aggregated solutions it has been found that initially fluorescence intensity started to increase for 457.14 μM and 304.76 μM solutions and then gradually started to decrease upon dilution for both the hydrogels (Fig. S2, ESI†). Overall, the fluorescence intensities have been enhanced upon dilution compared to their concentrated solutions up to a certain concentration of dilutions. The enhancement of fluorescence emission intensities suggests higher ordered aggregation62,63 in their self-assembled states.Structural studies
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching frequency of the aromatic ring for the Fmoc-1Nap-A bulk solid (Fig. S3 and Table S1, ESI†). Significant peaks at 3317.56 cm−1 (strong) due to N–H stretching frequency for the amide bond, 1691.57 cm−1 (strong) for carbonyl stretching frequency for the amide group, 1533.40 cm−1 (strong) for N–H bending of the amide group and a very weak peak at 1600.91 cm−1 for C
C stretching frequency of the aromatic ring for the Fmoc-1Nap-A bulk solid (Fig. S3 and Table S1, ESI†). Significant peaks at 3317.56 cm−1 (strong) due to N–H stretching frequency for the amide bond, 1691.57 cm−1 (strong) for carbonyl stretching frequency for the amide group, 1533.40 cm−1 (strong) for N–H bending of the amide group and a very weak peak at 1600.91 cm−1 for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching frequency of the aromatic group for the Fmoc-2Nap-A bulk solid have been observed. The Fmoc-1Nap-A xerogel shows characteristic peaks at 3334.92 cm−1, 1691.57 cm−1 and 1535.33 cm−1 for N–H stretching of the amide bond, carbonyl stretching of the amide bond and N–H bending of the amide bond respectively. The Fmoc-2Nap-A xerogel shows significant peaks at 3379.28 cm−1, 1691.57 cm−1, and 1535.33 cm−1 for NH stretching of the amide bond, carbonyl stretching of the amide bond and N–H bending of the amide bond respectively. It has been understood from the significant changes in their peak positions for Fmoc-1Nap-A and Fmoc-2Nap-A xerogels compared to their native bulk solid that due to the involvement of hydrogen bonding interaction hydrogel formation has been observed (Fig. S3, ESI†) for both the hydrogelators.
C stretching frequency of the aromatic group for the Fmoc-2Nap-A bulk solid have been observed. The Fmoc-1Nap-A xerogel shows characteristic peaks at 3334.92 cm−1, 1691.57 cm−1 and 1535.33 cm−1 for N–H stretching of the amide bond, carbonyl stretching of the amide bond and N–H bending of the amide bond respectively. The Fmoc-2Nap-A xerogel shows significant peaks at 3379.28 cm−1, 1691.57 cm−1, and 1535.33 cm−1 for NH stretching of the amide bond, carbonyl stretching of the amide bond and N–H bending of the amide bond respectively. It has been understood from the significant changes in their peak positions for Fmoc-1Nap-A and Fmoc-2Nap-A xerogels compared to their native bulk solid that due to the involvement of hydrogen bonding interaction hydrogel formation has been observed (Fig. S3, ESI†) for both the hydrogelators.
X-ray diffraction study
To investigate the molecular packing arrangements and the involvement of various non-covalent interactions in supramolecular hydrogelation, X-ray diffraction analysis has been performed. Molecular lengths for Fmoc-1Nap-A and Fmoc-2Nap-A have been found at 17.03 Å and 17. 97 Å (at a lower energy state obtained from ChemDraw 3D) respectively. Both hydrogelators, Fmoc-1Nap-A and Fmoc-2Nap-A, formed anti-parallel beta-sheet-like structures in their hydrogel states. Peaks at a d-spacing of 4.61 Å (2θ = 19.22°) represent the distance between two adjacent beta-strands and a d-spacing value of 9.33 Å (2θ = 9.47°) represents the distance between two neighbouring layers of the beta-sheet for Fmoc-1Nap-A (Fig. 4). The peak at a d-spacing value of 4.66 Å (2θ = 19.00°) represents the distance between two adjacent beta strands and that at a d-spacing value of 10.07 Å (2θ = 8.77°) represents the distance between two neighbouring layers of the beta-sheet for Fmoc-2Nap-A (Fig. 4). Both the hydrogelators' (Fmoc-1Nap-A and Fmoc-2Nap-A) pi–pi stacking interaction peaks in their xerogel states have appeared at a d-spacing of 3.81 Å. Both the hydrogelators have formed lamellar structures in their self-assembled hydrogel states due to the presence of peaks at D/2, D/3, D/4, and D/5 respectively (D obtained from ChemDraw 3D at their lower energy states) (Table S2, ESI†).Morphology study
The FE-SEM experiment has been performed to understand the morphological characteristics of Fmoc-1Nap-A and Fmoc-2Nap-A in their hydrogel states. Cross-linked nanofibrillar network structures have been found for both Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels. Also, it has been found that the nanofiber density of Fmoc-2Nap-A is more compared to that of Fmoc-1Nap-A. The average diameter has been estimated to be 8.77 ± 1.21 nm and 11.698 ± 3 nm for Fmoc-1Nap-A and Fmoc-2Nap-A in their hydrogel states (Fig. 5) respectively. Entangled nanofibrillar morphology for both Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels has also been obtained from a high resolution transmission electron microscopic (HR-TEM) imaging study (Fig. S4, ESI†).FE-SEM images reveal the presence of more cross-linked nanofibrillar morphology for the Fmoc-2Nap-A hydrogel compared to Fmoc-1Nap-A hydrogel. This suggests stronger hydrogel formation for Fmoc-2Nap-A compared to Fmoc-1Nap-A hydrogel at the same concentration [0.1% (w/v)] which has also evident from rheological measurements. This could also be due to the higher MGC of the Fmoc-1Nap-A hydrogel compared to that of the Fmoc-2Nap-A hydrogel.
Syneresis of hydrogels in the presence of toxic metal ions
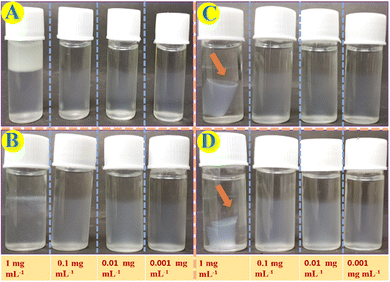
Various metal ion aqueous solutions including AgI, NaI, KI, CuII, NiII, CaII, ZnII, CdII, PbII, HgII, BaII, MgII and FeII have been added on top of both the Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels to investigate external stimuli induced syneresis. Interestingly, the Fmoc-1Nap-A hydrogel shows syneresis in the presence of NiII and HgII metal ions; however, Fmoc-2Nap-A did not show syneresis in the presence of a series of metal ions used for syneresis. The effects of concentrations and volume of the external stimuli have been investigated to know the critical concentrations and volume required for the syneresis.A control experiment has been performed with Milli Q water for comparison with HgII and NiII metal ion solutions to determine whether the syneresis happened only in the presence of these salt solutions or it happened in the presence of water only (Fig. S5, ESI†). In this experiment, 2 mL (0.08% w/v) of the Fmoc-1Nap-A hydrogel has been taken in three different vials. 1 mL of HgII and NiII metal ion solutions and 1 mL of Milli Q water have been added on top on these three Fmoc-1Nap-A hydrogel vials. Vials were kept undisturbed for three days. It has been found that syneresis has been observed in the vials with HgII and NiII metal ions. However, dissolution of the hydrogelators has been observed for all these systems as evident from the UV-Vis spectroscopic study (Fig. S6, ESI†). The amount of hydrogelator dissolution happened after 3 days for these three glass vials has been estimated using a standard calibration curve (Fig. S7, ESI†), Fig. S6 and Table S3 (ESI†).
ICP-MS analysis
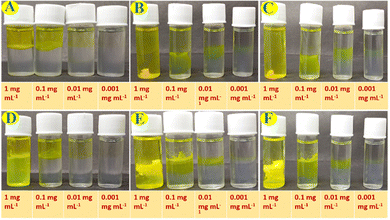
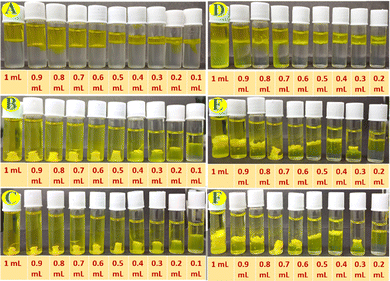
Percent removal capacity of HgII ions from HgII ions contaminated water by the Fmoc-1Nap-A hydrogel has been estimated by using ICP-MS analysis on the released water part after the HgII ions induced syneresis of the hydrogel. Estimation of % removal of the HgII ions from the contaminated water by the Fmoc-1Nap-A hydrogel has been shown in Table S4 and Fig. S8 (ESI†). It has been found that due to syneresis (Fig. S8, ESI†), the Fmoc-1Nap-A hydrogel is capable of removing 47.20% (Table S4, ESI†) of the HgII metal ions from water. Each 2 mL of the Fmoc-1Nap-A hydrogel is capable of removing 208.29 μg of HgII ions from a total of 440.61 μg of HgII metal ions contaminated water (Table S4 and Fig. S8, ESI†).The UV-Vis absorption spectroscopic profiles of the released water by concentration and volume dependent syneresis of Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels are shown in Fig. S11–S14 (ESI†) obtained from Fig. 8 and 9. The volume corrections of the released water have also been considered after syneresis in the calculation. It has been found that the TT dye absorption capacity of the Fmoc-2Nap-A hydrogel is more than that of the Fmoc-1Nap-A hydrogel (Tables S5–S9, ESI†). At the concentrations of 0.001 mg mL−1 and 0.01 mg mL−1 for dye solutions, both the hydrogels (Fmoc-1Nap-A and Fmoc-2Nap-A) have absorbed 100% dye from the solution. The minimum volume of TT dye required at a concentration of 1 mg mL−1 for the syneresis to happen has been found to be 0.2 mL for the Fmoc-1Nap-A hydrogel [2 mL at 0.08% (w/v)] and 0.3 mL for the Fmoc-2Nap-A hydrogel [2 mL at 0.08% (w/v)].
Morphology and structural study of the co-assembled shrink hydrogel matrixes and released water after syneresis induced by metal ions and TT dye
FT-IR analysis
The Fmoc-1Nap-A xerogel's characteristic peaks in the FT-IR spectrum at 3334.92 cm−1, 1691.57 cm−1, 1598.98 cm−1, 1535.33 cm−1 for N–H stretching of amide, carbonyl stretching frequency of the amide bond, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and N–H bending of the amide group respectively have been observed. However, for the Fmoc-1Nap-A-HgII matrix, N–H stretching frequency of the amide bond, carbonyl stretching frequency of the amide bond, C
C and N–H bending of the amide group respectively have been observed. However, for the Fmoc-1Nap-A-HgII matrix, N–H stretching frequency of the amide bond, carbonyl stretching frequency of the amide bond, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching frequency of the aromatic moiety and N–H bending of the amide bond have been found at 3323.34 cm−1, 1691.57 cm−1, 1597.05 cm−1 and 1535.33 cm−1 respectively and for the Fmoc-1Nap-A-NiII matrix N–H stretching frequency of the amide bond, carbonyl stretching frequency of the amide bond, C
C stretching frequency of the aromatic moiety and N–H bending of the amide bond have been found at 3323.34 cm−1, 1691.57 cm−1, 1597.05 cm−1 and 1535.33 cm−1 respectively and for the Fmoc-1Nap-A-NiII matrix N–H stretching frequency of the amide bond, carbonyl stretching frequency of the amide bond, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching frequency of the aromatic moiety and N–H bending of the amide bond have been found at 3325.27 cm−1, 1693.50 cm−1, 1597.05 cm−1 and 1535.33 cm−1 respectively. It has been found that for both Fmoc-1Nap-A-HgII and Fmoc-1Nap-A-NiII shrink hydrogel matrixes, the N–H stretching frequencies have changed notably compared to the Fmoc-1Nap-A native xerogel, suggesting hydrogelator–metal ion interactions through amide N–H (Fig. S15, ESI†). Thus, the released water that contains leached hydrogel matrix nanofibers (dissolution) has been more affected due to effective interactions in the solution state over the gel state between the hydrogelator nanofibers and the metal ions (HgII and NiII). This may be the probable reason for the destroyed structure of the nanofibers observed in the released water in the presence of metal ions (HgII and NiII).
C stretching frequency of the aromatic moiety and N–H bending of the amide bond have been found at 3325.27 cm−1, 1693.50 cm−1, 1597.05 cm−1 and 1535.33 cm−1 respectively. It has been found that for both Fmoc-1Nap-A-HgII and Fmoc-1Nap-A-NiII shrink hydrogel matrixes, the N–H stretching frequencies have changed notably compared to the Fmoc-1Nap-A native xerogel, suggesting hydrogelator–metal ion interactions through amide N–H (Fig. S15, ESI†). Thus, the released water that contains leached hydrogel matrix nanofibers (dissolution) has been more affected due to effective interactions in the solution state over the gel state between the hydrogelator nanofibers and the metal ions (HgII and NiII). This may be the probable reason for the destroyed structure of the nanofibers observed in the released water in the presence of metal ions (HgII and NiII).
Both the xerogel Fmoc-1Nap-A-TT and Fmoc-2Nap-A-TT-matrixes show N–H stretching frequencies of 3334.92 cm−1 and 3325.27 cm−1 respectively. Other characteristic peaks observed for the Fmoc-1Nap-A-TT matrix include 1691.57 cm−1 for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency of the amide bond, 1598.98 cm−1 for C
O stretching frequency of the amide bond, 1598.98 cm−1 for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching frequency of the aromatic ring, and 1535.35 cm−1 for the NH bending of the amide bond. C
C stretching frequency of the aromatic ring, and 1535.35 cm−1 for the NH bending of the amide bond. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency observed at 1691.57 cm−1, C
O stretching frequency observed at 1691.57 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching frequency of the aromatic ring observed at 1600.91 cm−1 and N–H bending of the amide bond observed at 1535.33 cm−1 for the Fmoc-2Nap-A-TT matrix. The N–H stretching frequencies of both xerogel Fmoc-1Nap-A-TT and Fmoc-2Nap-A-TT-matrixes have been red shifted from their native solids (Fig. S3, ESI†). The pure solid TT dye shows two characteristic peaks at 1600.91 cm−1 and 1504.47 cm−1, for the aromatic moiety (Fig. S15, ESI†). The metal ion (HgII and NiII)/dye (TT)–hydrogelator interaction through amide N–H probably increased the local hydrophobicity of the hydrogels and thus syneresis happened.
C stretching frequency of the aromatic ring observed at 1600.91 cm−1 and N–H bending of the amide bond observed at 1535.33 cm−1 for the Fmoc-2Nap-A-TT matrix. The N–H stretching frequencies of both xerogel Fmoc-1Nap-A-TT and Fmoc-2Nap-A-TT-matrixes have been red shifted from their native solids (Fig. S3, ESI†). The pure solid TT dye shows two characteristic peaks at 1600.91 cm−1 and 1504.47 cm−1, for the aromatic moiety (Fig. S15, ESI†). The metal ion (HgII and NiII)/dye (TT)–hydrogelator interaction through amide N–H probably increased the local hydrophobicity of the hydrogels and thus syneresis happened.
Effect of other metal ions on Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels
Syneresis of the hydrogels Fmoc-1Nap-A and Fmoc-2Nap-A has also been tested in the presence of 20 mg mL−1 of different metal salts including AgNO3 (A), BaCl2 (B), CaCl2 (C), Cd(OAc)2 (D), Co(NO3)2 (E), CuSO4 (F), FeSO4 (G), Hg(OAc)2 (H), HgCl2 (I), K2CrO4 (J), KBr (K), MgSO4 (L), NaCl (M), NaOAc (N), NiCl2 (O), Pb(OAc)2 (P), and ZnSO4 (Q). The fate of both Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels in the presence of 20 mg mL−1 of different metal salts is shown in Fig. S17 and S18 (ESI†). It was found that only the Fmoc-1Nap-A hydrogel showed syneresis in the presence of Hg(OAc)2, HgCl2, and NiCl2 selectively. No syneresis has been observed for Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels in the presence of other metal ions.Biological evaluation
Against P. aeruginosa and K. pneumoniae, the positive controls levofloxacin and ciprofloxacin exhibited inhibition zones of 27 ± 1 mm and 21 ± 1 mm, respectively. In comparison, Fmoc-1Nap-A showed strong antibacterial activity against P. aeruginosa, with an inhibition zone of 24 ± 1 mm, approaching the efficacy of levofloxacin. However, its activity against K. pneumoniae was significantly less, with an inhibition zone of 11 ± 2 mm. Fmoc-2Nap-A; while less effective than Fmoc-1Nap-A, it still showed better antibacterial activity, with inhibition zones of 21 ± 2 mm for P. aeruginosa while poor activity with an inhibition zone of 8 ± 1 mm for K. pneumoniae. The blood agar shows no hemolysis by Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels, with Tween 80 as the positive control, and PBS as the negative control. The difference in antibacterial activity between Fmoc-1Nap-A and Fmoc-2Nap-A could be attributed to variations in their chemical composition or structural properties, which may influence their ability to penetrate bacterial cell walls or disrupt bacterial processes. The stronger antibacterial efficacy of Fmoc-1Nap-A against both Gram-positive and Gram-negative bacteria suggests that it may possess more favorable physicochemical characteristics that enhance its antibacterial efficacy. Gram-positive bacteria, with their thicker peptidoglycan layer,64 tend to be more susceptible to antibacterial agents that can disrupt cell wall synthesis, which might explain the effectiveness of Fmoc-1Nap-A. On the other hand, Gram-negative bacteria possess an additional outer membrane that acts as a barrier to many antibiotics, making them more resistant;65 however, the ability of Fmoc-1Nap-A to inhibit P. aeruginosa suggests that it may have mechanisms that enable it to overcome this barrier to some extent. Therefore, the promising antibacterial activity of hydrogel Fmoc-1Nap-A, particularly against P. aeruginosa and S. aureus, makes it a potential candidate for further development as an antibacterial agent.
Hemolytic assay
The hemolytic activity of hydrogels Fmoc-1Nap-A and Fmoc-2Nap-A has been assessed using a blood agar plate, alongside positive and negative controls, Tween 80 and PBS, respectively to assess their potential cytotoxicity towards erythrocytes. The results demonstrated that Tween 80 used as the positive control exhibited a clear zone of hemolysis, confirming its cytotoxic nature. In contrast, PBS as the negative control showed no hemolysis validating its non-hemolytic nature. Both hydrogels, Fmoc-1Nap-A and Fmoc-2Nap-A, displayed no visible hemolysis on the blood agar plate, suggesting that neither hydrogel induces red blood cell lysis (Fig. 12). This lack of hemolysis confirms the biocompatibility of Fmoc-1Nap-A and Fmoc-2Nap-A, making them suitable candidates for biomedical applications where non-cytotoxicity is crucial, such as in wound dressings or drug delivery systems. The non-hemolytic nature of these hydrogels, combined with the previously observed antibacterial efficacy of Fmoc-1Nap-A, further supports their potential for safe and effective use in clinical settings.Antioxidant assay
This is the most commonly used method for estimating antioxidant activity, where DPPH changes from purple to yellow, indicating the scavenging properties of the synthesized compound.66 In the DPPH assay, the purple DPPH radical is reduced when an antioxidant donates a hydrogen atom or electron, neutralizing it and forming a non-radical compound (DPPH-H). This reaction results in a color change from purple to yellow. The decrease in color intensity, measured at 517 nm, is directly correlated with the antioxidant's radical scavenging capability. The positive and negative control used were methanol and ascorbic acid, whereas the hydrogels of both Fmoc-1Nap-A and Fmoc-2Nap-A were both evaluated at concentrations of 250 μg mL−1, 500 μg mL−1, 750 μg mL−1, 1000 μg mL−1, 1250 μg mL−1, 1500 μg mL−1, 1750 μg mL−1, 2000 μg mL−1, 2250 μg mL−1, and 2500 μg mL−1 for the antioxidant potential. The hydrogels Fmoc-1Nap-A and Fmoc-2Nap-A both demonstrated effective antioxidant activity, with Fmoc-1Nap-A having a scavenging activity of 67% and Fmoc-2Nap-A having a scavenging activity of 54% while comparing it with ascorbic acid having 88%. The results are summarized in Fig. 13. Hydrogels Fmoc-1Nap-A and Fmoc-2Nap-A showed 50% scavenging activity at 1000 μg μL−1 and 2000 μg mL−1 respectively, whereas comparing it with ascorbic acid showed 50% scavenging activity at 750 μg mL−1. The antioxidant properties of the compound depend on its reducing capability; hydrogel Fmoc-1Nap-A has the best antioxidant potential as it reduces the free radical to the greatest extent, while the compound Fmoc-2Nap-A has demonstrated promising antioxidant potential.Effect of the compounds on the proliferation of pancreatic cancer cells
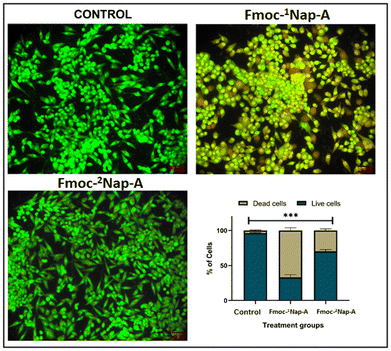
To determine if the compounds had elicited any cytotoxic effect in pancreatic cancer cells, an MTT assay has been performed using PANC-1 cell lines. The individual IC50 values of the compounds Fmoc-1Nap-A and Fmoc-2Nap-A were 77.97 μL and 145.51 μL, respectively. Among the compounds, Fmoc-1Nap-A displayed the most potent minimal effective concentration at an average of 77. 97 μL (Fig. 14). However, Fmoc-2Nap-A depicted only a moderate anti-proliferative effect with the highest minimal IC50 value (Fig. 14).Effect of the compounds on the morphology of pancreatic cancer cells
To elucidate if the growth-inhibitory effect observed by the cell viability assay was due to the induction of apoptosis, AO/EB dual staining has been performed. Following staining, it has been observed that about 95% of the control cells were spindle-shaped, emitting green fluorescence with clear demarcation and whole nucleus (Fig. 15). Upon exposure to the compounds, a gradual reduction in the percentage of viable cells (emitting green fluorescence) with a concomitant increase in the apoptotic cells (emitting orange-red fluorescence) has been observed. Markedly, among the treatment groups employed, Fmoc-1Nap-A depicted a significant increase in the number of apoptotic cells, with approximately 64% of apoptotic bodies emitting orange-red EB fluorescence when compared with Fmoc-2Nap-A and the mock group (p < 0.001) (Fig. 15). However, about 72% of the cells treated with Fmoc-2Nap-A portrayed a green fluorescence (Fig. 15). Overall, the AO/EB staining results suggested that the compound Fmoc-1Nap-A was more effective in the morphological induction of apoptosis in the chosen pancreatic cancer cell line.Conclusion
Fmoc-1Nap-A and Fmoc-2Nap-A form stable hydrogels in 50 mM phosphate buffer of pH 7.4. External stimuli responsive syneresis in the presence of HgII and NiII metal ions for the Fmoc-1Nap-A hydrogel and thioflavin T induced syneresis for both the Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels have been selectively reported in this study. This study reveals that the Fmoc-1Nap-A hydrogel can remove toxic heavy HgII metal ions through absorption of metal ions within the gel matrix followed by syneresis induced by a high amount of toxic HgII metal ions containing waste-water effectively. ICP-MS data show the effectiveness of this toxic metal ion removal by the Fmoc-1Nap-A hydrogel. Both the Fmoc-1Nap-A and Fmoc-2Nap-A hydrogels can absorb thioflavin T very efficiently from dye contaminated waste-water in the gel matrix and release almost pure water by the syneresis effect. Fmoc-2Nap-A shows higher efficiency for the removal of the TT dye from the water compared to Fmoc-1Nap-A. The MTT study shows that both the hydrogels are biocompatible and show anticancer activity toward the pancreatic cancer cell line. Both of these hydrogels show potent anti-bacterial and anti-oxidant properties. All of these experiments uphold the importance of supramolecular chemistry and gelation study, where weak interactions including pi–pi stacking, hydrogen bonding and indeed hydrophobic interactions play key roles in hydrogelation followed by external stimuli responsive syneresis, suggesting application in environmental remediation. The presence of high content of HgII toxic metal ions and toxic dyes in waste-water can be detected by using these hydrogels and their corresponding water treatment can be performed to get clean water following green and sustainable pathways.Author contributions
All authors have approved the final version of the manuscript. A. B. was responsible for the hydrogelation, methodology, investigations, characterization, graph preparation from raw data and their calculations, writing the early-stage draft, and formatting. R. R. P. was responsible for performing antibacterial, hemolytic, and antioxidant studies. S. D. P. was responsible for the MTT assay and cell line studies. M. S. was responsible for the supervision of the antibacterial, hemolytic, and antioxidant studies and this part writing. B. A. K. and S. B. were responsible for the supervision and the writing of the MTT assay and cell line studies. M. V. was responsible for the rheological measurements. S. R. was responsible for conceptualization, supervision, writing of the manuscript, review, editing, and finalization of the manuscript and ESI.†Data availability
The data used to support this study findings are included within the paper and in the supplementary information file (ESI†).Conflicts of interest
The authors declare there are no conflicts of interest.Acknowledgements
ANRF (previously known as SERB)-SURE Research Grant, DST, New Delhi, India (Project no. SUR/2022/000101) is gratefully acknowledged for the financial support. S. R. sincerely thanks ANRF for the research grant. A. B. sincerely thanks BITS-Pilani, K K Birla Goa Campus for PhD position and PhD fellowship. We acknowledge the central sophisticated instrumentation facility (CSIF) and Department of Chemistry of BITS-Pilani, K K Birla Goa Campus for the instrumental facilities.Notes and references
- C. Ren, L. Chu, F. Huang, L. Yang, H. Fan, J. Liu and C. Yang, RSC Adv., 2017, 7, 1313–1317 RSC.
- H. Najafi, A. M. Tamaddon, S. Abolmaali, S. Borandeh and N. Azarpira, Soft Matter, 2021, 17, 57–67 RSC.
- J. E. P. Sun, B. Stewart, A. Litan, S. J. Lee, J. P. Schneider, S. A. Langhans and D. J. Pochan, Biomater. Sci., 2016, 4, 839–848 RSC.
- B. Mondal, V. K. Gupta, B. Hansda, A. Bhoumik, T. Mondal, H. K. Majumder, C. J. C. Edwards-Gayle, I. W. Hamley, P. Jaisankar and A. Banerjee, Soft Matter, 2022, 18, 7201–7216 RSC.
- R. Huang, W. Qi, L. Feng, R. Su and Z. He, Soft Matter, 2011, 7, 6222–6230 RSC.
- K. Gayen, K. Basu, D. Bairagi, V. Castelletto, I. W. Hamley and A. Banerjee, ACS Appl. Bio Mater., 2018, 1, 1717–1724 CrossRef CAS PubMed.
- T. De Serres-Bérard, T. B. Becher, C. B. Braga, C. Ornelas and F. Berthod, ACS Appl. Polym. Mater., 2020, 2, 5790–5797 CrossRef.
- L. Wang, J. Li, Y. Xiong, Y. Wu, F. Yang, Y. Guo, Z. Chen, L. Gao and W. Deng, ACS Appl. Mater. Interfaces, 2021, 13, 58329–58339 CrossRef CAS PubMed.
- M. Criado-Gonzalez, E. Espinosa-Cano, L. Rojo, F. Boulmedais, M. R. Aguilar and R. Hernández, ACS Appl. Mater. Interfaces, 2022, 14, 10068–10080 CrossRef CAS PubMed.
- Q. Zhao, Y. Zhao, Z. Lu and Y. Tang, ACS Appl. Mater. Interfaces, 2019, 11, 16320–16327 CrossRef CAS PubMed.
- S. R. Nelli, R. D. Chakravarthy, M. Mohiuddin and H.-C. Lin, RSC Adv., 2018, 8, 14753–14759 RSC.
- R. Bassan, M. Varshney and S. Roy, ChemistrySelect, 2023, 8, e202203317 CrossRef CAS.
- S.-Y. Qin, Y. Pei, X.-J. Liu, R.-X. Zhou and X.-Z. Zhang, J. Mater. Chem. B, 2013, 1, 668–675 RSC.
- I. Maity, T. K. Mukherjee and A. K. Das, New J. Chem., 2014, 38, 376–385 RSC.
- J. Nanda, B. Adhikari, S. Basak and A. Banerjee, J. Phys. Chem. B, 2012, 116, 12235–12244 CrossRef CAS PubMed.
- B. Adhikari and A. Banerjee, Soft Matter, 2011, 7, 9259–9266 RSC.
- D. M. Ryan, S. B. Anderson and B. L. Nilsson, Soft Matter, 2010, 6, 3220–3231 RSC.
- W. Zhang, K. Zhang, S. Yan, J. Wu and J. Yin, J. Mater. Chem. B, 2018, 6, 6865–6876 RSC.
- Y. Zhu, L. Wang, Y. Li, Z. Huang, S. Luo, Y. He, H. Han, F. Raza, J. Wu and L. Ge, Biomater. Sci., 2020, 8, 5415–5426 RSC.
- R. Liang, Z. Luo, G. Pu, W. Wu, S. Shi, J. Yu, Z. Zhang, H. Chen and X. Li, RSC Adv., 2016, 6, 76093–76098 RSC.
- M. L. Jagrosse, P. Agredo, B. L. Abraham, E. S. Toriki and B. L. Nilsson, ACS Biomater. Sci. Eng., 2023, 9, 784–796 CrossRef CAS PubMed.
- B. L. Abraham, E. S. Toriki, N. J. Tucker and B. L. Nilsson, J. Mater. Chem. B, 2020, 8, 6366–6377 RSC.
- A. Baral, S. Roy, A. Dehsorkhi, I. W. Hamley, S. Mohapatra, S. Ghosh and A. Banerjee, Langmuir, 2014, 30, 929–936 CrossRef CAS PubMed.
- H. Vilaça, T. Castro, F. M. G. Costa, M. Melle-Franco, L. Hilliou, I. W. Hamley, E. M. S. Castanheira, J. A. Martins and P. M. T. Ferreira, J. Mater. Chem. B, 2017, 5, 8607–8617 RSC.
- K. Ghosh and S. Panja, ChemistrySelect, 2016, 1, 3667–3674 CrossRef CAS.
- D. Ghosh, D. Deepa and K. K. Damodaran, Supramol. Chem., 2020, 32, 276–286 CrossRef CAS.
- K. Ghosh, S. Panja and S. Bhattacharya, RSC Adv., 2015, 5, 72772–72779 RSC.
- Y. Zhang, Y.-C. Pan, Y. Wang, D.-S. Guo, J. Gao and Z. Yang, Nanoscale, 2018, 10, 18829–18834 RSC.
- S. Basak, N. Nandi, S. Paul, I. W. Hamley and A. Banerjee, Chem. Commun., 2017, 53, 5910–5913 RSC.
- B. Mondal, D. Bairagi, N. Nandi, B. Hansda, K. S. Das, C. J. C. Edwards-Gayle, V. Castelletto, I. W. Hamley and A. Banerjee, Langmuir, 2020, 36, 12942–12953 CrossRef CAS PubMed.
- Z. Li, Z. Luo, J. Zhou, Z. Ye, G.-C. Ou, Y. Huo, L. Yuan and H. Zeng, Langmuir, 2020, 36, 9090–9098 CrossRef CAS PubMed.
- M. Abbas, H. H. Susapto and C. A. E. Hauser, ACS Omega, 2022, 7, 2082–2090 CrossRef CAS PubMed.
- S. Roy and A. Banerjee, Soft Matter, 2011, 7, 5300–5308 RSC.
- M. Criado-Gonzalez, J. Rodon Fores, A. Carvalho, C. Blanck, M. Schmutz, L. Kocgozlu, P. Schaaf, L. Jierry and F. Boulmedais, Langmuir, 2019, 35, 10838–10845 CrossRef CAS PubMed.
- B. Das Gupta, A. Halder, T. Vijayakanth, N. Ghosh, R. Konar, O. Mukherjee, E. Gazit and S. Mondal, J. Mater. Chem. B, 2024, 12, 8444–8453 RSC.
- A. Baral, S. Roy, S. Ghosh, D. Hermida-Merino, I. W. Hamley and A. Banerjee, Langmuir, 2016, 32, 1836–1845 CrossRef CAS PubMed.
- A. P. McCloskey, M. Lee, J. Megaw, J. McEvoy, S. M. Coulter, S. Pentlavalli and G. Laverty, ACS Omega, 2019, 4, 2584–2589 CrossRef CAS.
- F. Cao, G. Ma, M. Song, G. Zhu, L. Mei and Q. Qin, Soft Matter, 2021, 17, 4445–4451 RSC.
- S. Roy, D. K. Maiti, S. Panigrahi, D. Basak and A. Banerjee, RSC Adv., 2012, 2, 11053–11060 RSC.
- R. Bassan, B. Mondal, M. Varshney and S. Roy, Nanoscale Adv., 2024, 6, 3399–3409 RSC.
- I. Maity, D. B. Rasale and A. K. Das, Soft Matter, 2012, 8, 5301–5308 RSC.
- D. K. K. Kori, T. Ghosh and A. K. Das, Catal. Sci. Technol., 2023, 13, 2540–2550 RSC.
- J. Wang, H. Wang, Z. Song, D. Kong, X. Chen and Z. Yang, Colloids Surf., B, 2010, 80, 155–160 CrossRef CAS PubMed.
- S. Kiyonaka, K. Sugiyasu, S. Shinkai and I. Hamachi, J. Am. Chem. Soc., 2002, 124, 10954–10955 CrossRef CAS PubMed.
- Z. Azoulay and H. Rapaport, J. Mater. Chem. B, 2016, 4, 3859–3867 RSC.
- D. K. Duraisamy, P. D. Sureshbhai, P. Saveri, A. P. Deshpande and G. Shanmugam, Chem. Commun., 2022, 58, 13377–13380 RSC.
- T. Divoux, B. Maoa and P. Snabre, Soft Matter, 2015, 11, 3677–3685 RSC.
- M. P. Conte, N. Singh, I. R. Sasselli, B. Escuder and R. V. Ulijn, Chem. Commun., 2016, 52, 13889–13892 RSC.
- L. Qin, P. Duan, F. Xie, L. Zhang and M. Liu, Chem. Commun., 2013, 49, 10823–10825 RSC.
- B. K. Das, B. Pramanik, S. Chowdhuri, O. A. Scherman and D. Das, Chem. Commun., 2020, 56, 3393–3396 RSC.
- S. Panja, B. Dietrich and D. J. Adams, Angew. Chem., Int. Ed., 2022, 61, e202115021 CrossRef CAS PubMed.
- T. Sugiura, T. Kanada, D. Mori, H. Sakai, A. Shibata, Y. Kitamura and M. Ikeda, Soft Matter, 2020, 16, 899–906 RSC.
- D. J. Adams, L. M. Mullen, M. Berta, L. Chen and W. J. Frith, Soft Matter, 2010, 6, 1971–1980 RSC.
- A. M. Castilla, M. Wallace, L. L. E. Mears, E. R. Draper, J. Doutch, S. Rogers and D. J. Adams, Soft Matter, 2016, 12, 7848–7854 RSC.
- S. C. Lange, J. Unsleber, P. Drücker, H.-J. Galla, M. P. Waller and B. J. Ravoo, Org. Biomol. Chem., 2015, 13, 561–569 RSC.
- F. Xie, L. Qin and M. Liu, Chem. Commun., 2016, 52(5), 930–933 RSC.
- M. Bastrop, A. Meister, H. Metz, S. Drescher, B. Dobner, K. Mäder and A. Blume, J. Phys. Chem. B, 2011, 115(1), 14–22 CrossRef CAS PubMed.
- B. D. Pinto, O. Ronsin and T. Baumberger, Soft Matter, 2023, 19, 1720–1731 RSC.
- Y. Ma, B. Li, K. Zhang, Q. Wan, Z. Džolić and B. Z. Tang, J. Mater. Chem. C, 2020, 8, 13705–13712 RSC.
- J. Liu, F. Yin, J. Hu and Y. Ju, Mater. Chem. Front., 2021, 5(16), 4764–4771 RSC.
- F. Li, L. Gao, X. Zhang, P. Wang, Y. Liu, J. Feng, C. Zhang, C. Zhao and S. Zhang, Nanoscale Adv., 2021, 3, 6056–6062 RSC.
- L. Zhu, C. Yang and J. Qin, Chem. Commun., 2008, 6303–6305 RSC.
- M. Más-Montoya and R. A. J. Janssen, Adv. Funct. Mater., 2017, 27, 1605779 CrossRef.
- G. K. Auer and D. B. Weibel, Biochemistry, 2017, 56(29), 3710–3724 CrossRef CAS PubMed.
- Z. Breijyeh, B. Jubeh and R. Karaman, Molecules, 2020, 25(6), 1340 CrossRef CAS PubMed.
- M. Javaid, I.-U. Haq, H. Nadeem, H. Fatima, A.-U. Khan and N. Irshad, Front. Pharmacol., 2023, 14, 1084181 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ma00274e |
| This journal is © The Royal Society of Chemistry 2025 |