DOI:
10.1039/D4MA01278J
(Paper)
Mater. Adv., 2025,
6, 1478-1496
A family of zinc compounds of an anthracene-appended new multifunctional organic scaffold as potent chemotherapeutics against cervical cancer†
Received
25th December 2024
, Accepted 21st January 2025
First published on 21st January 2025
Abstract
A family of biologically active novel zinc(II) compounds, namely [Zn(ahpa)(Cl)(H2O)] (1), [Zn(ahpa)(NO3)(H2O)] (2) and [Zn(ahpa)(H2O)2](ClO4) (3), of an anthracene-appended multifunctional organic scaffold, Hahpa (Hahpa = 3-((anthracene-10-ylmethyl)(2-hydroxyethyl)amino)propanoic acid), were synthesized and characterized. Synthesis of 1–3 was accomplished by reacting Hahpa with zinc(II) precursors such as ZnCl2, Zn(NO3)2·6H2O and Zn(ClO4)2·6H2O, respectively, in the presence of NaOH at room temperature. Compounds 1–3 were characterized by elemental analysis, FTIR, electronic absorption and emission spectroscopy, molar conductivity analysis, and TGA studies. Elemental analysis, molar conductivity analysis, and UV-vis and fluorescence titration results unambiguously confirm the integrity of the compound frameworks. Moreover, the structures of 1–3 were ascertained by density functional theory (DFT) computation using the B3LYP/6-311G level of theory, indicating a distorted square pyramidal geometry around the zinc centers. Furthermore, the anticancer properties of 1–3 were assessed in human cervical cancer (HeLa) cell lines, revealing a significantly high cytotoxicity with IC50 values ranging from 1.09 to 2.11 μM. They showed high selectivity between the normal and cancer cells despite this potency. The anticancer activity of 1–3 was possibly due to an increase in cellular reactive oxygen species (ROS), destruction of cell membrane integrity, and DNA damage occurring via nuclear condensation. Electronic absorption spectroscopy, ethidium bromide (EB) displacement assay and circular dichroism (CD) spectroscopy confirmed the binding affinity and binding mode of 1–3 with DNA in a dose-dependent manner. All three compounds were also able to modulate the expression of p53 tumour suppressor protein and exhibited antitumorigenic activity, whereas their activity remained unaltered in the normal cell. When a comparative assessment of anticancer properties of 1–3 was made, 1 showed a higher cytotoxicity towards the cancer cells in comparison to 2 and 3.
Introduction
The design and synthesis of therapeutically active new multifunctional organic scaffolds and their metal compounds for cancer treatment are important areas of interdisciplinary research.1,2 Over the past few decades, significant achievements have been made in this area, including the development of organic, inorganic and composite material-based nanodrugs for preclinical and clinical cancer treatment.3–6 However, the early-stage diagnosis and effective treatment of cancer are still challenging, specifically due to the multidrug resistance and inadequate availability of cancer-targeting therapeutic drugs.7,8 In this context, the nanosized antitumor agents have been widely studied owing to their good lesion specificity and improved bioavailability and biodistribution; however they suffer from poor efficacy and serious side effects.9,10 Hence, there is a demanding need to develop new anticancer drugs to overcome such drawbacks. It is worth mentioning that the success of cisplatin and its derivatives such as oxaliplatin and carboplatin as anticancer drugs has paved the way for discovery of newer and more effective metal-based anticancer drugs.11 The multifunctional organic scaffold-based metal compounds have prominent roles in cancer chemotherapy since several metal ions are crucial for numerous biological processes.12 In particular, several ruthenium-based luminescent compounds are identified as potential theragnostic probes due to their easy synthesis, structural modification and functional tunability.13,14 However, their poor selectivity and dose-dependent toxicity limit their long-term usage for cancer treatment.15 Recently, some other ruthenium-based compounds such as NAMI-A, KP1019 and (N)KP1339 have been discovered as potential anticancer agents because of their improved cancer selectivity.16,17
Zinc is the second most abundant transition metal in our body and considered as an indispensable element for human physiology. It is very crucial for cell survival, proliferation, and differentiation.18 Zn(II) ions play an important role as a cofactor of several intracellular enzymes.19,20 Thus, zinc is required for critical functions in humans such as immunity and reproduction.21,22 It is also established that cancer is strongly related to zinc deficiency. For example, zinc enhances the immune response and function of white blood cells in cancer patients.23 Zinc ions can mitigate the cardiotoxicity and hepatotoxicity of anticancer drugs due to its potential to enable Lewis acid activation, nucleophile formation, and fast ligand exchange.24 However, a very few zinc-based chemotherapeutic drugs are reported to serve as alternatives to platinum-based anticancer drugs.25,26 It is also believed that the binding of Zn(II) with appropriately designed multifunctional organic scaffolds improves bioavailability and biodistribution and reduces the overall cost of drug formulation while availing the beneficial outcome against cancer. Moreover, the cellular uptake of Zn(II) compounds is supposed to be driven by specific binding to transferrin and human serum albumin so that they can cause less toxicity to healthy cells, while the uptake of other transition metal compounds involves passive diffusion.27,28 Unfortunately, significantly fewer research investigations into anticancer Zn(II) compounds have been conducted. The present study originates from our ongoing research endeavour that is undertaken to systematically study 3d transition metal-based compounds of carboxylate-incorporated multifunctional organic assemblies.29–36 In our current research, we have successfully introduced an anthracene backbone as a signalling agent within a multifunctional organic scaffold, thus resulting in the formation of Hahpa (Hahpa = 3-((anthracene-10-ylmethyl)(2-hydroxyethyl)amino)propanoic acid), with a desired degree of flexibility of the donor atoms for metal binding (Scheme 1). Thorough literature search reveals that the currently available and clinically active anticancer drugs such as doxorubicin, mitoxantrone, and camptothecin contain the anthracene backbone as a significant part of their molecular structures.37–39 These drugs are believed to function via the inhibition of DNA topoisomerases, particularly topoisomerase II. Therefore, motivated by the challenges and our continuing research interest in designing and synthesizing some smart organic scaffolds for exploration of promising biological properties, the main focus of our interest was to develop a family of Zn(II) compounds incorporating the anthracene backbone. In the present article, the design and synthesis, structural and physicochemical characterization, and evaluation of three novel Zn(II) compounds [Zn(ahpa)(Cl)(H2O)] (1), [Zn(ahpa)(NO3)(H2O)] (2) and [Zn(ahpa)(H2O)2](ClO4) (3) as promising chemotherapeutic agents against cervical cancer have been explored through well-established scientific evidence.
 |
| | Scheme 1 Synthetic pathway of the multifunctional organic scaffold, Hahpa. | |
Results and discussion
Design, synthesis and general characterization of a multifunctional organic scaffold (Hahpa) and its Zn(II) compounds (1–3)
The synthesis of an anthracene-appended new multifunctional organic scaffold, Hahpa, is illustrated in Scheme 1. This organic scaffold has one alcohol, one carboxylate and one tertiary amine-based O2N donor atom as the metal binding sites and one pendant anthracene backbone as the signalling component. Hahpa has been characterized by standard analytical and spectroscopic techniques such as elemental analysis, FTIR (Fig. S1, ESI†), 1H and 13C NMR (Fig. S2 and S3, ESI†) and ESI-MS (Fig. S4, ESI†). Compounds 1–3 were synthesized in excellent yield (70–80%) by adding a mixture of Hahpa and NaOH to the respective Zn(II) salts separately, in methanol solution. A general synthetic protocol of compounds 1–3 is depicted in Scheme 2. Briefly, the room temperature reactions of one equiv. of Hahpa with one equiv. of ZnCl2, Zn(NO3)2·6H2O and Zn(ClO4)2·6H2O, separately, in the presence of NaOH in methanol produced compounds [Zn(ahpa)(Cl)(H2O)] (1), [Zn(ahpa)(NO3)(H2O)] (2) and [Zn(ahpa)(H2O)2](ClO4) (3), respectively. Characterization of these compounds was performed by elemental analysis, molar conductance analysis, FTIR, UV-vis and fluorescence spectroscopy, TGA analysis, including molecular structure optimization using density functional theory (DFT) calculations. The DFT calculations provided the metric parameters and geometrical arrangements around the zinc centers in 1–3. The molar conductance (ΛM) values of 1, 2 and 3 measured in DMF solution are 17, 10 and 80 Ω cm2 mol−1, respectively.
 |
| | Scheme 2 Synthetic pathway of compounds 1–3. | |
As indicated by these values, compounds 1 and 2 are non-electrolytic formulations, while compound 3 is of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrolyte-type.40 The FTIR spectra (Fig. S5–S7, ESI†) of 1–3 showed characteristic asymmetric (νas) and symmetric (νs) stretching bands of the carboxylates: 1570 (νas) and 1403 cm−1 (νs) for 1, 1583 (νas) and 1384 cm−1 (νs) for 2, and 1594 (νas) and 1407 cm−1 (νs) for 3. The relatively higher value of Δ (Δ = νas(COO−) − νs(COO−)) at ∼167, 199 and 187 cm−1 for 1, 2 and 3, respectively strongly suggests the monodentate terminal binding of the carboxylate functionalities.41 The bands observed between 3424 and 3453 cm−1 correspond to the ν(O–H) vibrations of the coordinated H2O molecules in 1–3. In addition, the terminal coordination of the NO3− group in compound 2 is suggested by the occurrence of sharp bands at 1789 and 1325 cm−1.42 Verification of an uncoordinated ClO4− ion outside the coordination sphere in compound 3 is indicated by a sharp unsplit band at 1090 cm−1.43
1 electrolyte-type.40 The FTIR spectra (Fig. S5–S7, ESI†) of 1–3 showed characteristic asymmetric (νas) and symmetric (νs) stretching bands of the carboxylates: 1570 (νas) and 1403 cm−1 (νs) for 1, 1583 (νas) and 1384 cm−1 (νs) for 2, and 1594 (νas) and 1407 cm−1 (νs) for 3. The relatively higher value of Δ (Δ = νas(COO−) − νs(COO−)) at ∼167, 199 and 187 cm−1 for 1, 2 and 3, respectively strongly suggests the monodentate terminal binding of the carboxylate functionalities.41 The bands observed between 3424 and 3453 cm−1 correspond to the ν(O–H) vibrations of the coordinated H2O molecules in 1–3. In addition, the terminal coordination of the NO3− group in compound 2 is suggested by the occurrence of sharp bands at 1789 and 1325 cm−1.42 Verification of an uncoordinated ClO4− ion outside the coordination sphere in compound 3 is indicated by a sharp unsplit band at 1090 cm−1.43
Photophysical properties: UV-vis absorption and fluorescence titration studies
In order to understand the binding events of the newly designed multifunctional organic scaffold Hahpa with Zn(II), the changes in its photophysical properties induced by addition of different Zn(II) salts were studied in solution by employing UV-vis absorption and fluorescence emission titrations. The binding of different Zn(II) salts with Hahpa brought about significant changes in both the absorption and emission features of the attached anthracene backbone. These studies also indicated the composition and stability of compounds 1–3 formed upon binding of Hahpa with Zn(II).
Fig. 1 illustrates the UV-vis titration spectra of Hahpa with different Zn(II) salts in methanol solution. The electronic absorption spectrum of Hahpa exhibits intense absorption bands at 385, 365, 347 and 331 nm due to π–π* transitions of the anthracene backbone.44 Upon gradual addition of ZnCl2, Zn(NO3)2·6H2O and Zn(ClO4)2·6H2O separately to a methanol solution of Hahpa, the absorption bands at 385, 365, 347 and 331 nm increased progressively, with a significant red shift of the absorption maxima by 2–4 nm in the case of all three Zn(II) salts, indicating that the (Zn2+)/(ahpa)− compounds were formed in solution. The increase in the absorption bands continued up to a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for (Zn2+)/(ahpa)−. Fig. 1 insets show the titration profiles for ZnCl2, Zn(NO3)2·6H2O and Zn(ClO4)2·6H2O confirming the formation of the 1
1 for (Zn2+)/(ahpa)−. Fig. 1 insets show the titration profiles for ZnCl2, Zn(NO3)2·6H2O and Zn(ClO4)2·6H2O confirming the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Zn2+)/(ahpa)− bound assemblies in solution. The spectral changes observed upon binding of Zn(II) with the (ahpa)− scaffold are very similar to the results reported by our research group for the interaction of Zn(II) with anthracene-incorporated μ-bis(tridentate) organic assembly.36 Addition of increasing concentrations of Zn(II) ions to the (ahpa)− scaffold induced the red-shift of the absorption bands, which clearly explained the high stability of the as-synthesized Zn(II) compounds (1–3). Accordingly, the binding constant (Kb) values were calculated using the UV-vis titration data by applying the method of Rose and Drago.45 The calculated Kb values of the organic scaffold-bound Zn(II) compounds are (2.31 ± 0.14) × 104 M−1, (2.10 ± 0.17) × 104 M−1 and (3.66 ± 0.10) × 104 M−1 for 1, 2 and 3, respectively.
1 (Zn2+)/(ahpa)− bound assemblies in solution. The spectral changes observed upon binding of Zn(II) with the (ahpa)− scaffold are very similar to the results reported by our research group for the interaction of Zn(II) with anthracene-incorporated μ-bis(tridentate) organic assembly.36 Addition of increasing concentrations of Zn(II) ions to the (ahpa)− scaffold induced the red-shift of the absorption bands, which clearly explained the high stability of the as-synthesized Zn(II) compounds (1–3). Accordingly, the binding constant (Kb) values were calculated using the UV-vis titration data by applying the method of Rose and Drago.45 The calculated Kb values of the organic scaffold-bound Zn(II) compounds are (2.31 ± 0.14) × 104 M−1, (2.10 ± 0.17) × 104 M−1 and (3.66 ± 0.10) × 104 M−1 for 1, 2 and 3, respectively.
 |
| | Fig. 1 UV-vis titration spectra of the multifunctional organic scaffold (ahpa)− (1.013 × 10−4 M) acquired on adding (A) ZnCl2, (B) Zn(NO3)2·6H2O, and (C) Zn(ClO4)2·6H2O in MeOH solution at room temperature; the concentrations of Zn(II) salts were varied from 0 to 4.526 × 10−4 M. (inset) The corresponding titration curves of (A) ZnCl2, (B) Zn(NO3)2·6H2O, and (C) Zn(ClO4)2·6H2O according to the absorption intensity, indicating 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for (Zn2+)/(ahpa)− (1). 1 stoichiometry for (Zn2+)/(ahpa)− (1). | |
In order to further demonstrate the ability of Hahpa to bind with Zn(II), the fluorescence titration experiments of Hahpa with different zinc salts were performed in methanol solution. The emission spectrum of Hahpa, which was excited at 365 nm, showed strong emission bands at 436, 412 and 394 nm at room temperature in methanol solution. These emission bands are typical for the attached anthracene backbone.36 The fluorescence titration spectra acquired from the titration of Hahpa with increasing concentrations of ZnCl2, Zn(NO3)2·6H2O and Zn(ClO4)2·6H2O are displayed in Fig. 2. As seen, the gradual addition of the respective Zn(II) salt to the methanol solution of Hahpa causes significant enhancement of its fluorescence intensities, with a considerable red shift of the emission maxima by 2–3 nm. The observed fluorescence intensity was virtually proportional to the concentration of the Zn(II) salt. The saturation behavior of the emission intensity after addition of 1 equiv. of Zn(II) indicates that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Zn2+)/(ahpa)− bound assemblies (1–3) are formed in solution (Fig. 2 insets). The chelation promoted fluorescence-on can be justified by considering that the Zn(II) ions with completely filled d-orbitals are known to increase the fluorescence intensity via either the photo-induced electron transfer or energy transfer mechanism.46 The binding constant (Kb) values of (Zn2+)/(ahpa)− assemblies calculated from the fluorescence titration data are found to be (5.27 ± 0.20) × 103 M−1, (4.86 ± 0.28) × 103 M−1 and (6.38 ± 0.16) × 103 M−1 for 1, 2 and 3, respectively. The fluorescence titration outcomes suggest that the multifunctional organic scaffold Hahpa forms 1
1 (Zn2+)/(ahpa)− bound assemblies (1–3) are formed in solution (Fig. 2 insets). The chelation promoted fluorescence-on can be justified by considering that the Zn(II) ions with completely filled d-orbitals are known to increase the fluorescence intensity via either the photo-induced electron transfer or energy transfer mechanism.46 The binding constant (Kb) values of (Zn2+)/(ahpa)− assemblies calculated from the fluorescence titration data are found to be (5.27 ± 0.20) × 103 M−1, (4.86 ± 0.28) × 103 M−1 and (6.38 ± 0.16) × 103 M−1 for 1, 2 and 3, respectively. The fluorescence titration outcomes suggest that the multifunctional organic scaffold Hahpa forms 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 compounds with Zn(II) ions in solution.
1 compounds with Zn(II) ions in solution.
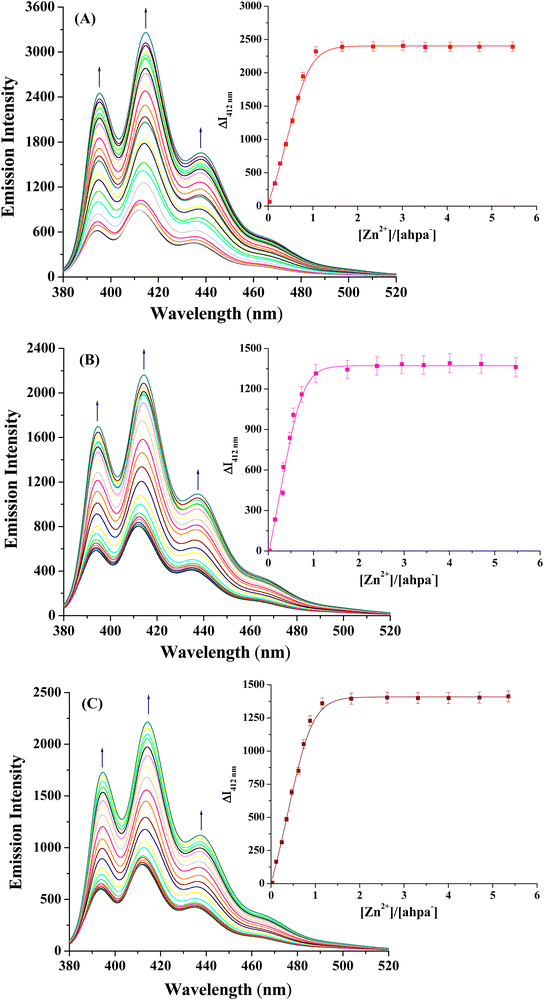 |
| | Fig. 2 Fluorescence titration spectra of the multifunctional organic scaffold (ahpa)− (1.013 × 10−4 M) acquired on adding (A) ZnCl2, (B) Zn(NO3)2·6H2O, and (C) Zn(ClO4)2·6H2O in MeOH solution at room temperature; the concentrations of Zn(II) salts were varied from 0 to 5.480 × 10−4 M. (inset) The corresponding titration curves of (A) ZnCl2, (B) Zn(NO3)2·6H2O, and (C) Zn(ClO4)2·6H2O according to the emission intensity, indicating 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for (Zn2+)/(ahpa)− (1). 1 stoichiometry for (Zn2+)/(ahpa)− (1). | |
Thermal studies
The thermogravimetric analysis (TGA) of compounds 1–3 was performed to understand their thermal behavior. The TGA curves of 1–3 are displayed in Fig. 3. The first decomposition step of 1 shows a mass loss between 43 and 74 °C due to the removal of a coordinated water molecule. The mass loss detected in this step is 3.84% (calcd 4.08%). The second decomposition step observed between 308 and 400 °C is due to the release of one CO2 and half Cl2 gases corresponding to a mass loss of 18.01% (calcd 18.05%). The decomposition of the overall metal–organic framework takes place between 480 and 850 °C. For thermal decomposition of 2, the first step proceeds between 55 and 97 °C with a mass loss of 4.51% (calcd 3.86%), attributed to the loss of a coordinated water molecule. The second decomposition step that occurred between 270 and 351 °C is attributed to the release of one CO2 and one NO gas molecules, which corresponds to a mass loss of 15.75% (calcd 15.85%). The third decomposition step that occurred between 477 and 853 °C is associated with the disintegration of the overall metal–organic framework. Similarly, compound 3 shows a three-step thermal decomposition reaction. The first decomposition step happens between 35 and 195 °C with a mass loss of 7.89% (calcd 8.52%), corresponding to the removal of two coordinated water molecules. The second decomposition step observed between 264 and 398 °C with a mass loss of 17.66% (calcd 17.97%) is attributed to the removal of one CO2 and one O2 gas molecules. The third and final step takes place between 460 and 878 °C showing the decomposition of the overall metal–organic framework. ZnO is formed as the most possible residual end product for all three complexes.47 A careful literature study reveals that analogous thermal features are observed for carboxylate, chloride, nitrate and perchlorate-based metal compounds that release CO2, Cl2, NO and O2 gases, respectively.48–51
 |
| | Fig. 3 TGA curves of [Zn(ahpa)(Cl)(H2O)] (1), [Zn(ahpa)(NO3)(H2O)] (2) and [Zn(ahpa)(H2O)2](ClO4) (3) under an inert atmosphere of nitrogen gas at a heating rate of ∼12 °C min−1. | |
Structural optimization by density functional theory (DFT) calculations
The structures of all three Zn(II) compounds were optimized by density functional theory (DFT) calculations in order to obtain insight into their molecular arrangements, along with the metric parameters and geometry around the metal centers. Tables 1–3 summarize the selected bond distances and angles of 1–3. Views of their optimized structures are depicted in Fig. 4. The structures of 1–3 are in good agreement with the spectroscopic and analytical data. As shown in Fig. 4, the zinc centers lie in a distorted square pyramidal geometry, as indicated by the values of the Addison parameter, τ (Zn45, 0.085 in 1; Zn52, 0.049 in 2; and Zn45, 0.014 in 3).52 On the other hand, the square pyramidal geometry around Zn45 in 1 is defined by one nitrogen and two oxygen donor groups (N7, O1 and O2) of the (ahpa)− scaffold, one oxygen donor from a monodentate water molecule (O4) and an auxiliary chloride ligand (Cl49); the square pyramidal geometry around Zn52 in 2 is defined by one nitrogen and two oxygen donor groups (N7, O1 and O2) of the (ahpa)− scaffold, and two oxygen donor groups from a monodentate water molecule (O4) and an auxiliary nitrate ligand (O46). In contrast, the square pyramidal geometry around Zn45 in 3 is defined by one nitrogen and two oxygen donor groups (N7, O1 and O2) of the (ahpa)− scaffold, and two oxygen donor groups from two monodentate water molecules (O4 and O49). The zinc center deviates from the respective square plane by 0.455, 0.458 and 0.234 Å in 1, 2 and 3, respectively. The Zn–N and Zn–O bond distances range from 1.9088 to 2.0180 Å (Tables 1–3), which are comparable to the analogous five-coordinate zinc(II) complexes.53,54 The five- and six-membered chelate rings are markedly non-planar with the bite angles of O(1)–Zn(45)–N(7) = 83.51 and O(2)–Zn(45)–N(7) = 89.30° in 1; O(1)–Zn(52)–N(7) = 83.16 and O(2)–Zn(52)–N(7) = 89.31° in 2; O(1)–Zn(45)–N(7) = 79.50 and O(2)–Zn(45)–N(7) = 90.48° in 3. The zinc centers in 1–3 are linked to the planar anthracene backbone by the –CH2–N< linker, showing a virtually perpendicular orientation to each other. Careful structural analysis also disclosed that the distances between the Zn(II) ions and the centroid of anthracene rings are 3.903, 3.936 and 3.959 Å in 1, 2 and 3, respectively, suggesting a weak intramolecular cation⋯π interaction.55
Table 1 Selected bond distances (Å) and angles (deg) for [Zn(ahpa)(Cl)(H2O)] (1) acquired from the DFT-B3LYP/6-311G level of theory
| Bond distances [Å] |
Bond angles [deg] |
| Zn(45)–O(1) |
1.9304 |
O(1)–Zn(45)–O(2) |
149.77 |
| Zn(45)–O(2) |
1.9288 |
O(1)–Zn(45)–O(4) |
95.88 |
| Zn(45)–O(4) |
1.9088 |
O(1)–Zn(45)–N(7) |
83.51 |
| Zn(45)–N(7) |
1.9564 |
O(1)–Zn(45)–Cl(49) |
104.95 |
| Zn(45)–Cl(49) |
2.2398 |
O(2)–Zn(45)–O(4) |
78.59 |
|
|
|
O(2)–Zn(45)–N(7) |
89.30 |
|
|
|
O(2)–Zn(45)–Cl(49) |
105.22 |
|
|
|
O(4)–Zn(45)–N(7) |
154.85 |
|
|
|
O(4)–Zn(45)–Cl(49) |
104.03 |
|
|
|
N(7)–Zn(45)–Cl(49) |
100.37 |
Table 2 Selected bond distances (Å) and angles (deg) of [Zn(ahpa)(NO3)(H2O)] (2) acquired at the DFT-B3LYP/6-311G level of theory
| Bond distances [Å] |
Bond angles [deg] |
| Zn(52)–O(1) |
1.9348 |
O(1)–Zn(52)–O(2) |
150.75 |
| Zn(52)–O(2) |
1.9251 |
O(1)–Zn(52)–O(4) |
95.91 |
| Zn(52)–O(4) |
1.9105 |
O(1)–Zn(52)–O(46) |
104.16 |
| Zn(52)–O(46) |
1.9091 |
O(1)–Zn(52)–N(7) |
83.16 |
| Zn(52)–N(7) |
1.9580 |
O(2)–Zn(52)–O(4) |
78.68 |
|
|
|
O(2)-Zn(52)–O(46) |
104.99 |
|
|
|
O(2)–Zn(52)–N(7) |
89.31 |
|
|
|
O(4)–Zn(52)–N(7) |
153.69 |
|
|
|
O(4)–Zn(52)–O(46) |
105.43 |
|
|
|
N(7)–Zn(52)–O(46) |
100.24 |
Table 3 Selected bond distances (Å) and angles (deg) of [Zn(ahpa)(H2O)2](ClO4) (3) acquired at the DFT-B3LYP/6-311G level of theory
| Bond distances [Å] |
Bond angles [deg] |
| Zn(45)–O(1) |
1.9449 |
O(1)–Zn(45)–O(2) |
163.39 |
| Zn(45)–O(2) |
1.9184 |
O(1)–Zn(45)–O(4) |
107.04 |
| Zn(45)–O(4) |
1.9099 |
O(1)–Zn(45)–O(49) |
65.78 |
| Zn(45)–O(49) |
1.9101 |
O(1)–Zn(45)–N(7) |
79.50 |
| Zn(45)–N(7) |
2.0180 |
O(2)–Zn(45)-O(4) |
79.59 |
|
|
|
O(2)–Zn(45)–O(49) |
105.57 |
|
|
|
O(2)–Zn(45)–N(7) |
90.48 |
|
|
|
O(4)–Zn(45)–N(7) |
164.24 |
|
|
|
O(4)–Zn(45)–O(49) |
63.90 |
|
|
|
N(7)–Zn(45)–O(49) |
107.88 |
 |
| | Fig. 4 Geometric and electronic structures of (A) [Zn(ahpa)(Cl)(H2O)] (1); (B) [Zn(ahpa)(NO3)(H2O)] (2); and (C) [Zn(ahpa)(H2O)2](ClO4) (3) calculated at the DFT-B3LYP/6-311G level of theory. Color code: Zn, pink; N, blue; O, red; C, black; H, light blue. | |
Anticancer properties: quantification of % cytotoxicity
In order to examine the anticancer properties of the Zn(II) compounds, the in vitro cytotoxicity of 1–3 was determined first by a trypan blue dye exclusion method using the human cervical cancer (HeLa) cells as they are the most commonly used model human cancer cell lines for the investigation of chemotherapy. The proliferation of HeLa cells treated with 1–3 appeared to be reduced significantly after 24 h of treatment (Fig. 5). The % cytotoxicity was markedly higher in all three compound-treated groups when compared to the control (***p < 0.001 vs. control). The IC50 values were calculated and found to be 1.09 μM for 1, 1.54 μM for 2, and 2.11 μM for 3. Furthermore, these compounds when checked in the normal L6 skeletal muscle cell line did not show any significant change in % cell viability as compared to untreated normal cells (Fig. S8, ESI†). A comparison of these results indicated that 1 is much more effective in reducing cell proliferation, thus resulting in diminished cancer cell growth compared to 2 and 3. Moreover, the treatment of HeLa cells with precursor zinc salts used in the present study resulted in a very insignificant change in % cytotoxicity (Fig. S9, ESI†).
 |
| | Fig. 5 Graph showing the % cytotoxicity for each experimental group. **p < 0.01 vs. control; ***p < 0.001 vs. control. | |
Studies of cell morphology and cell membrane integrity
The surface morphology of HeLa cells was analyzed by phase contrast and scanning electron microscopy studies. Both the phase contrast and scanning electron microscopic images (Fig. 6 and 7) showed that compounds 1–3 treated groups contained a significant amount of dead HeLa cells, which is in good agreement with the results obtained from the assessment of % cytotoxicity. Critical analysis of these microscopic images revealed the following observations for treatments using compounds 1–3: (i) the compound 1-treated group showed the highest number of apoptotic cells (Fig. 6C and 7C), (ii) the compound 2-treated group showed the cells that acquired an ovoid morphology due to the loss of cellular matrix adherence and an increased number of membrane perforations (Fig. 6D and 7D), (iii) the compound 3-treated group also showed numerous apoptotic cells; however, in some cases, the cells were completely devoured and cellular remnants were left (Fig. 6E and 7E). When analyzed concertedly, the formation of beaded apoptopodia and other apoptotic bodies, membrane blebbing, membrane disruption and a large number of plasma membrane perforations were observed, indicating the detrimental effects of the Zn(II) compounds that imposed cellular death in the cancer cells.
 |
| | Fig. 6 Phase contrast light microscopic images showing cellular morphology in different experimental groups. (A) control, (B) DMSO, (C) 1, (D) 2, and (E) 3. | |
 |
| | Fig. 7 Scanning electron microscopic (SEM) images showing cellular morphology in different experimental groups. (A) control, (B) DMSO, (C) 1, (D) 2, and (E) 3. | |
Monitoring the production of intracellular reactive oxygen species (ROS)
Reactive oxygen species (ROS) play a significant role in controlling the cell proliferation and apoptosis, and hence the mechanistic action of cell death. Extreme and uncontrolled production of ROS leads to oxidative stress, thus accelerating the damage of intracellular components. In fact, due to the abnormal cellular functions, the cancer cells remain under amplified oxidative stress. The H2DCFDA (H2DCFDA = 2′,7′-dichlorodihydrofluorescein diacetate) assay revealed that the HeLa cells treated with compounds 1–3 showed significant ROS production after 24 h (Fig. 8). At a glance, the produced ROS break down the two acetate linkages of non-fluorescent H2DCFDA to yield green fluorescence-based DCF.56 On the other hand, 1 showed an ∼25% increase in ROS production compared to the control, which was the highest among the three compounds, followed by 2 and 3 with ∼15% and ∼3% increase in ROS production, respectively (Fig. 8). The intensity profiles of ROS mediated by all three Zn(II) compounds suggest their roles in ROS generation, which is followed by cellular apoptosis.
 |
| | Fig. 8 Spectrofluorometric studies of ROS production in different experimental groups using the H2DCFDA dye (λexi = 485 nm; λemi = 527 nm) and compared against the control. | |
DNA binding studies and UV-vis spectroscopy
Electronic absorption spectroscopy was employed for investigating the binding interactions of compounds 1–3 with DNA. The interactions between compounds 1–3 and CT-DNA were followed by spectral changes arising in the range of 320–400 nm. Fig. S10–S12 of the ESI† depict the UV-vis spectra obtained upon titration of a fixed concentration of 1–3 with increasing concentrations of CT-DNA. The spectra showed the occurrence of ligand-based π–π* transition bands showing a significant hypochromism with a blue shift of 2–3 nm. It is pertinent to mention that the extent of hypochromism is directly proportional to the strength of DNA interaction. A strong stacking interaction between the aromatic chromophore of 1–3 and the adjacent base pairs of CT-DNA resulted in the reduction of transition probability and hence hypochromism.57 Significant hypochromism and blue shift in the UV-vis titration spectra of 1–3 indicated their interactions with CT-DNA via partial intercalation.58 The following equation (eqn (1)) was used to determine the DNA binding constant (Kb) values:| | | [DNA]/(εa − εf) = [DNA]/(εb − εf) + 1/Kb (εb − εf) | (1) |
where [DNA] = concentration of CT-DNA, εa = ratio of the absorbance/[compound], εf = extinction coefficient of the free metal compound, and εb = extinction coefficient of the metal compound present in the completely bound form. The Kb values were calculated to be (5.07 ± 0.23) × 104 M−1, (2.62 ± 0.19) × 104 M−1 and (1.83 ± 0.15) × 104 M−1 for 1, 2 and 3, respectively (Fig. S10–S12 insets, ESI†). A comparative analysis of these binding constant values of the studied Zn(II) compounds revealed the order of their binding affinity towards DNA as 1 > 2 > 3.
DNA binding studies and fluorescence spectroscopy
The binding interactions of compounds 1–3 with DNA were further studied by carrying out ethidium bromide (EB) displacement assay. The emission intensity of free EB is relatively low, but the intensity is significantly enhanced by stacking interaction between the adjacent DNA base pairs. So, the competitive DNA binding experiments of 1–3 were performed by monitoring the changes in the emission intensity of EB bound to CT-DNA as a function of the complex concentration to obtain insight into the binding interactions of the Zn(II) compounds with DNA via intercalation.59 Fig. S13–S15 of the ESI† display the emission spectra of EB bound to CT-DNA acquired upon titration of a fixed concentration of EB/DNA adduct with increasing concentrations of 1–3 separately. The emission intensity of the EB/DNA adduct reduced substantially because of the competition by the corresponding Zn(II) compound in the EB intercalated sites. In the emission titration spectra (Fig. S13–S15, ESI†), the observed hypochromic shift at ∼598 nm indicated that EB was partially replaced by the EB/DNA adduct by the Zn(II) compounds via the intercalative interactions. Furthermore, the emission spectra of the Zn(II) compound–DNA adduct also revealed that the emission intensities were reduced by 77%, 75% and 73% for 1, 2 and 3, respectively. The DNA binding constant (Kb) values were evaluated using the Scatchard equation (eqn (2))60,61 as described below:| | log[(I0 − I)/I] = log[Kb] + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) log[Q] log[Q] | (2) |
where n = the number of binding sites, I0 = fluorescence intensity in the absence of the quencher, and I = fluorescence intensity in the presence of the quencher. Therefore, the Kb values obtained from the plots of log[(I0 − I)/I] vs. log[Q] (Fig. S16–S18, ESI†) were found to be (8.58 ± 0.22) × 103 M−1, (5.14 ± 0.21) × 103 M−1 and (3.87 ± 0.19) × 103 M−1 for 1, 2 and 3, respectively. These binding constant values suggest the order of the binding strength of the Zn(II) compounds as 1 > 2 > 3, which agrees well with the observation made in the UV-vis spectral studies.
DNA binding studies and viscosity measurement
In order to explore the mode of binding interactions of 1–3 with DNA, viscosity measurement was performed using the solutions of CT-DNA incubated with the Zn(II) compounds individually. It is well known that intercalative DNA binding would cause an elongation of DNA by increasing the separation of DNA base pairs, leading to an enhancement of viscosity. On the other hand, DNA may bend or twist due to partial or non-classical intercalation of the compound, which would reduce its effective length and viscosity simultaneously. Thus, the relative specific viscosity (η/η0) of DNA reveals an increase in contour length associated with the separation of DNA base pairs caused by intercalation. Fig. S19 of the ESI† shows the increase in the relative specific viscosity of DNA when 1–3 are added into CT-DNA solution separately. Hence, these results clearly suggest that 1–3 bind to DNA via intercalation, which is in accordance with the absorption and fluorescence spectral studies. The ability of the compounds to increase the viscosity of DNA follows the order 1 > 2 > 3.
DNA binding studies and circular dichroism (CD) spectroscopy
Binding of a small molecule with the right-handed B-form of DNA leads to the change in molar ellipticity, when analysed by circular dichroism (CD) spectroscopy. In the CD spectrum of CT-DNA, the positive band at 275 nm corresponds to base stacking, and the negative band at 245 nm corresponds to helicity, which are the typical characteristics of the right-handed B-form of DNA.62 Thus, the mode of DNA binding with compounds 1–3 was ascertained by comparing the changes in the positive and negative bands upon addition of the respective Zn(II) compound at different concentrations (Fig. 9). The decrease in the amplitude of the positive CD bands in the range of 275–290 nm accompanied by a minor red-shift corresponded to the change in base stacking interactions of DNA in a concentration-dependent manner for 1–3.63 On the other hand, the negative CD bands in the range of 235–250 nm showed a decrease of molar ellipticity in a concentration-dependent manner, with a minor blue-shift, indicating the destabilisation of the right-handed DNA structure.64 However, a comparison of the CD spectral results specified that compound 1 exhibited the highest change in molar ellipticity in both the positive and negative bands, while compound 2 exhibited the lowest change in molar ellipticity. Additionally, for compound 3, the negative band showed a significant change in molar ellipticity, which might indicate the dehydration of the B form of DNA and a transition from B to A form.
 |
| | Fig. 9 Circular dichroism (CD) spectra of (A) 1, (B) 2, and (C) 3 with CT-DNA showing changes in molar ellipticity in a dose-dependent manner. | |
Studies of p53 protein expression and nuclear condensation
It has been established that p53 is a tumor suppressor gene whose mutation occurs in >50% of tumor cells.65 Thus, the potential of this tumor protein (p53) has become a prominent research topic in cancer treatment.66 The immunofluorescence study revealed that all three Zn(II) compounds were found to be capable of inducing the expression of p53 protein in the treated HeLa cells (Fig. 10). The activated p53 protein promotes the cell cycle arrest, thus allowing apoptosis to stop the propagation of cancer cells. As observed from the fluorescence intensity histogram (Fig. 11), compound 1 showed the highest expression of p53 protein, followed by compounds 2 and 3.
 |
| | Fig. 10 Immunofluorescence study of p53 protein expression and nuclear condensation using DAPI staining in 1 (C1), 2 (C2), and 3 (C3) treated HeLa cells in comparison to the control group. The red arrows indicate the condensed nuclei. | |
 |
| | Fig. 11 Histogram profiles of the fluorescence intensities of the p53 protein expression level and DAPI stained nucleus in HeLa cells in different experimental groups. | |
Furthermore, the HeLa cells of experimental and control sets were stained with DAPI (DAPI = 4′,6-diamidino-2-phenylindole) and monitored under a confocal microscope. The microscopic images (Fig. 10) showed that the nuclear morphology became substantially condensed for all three Zn(II) compound-treated cells as indicated by the red arrows, in comparison to the untreated control sets. Generally, apoptosis is strongly believed to be associated with the modification of the structure, function and stability of DNA.67 The results obtained in the present study supported this observation because one of the advanced steps in apoptosis is DNA damage that results from the nuclear condensation during the apoptotic cell death.68
Comparative study of anticancer properties of the Zn(II) compounds (1–3)
The relevance of Zn(II) compounds in biological systems is definitely related to their unique chemical features such as (a) Zn(II) is redox inactive, (b) it is a strong Lewis acid, (c) it has a d10 electronic configuration, and hence diamagnetic, (d) it can adopt a varied coordination geometry, and (e) it is prone to a fast exchange of ancillary ligands. The electron affinity of zinc resembles that of nickel or copper, but the lack of redox activity of Zn(II) eliminates any possibility of free radical reactions and makes it vital for the body's antioxidant protection system. On the other hand, literature review also reveals that incorporation of an anthracene backbone within a multifunctional organic scaffold leads to enhanced anticancer activities of their metal compounds due to the increased lipophilic and intercalating properties, which in succession result in their higher cellular uptake.69 It is well established that increased lipophilicity and intercalating properties of several chelating compounds stimulate the antiproliferative activity.70 Our results demonstrated that all three Zn(II) compounds exhibited significant anticancer properties when examined in human cervical cancer (HeLa) cell lines. However, among them, 1 showed the highest tumor cell proliferation inhibitory action and consequently anticancer activity. Particularly, while comparing with 2 and 3, compound 1 was found to exhibit (i) the topmost ROS generation, (ii) highest DNA binding affinity, and (iii) maximum p53 protein expression and nuclear condensation, followed by cellular apoptosis. It is noteworthy to mention that the stimulation of apoptosis in malignant cells is the most preferred mechanism for the treatment of different types of cancers.71 The results achieved in the present investigation may be attributed to the increased lipophilicity of Zn(II) compounds 1–3, thereby enhancing their ability to cross the cell membrane. However, it is believed that the chloride-bound metal compounds show superior anticancer properties in comparison to nitro- and aqua-bound metal compounds.72 Thus, 1 contains a Zn(II)-bound chloride ligand that leaves the coordination sphere easily as a result of the intracellular activation process. Comparing the experimentally obtained IC50 values (IC50 = 1.09–2.11 μM) with those reported for some analogous Zn(II) compounds, it can be emphasized that there are many reports on the Zn(II) compounds showing lower activity with IC50 values of >2.50 μM73–75 or higher activity with IC50 values of <2.50 μM.76,77 Nevertheless, from these results, very little can be said about the molecular mechanism by which Zn(II) compounds 1–3 elicited their anticancer activity on human cervical cancer (HeLa) cells. Based on these in vitro studies, detailed experiments on animals might provide insight into the biological activity of these compounds in tumor-bearing hosts and will help to build clinically relevant information for developing potential anticancer drugs.
Conclusion
In conclusion, the successful design and synthesis, physicochemical and spectroscopic characterization, and assessment of anticancer potential of a novel class of three Zn(II) compounds (1–3) of an anthracene-appended multifunctional organic scaffold bearing amine, alcohol and carboxylate functionalities have been described. Different analytical and spectroscopic techniques have been employed to establish the compositions of the compounds in solid and solution states. UV-vis absorption and fluorescence emission titration spectra confirm the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model between the (ahpa)− scaffold and Zn(II) ion in solution. The structures of 1–3 were optimized and ascertained by density functional theory (DFT) computation. On the other hand, 1–3 showed an excellent anticancer efficacy with significant inhibition of % cell viability by inducing apoptotic cell death in HeLa cells; they remained inefficient in inducing cytotoxicity and apoptosis when administered to normal cells. Furthermore, 1 displayed the highest ROS generation and DNA binding ability and maximum p53 protein expression and nuclear condensation, thus showing a much better anticancer activity in comparison to 2 and 3. Therefore, from our present exploration, it can be suggested that these novel Zn(II) compounds can be utilized for target-specific chemo-therapeutic drug design for interrupting the molecular mechanism involved in the advancement and propagation of the frightful ailment cancer.
1 binding model between the (ahpa)− scaffold and Zn(II) ion in solution. The structures of 1–3 were optimized and ascertained by density functional theory (DFT) computation. On the other hand, 1–3 showed an excellent anticancer efficacy with significant inhibition of % cell viability by inducing apoptotic cell death in HeLa cells; they remained inefficient in inducing cytotoxicity and apoptosis when administered to normal cells. Furthermore, 1 displayed the highest ROS generation and DNA binding ability and maximum p53 protein expression and nuclear condensation, thus showing a much better anticancer activity in comparison to 2 and 3. Therefore, from our present exploration, it can be suggested that these novel Zn(II) compounds can be utilized for target-specific chemo-therapeutic drug design for interrupting the molecular mechanism involved in the advancement and propagation of the frightful ailment cancer.
Experimental section
Synthesis of 3-((anthracene-10-ylmethyl)(2-hydroxyethyl)amino)propanoic acid (Hahpa)
A solution of 9-anthracenecarboxaldehyde (2.000 g, 9.69 mmol) in methanol (25 mL) was added dropwise to a solution of β-alanine (0.863 g, 9.69 mmol) and NaOH (0.388 g, 9.69 mmol) in methanol (15 mL) under stirring conditions. After complete addition, the reaction mixture was refluxed for 6 h. Thus, a deep yellow solution of Schiff base was obtained and subsequently it was cooled in an ice-bath. Afterwards, NaBH4 (0.551 g, 14.56 mmol) was added in excess to the cold solution under stirring conditions, resulting in a slight diminishing of the yellow color of the solution. Then, 2 mL of conc. HCl (12 M) was added dropwise to remove excess NaBH4. The solution was then acidified to a pH of ∼5 by adding extra conc. HCl (12 M). Next, the solution was filtered and the clear filtrate was rotary evaporated to isolate the precursor ligand 3-[(anthracene-9-ylmethyl)-amino]-propionic acid (Hapa) as a yellow crystalline solid. The product was washed with MeOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; v/v) and dried in a vacuum desiccator containing anhydrous CaCl2. Yield: 2.165 g (80%). Anal. calc. for C18H17NO2: C, 77.40%; H, 6.13%; N, 5.01%. Found: C, 77.27%; H, 5.99%; N, 5.09%. FTIR (KBr, cm−1): ν = 3386(b), 1624(s), 1449(s), 1385(s), 1344(s), 1288(s), 1262(s), 1218(s), 1160(s), 1053(s), 886(s), 844(s), 784(s), 772(s), 737(s), 702(s), 598(s). 1H NMR (400 MHz, DMSO-d6, room temperature, δ): 8.80 (s, 1H), 8.52 (d, 2H), 8.20 (d, 2H), 7.71 (t, 2H), 7.62 (t, 2H), 5.27 (s, 2H), 3.65 (s, 2H), 2.86 (t, 1H), 2.76 (t, 1H).
4; v/v) and dried in a vacuum desiccator containing anhydrous CaCl2. Yield: 2.165 g (80%). Anal. calc. for C18H17NO2: C, 77.40%; H, 6.13%; N, 5.01%. Found: C, 77.27%; H, 5.99%; N, 5.09%. FTIR (KBr, cm−1): ν = 3386(b), 1624(s), 1449(s), 1385(s), 1344(s), 1288(s), 1262(s), 1218(s), 1160(s), 1053(s), 886(s), 844(s), 784(s), 772(s), 737(s), 702(s), 598(s). 1H NMR (400 MHz, DMSO-d6, room temperature, δ): 8.80 (s, 1H), 8.52 (d, 2H), 8.20 (d, 2H), 7.71 (t, 2H), 7.62 (t, 2H), 5.27 (s, 2H), 3.65 (s, 2H), 2.86 (t, 1H), 2.76 (t, 1H).
A solution of 2-iodoethanol (1.847 g, 10.74 mmol) in water (15 mL) was added dropwise to a solution of 3-[(anthracene-9-ylmethyl)-amino]-propionic acid (Hapa) (3.000 g, 10.74 mmol) and NaOH (0.430 g, 10.74 mmol) in water (40 mL) under stirring conditions. After complete addition, the reaction mixture was refluxed for 6 h. While refluxing, more NaOH (0.143 g, 3.57 mmol) was added to retain the pH of ∼11–12. The resulting solution was cooled and acidified with conc. HCl (12 M) up to pH ∼5. Subsequently, a light yellow solid product was precipitated. The product was collected by filtration and washed with water and then dried in a vacuum desiccator containing anhydrous CaCl2. The final product was confirmed by elemental analysis, FTIR, 1H and 13C NMR spectroscopy, and mass spectrometry. Yield: 2.613 g (75%). Anal. calc. for C20H21NO3: C, 74.28%; H, 6.55%; N, 4.33%. Found: C, 74.35%; H, 6.67%; N, 4.22%. FTIR (KBr, cm−1): ν = 3427(b), 2925(s), 1709(s), 1621(s), 1588(s), 1449(s), 1387(s), 1259(s), 1181(s), 1051(s), 891(s), 844(s), 785(s), 735(s), 632(s), 600(s), 536(s). 1H NMR (400 MHz, D2O, room temperature, δ): 8.39 (s, 1H), 8.25 (d, 1H), 8.18 (d, 1H), 7.95-7.98 (m, 2H), 7.41-7.53 (m, 4H), 4.42 (s, 2H), 3.32 (t, 2H), 2.97 (t, 2H), 2.85 (t, 2H), 2.57 (t, 2H). 13C NMR (100 MHz, DMSO-d6, room temperature, δ): 174.22, 131.40, 131.35, 129.56, 127.44, 126.34, 125.95, 125.50, 124.67, 59.45, 55.13, 50.90, 50.16, 32.16. ESI-MS data: m/z 362.1169 {[Hahpa + K]+}, calcd for {[Hahpa + K]+} = 362.1159; m/z 453.1700 {[Hahpa + 5H2O + K]+}, calcd for {[Hahpa + 5H2O + K]+} = 453.1720.
Synthesis of Zn(II) compounds (1–3)
Zinc compounds (1–3) were prepared by employing the synthetic protocol as described below. For 1, ZnCl2 (0.105 g, 0.77 mmol) in 10 mL of MeOH was added dropwise to a mixture of Hahpa (0.250 g, 0.77 mmol) and NaOH (0.030 g, 0.77 mmol) in 10 mL of MeOH with magnetic stirring for a period of 10 min. For 2, Zn(NO3)2·6H2O (0.229 g, 0.77 mmol) in 10 mL of MeOH was added dropwise to a mixture of Hahpa (0.250 g, 0.77 mmol) and NaOH (0.030 g, 0.77 mmol) in 10 mL of MeOH with magnetic stirring for a period of 10 min. Similarly, for 3, Zn(ClO4)2·6H2O (0.287 g, 0.77 mmol) in 10 mL of MeOH was added dropwise to a mixture of Hahpa (0.250 g, 0.77 mmol) and NaOH (0.030 g, 0.77 mmol) in 10 mL of MeOH with magnetic stirring for a period of 10 min. The reaction mixture was stirred vigorously for all three reaction sets at ambient temperature for 1 h, resulting in a light yellow solution. The crystalline light yellow compounds were separated gradually upon stirring for 1 h. The compounds were isolated by filtration, washed with water to obtain pure compounds of 1–3, and dried in a vacuum desiccator containing anhydrous CaCl2.
[Zn(ahpa)(Cl)(H2O)] (1).
Yield: 0.272 g (80%). Anal. calcd for C20H21NO4ClZn: C, 54.57%; H, 4.81%; N, 3.18%; Zn, 14.85; found: C, 54.61; H, 4.67; N, 3.02; Zn, 14.99. FTIR (KBr, cm−1): ν = 3424(b), 1570(s), 1403(s), 1282(s), 1053(s), 887(s), 734(s), 654(s). Molar conductance, ΛM: (DMF) = 17 Ω−1 cm2 mol−1.
[Zn(ahpa)(NO3)(H2O)] (2).
Yield: 0.270 g (75%). Anal. calcd for C20H21N2O7Zn: C, 51.46; H, 4.53; N, 6.00; Zn, 14.01; found: C, 51.59; H, 4.59; N, 6.09; Zn, 13.78. FTIR (KBr, cm−1): ν = 3453(b), 1789(s), 1583(s), 1384(s), 1325(s), 1053(s), 895(s), 836(s), 738(s), 635(s). Molar conductance, ΛM: (DMF) = 10 Ω−1 cm2 mol−1.
[Zn(ahpa)(H2O)2](ClO4) (3).
Yield: 0.229 g (70%). Anal. calcd for C20H23NO5Zn: C, 56.82; H, 5.48; N, 3.31; Zn, 15.47; found: C, 56.71; H, 5.51; N, 3.42; Zn, 15.56. FTIR (KBr, cm−1): ν = 3434(b), 1594(s), 1407(s), 1347(s), 1261(s), 1199(s), 1090(s), 963(s), 891(s), 846(s), 737(s), 664(s), 627(s). Molar conductance, ΛM: (DMF) = 80 Ω−1 cm2 mol−1.
Reagents and solvents
All analytical grade chemicals, reagents and solvents were purchased from commercial sources and used without further purification. 9-Anthracenecarboxaldehyde, β-alanine, 2-iodoethanol and sodium borohydride were purchased from Sigma-Aldrich, Germany. ZnCl2, Zn(NO3)2·6H2O, and NaOH were purchased from Merck, India. It is important to mention that Zn(ClO4)2·6H2O is potentially explosive, and it should be handled in small quantity with great care. Calf thymus DNA (CT-DNA) was procured from SRL, India. The human cervical cancer (HeLa) cell line was acquired from National Centre for Cell Science (NCCS), Pune, India. The Tris–HCl buffer (pH ∼ 7.5) was prepared by using triply distilled and de-ionized water. All the antibiotics, antimycotics and DMEM were obtained from Himedia Laboratories Pvt. Ltd (Mumbai, India), and the foetal bovine serum (FBS) was obtained from Gibco BRL (Grand Island, NY, USA), respectively. The primary antibodies and FITC conjugated secondary antibodies were procured from Santa Cruz Biotechnology (USA) and Sigma-Aldrich (USA), respectively.
General instrumentation methods
The percentage of zinc contents in 1–3 was determined by carrying out a complexometric titration method using Na2H2EDTA in aqueous solution, after digesting their crystalline powder samples in a conc. HCl/HNO3 mixture. The percentages of C, H and N were determined using a PerkinElmer 2400 CHNS/O Series II elemental analyzer. FTIR spectra were recorded on a PerkinElmer L120-000A spectrometer (450–4000 cm−1). The nuclear magnetic resonance spectra were obtained on a Bruker AC 400 MHz NMR spectrometer. UV-vis spectra were performed by employing a Shimadzu UV 1800 (200–900 nm) spectrophotometer. Fluorescence spectra were acquired using a Hitachi F-7000 spectrofluorometer at room temperature. Molar conductivity in solution was measured with a Mettler Toledo Five Easy Plus FEP 30 digital conductivity meter. The TGA studies were performed using NETZSCH STA 449F3 thermal apparatus.
UV-vis absorption spectroscopy
All UV-vis absorption experiments were carried out using a Shimadzu UV 1800 spectrophotometer with a 1 cm quartz cell at room temperature over a wavelength range of 200 to 900 nm. The UV-vis experiments of the multifunctional organic scaffold Hahpa with different Zn(II) salts were performed separately in methanol solution by titrating a specified concentration of Hahpa (1.013 × 10−4 M) with varying concentrations of the respective Zn(II) salts, such as ZnCl2, Zn(NO3)2·6H2O and Zn(ClO4)2·6H2O, ranging from 0 to 4.526 × 10−4 M. The changes in absorption intensities of the multifunctional organic scaffold (ahpa)− were documented for the evaluation of stoichiometry and binding constant values of the compounds.
Fluorescence emission spectroscopy
All fluorescence emission experiments were carried out using a Hitachi F-7000 spectrofluorometer with a rectangular quartz cuvette of path length 1 cm at room temperature. The titration experiments of the multifunctional organic scaffold Hahpa with different Zn(II) salts were performed separately in methanol solution by titrating a specified concentration of Hahpa (1.013 × 10−4 M) with varying concentrations of the respective Zn(II) salts, such as ZnCl2, Zn(NO3)2·6H2O and Zn(ClO4)2·6H2O, ranging from 0 to 5.480 × 10−4 M. The solution was excited at 365 nm for each set of titrations. The changes in emission intensities of the multifunctional organic scaffold (ahpa)− were documented for the evaluation of stoichiometry and binding constant values of the compounds.
Theoretical calculations
Density functional theory (DFT) calculations78 regarding the optimization of the structures of all three Zn(II) compounds were performed by employing the B3LYP method79 and 6-311G basis set.80,81
Maintenance of the cell culture and treatment
The cervical cancer cells, HeLa, were cultured and maintained in Dulbecco's Modified Eagle's Medium (DMEM) with 10% FBS and 1% antibiotic solution with antimitotic supplements and kept in the incubator with 5% CO2 at 37 °C as per the standard procedure.82 Prior to the in vitro treatment, all three compounds were separately dissolved and diluted in PBS buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9; v/v) to make the working solution. The cells were administered with 1 μL per 1 mL of the working solution in fresh cell culture media and incubated for 30 min, after which they were fixed and analyzed for appropriate data. Independent experiments with precursor zinc salts used in the present study were carried out by employing the same method as specified above. The L6 cells (normal cell line), which were used only to check the % of cell viability, were also maintained applying the above-mentioned protocol.
9; v/v) to make the working solution. The cells were administered with 1 μL per 1 mL of the working solution in fresh cell culture media and incubated for 30 min, after which they were fixed and analyzed for appropriate data. Independent experiments with precursor zinc salts used in the present study were carried out by employing the same method as specified above. The L6 cells (normal cell line), which were used only to check the % of cell viability, were also maintained applying the above-mentioned protocol.
Measurement of % cytotoxicity
The % cytotoxicity in the cervical cancer HeLa cell line and normal L6 skeletal muscle cell line was evaluated by employing the trypan blue dye exclusion method by microscopic quantification of the number of dead cells relative to the total number of cells for each experimental set as per common practice, using the following formula.83
Morphological study of HeLa cells by phase contrast microscopy and scanning electron microscopy
The surface morphology of HeLa cells was studied by phase contrast microscopy and scanning electron microscopy. The cells were seeded on the glass coverslips and were allowed to grow and attain a desirable confluency (70–75%). Following the treatment, the cells were washed with PBS buffer and fixed with 2.5% glutaraldehyde for 2 h.84 The phase contrast features of the Carl Zeiss Axio Vert-A1 inverted fluorescent microscope were used to capture the images. To dehydrate the cells for the SEM study, the samples were passed through different grades of alcohols and finally placed in a vacuum desiccator overnight. After this, the samples were mounted on aluminium stubs using the double-sided tape and vacuum sputter coated with gold and palladium. The images were captured using a high-resolution scanning electron microscope (Carl Zeiss EVO LS10) that was set at 20 kV and 10.5 mm working distance.
Quantification of cellular reactive oxygen species (ROS)
The cellular reactive oxygen species (ROS) was quantified by H2DCFDA following the common practice.83 An equal number of cells in each group were incubated with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) in the dark, and the fluorescence intensity following the conversion of H2DCFDA to DCF (2′,7′-dichlorodihydrofluorescein) was measured using a fluorescence spectrophotometer (Hitachi F-7100).
DNA binding studies: UV-vis absorption spectroscopy
DNA binding studies of 1–3 were carried out by titrating a specified concentration of each compound (1.010 × 10−4 M) with rising concentrations of CT-DNA (0–8.712 × 10−5 M). The experimental solutions were made in 30 mM Tris–HCl buffer (pH ∼ 7.5) with 5% DMSO. Using electronic absorption spectroscopy, the DNA concentration in solution was determined with a molar extinction coefficient of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 M−1 cm−1 at 260 nm.85 The CT-DNA was examined for its purity by monitoring the ratio of the absorbance at 260 nm to that at 280 nm having the value of 1.8–1.9. By adding an equal amount of DNA to both compound and reference solutions, the absorbance of the DNA itself was subtracted.
600 M−1 cm−1 at 260 nm.85 The CT-DNA was examined for its purity by monitoring the ratio of the absorbance at 260 nm to that at 280 nm having the value of 1.8–1.9. By adding an equal amount of DNA to both compound and reference solutions, the absorbance of the DNA itself was subtracted.
DNA binding studies: ethidium bromide displacement assay
The DNA binding affinity of 1–3 was further examined by the ethidium bromide (EB) displacement assay. For this, the CT-DNA was incubated first with ethidium bromide ([EB]/[DNA] = 0.1) at 37 °C in the dark for 1/2 h. Then, the solution of the EB/DNA mixture was titrated with rising concentrations of each compound from 0 to 5.762 × 10−3 M after exciting at 510 nm. The emission bands were found at ∼598 nm for 1–3, respectively.
DNA binding studies: viscosity measurement
The viscosity experiments were carried out using an Ostwald viscometer at room temperature. The viscosity of CT-DNA solution (25 × 10−5 M) was measured in the absence and presence of increasing amounts of the compounds separately in 30 mM Tris–HCl buffer (pH ∼ 7.5) at 25 °C. Flow-time was measured thrice with a digital stopwatch. Viscosity values were presented as (η/η0)1/3versus [compound]/[DNA],86 where η is the viscosity of DNA in the presence of the zinc(II) compound and η0 is the viscosity of DNA in the absence of the zinc(II) compound. Calculation of the relative viscosities was achieved using the relation η = (t − t0)/t0 (t = flow-time of DNA solution in the presence or absence of the compound and t0 = flow-time of Tris–HCl buffer solution).
DNA binding studies: circular dichroism (CD) spectroscopic study
The CT-DNA was dissolved in tris-buffer saline at a concentration of 1.0 mg mL−1. Different concentrations of each compound were added to the different experimental sets, and the changes in molar ellipticity as a result of the binding interactions were measured by scanning in the wavelength window of 200–400 nm using a Jasco-720 circular dichroism (CD) spectrometer. The path length was 1 cm, the experiments were conducted under a steady nitrogen flush, and the system was maintained at 25 °C.82
Immunofluorescence study of p53 protein expression in HeLa cells
The expression of the tumour suppressor protein p53 in HeLa cells upon induction of the model Zn(II) compounds (1–3) in different experimental sets was assessed and quantified by the immunofluorescence study. Cells were incubated with the anti-p53 antibody (Santa Cruz Biotechnology, Inc., USA), followed by incubation with the FITC-conjugated secondary antibody (Thermo Fisher Scientific, USA), and then counter-stained with DAPI (Sigma Aldrich, USA) for visualizing the nucleus.
Data availability
We, the authors of this article, confirm that the data will be available on request.
Conflicts of interest
The authors declare that they have no known competing financial interest.
Acknowledgements
The Department of Science, Technology and Biotechnology (DSTBT), West Bengal, India is highly acknowledged for funding and financial support (Grant No. ST/P/S&T/15G-18/2019). The DST-FIST (Level-2; SR/FST/CS-II/2019/96) program is greatly acknowledged for supporting the instrumental facilities in the Department of Chemistry, University of Kalyani. S. S. is thankful to the UGC, New Delhi for offering him a Senior Research Fellowship (SRF).
References
- F. E. Poynton, S. A. Bright, S. Blasco, D. C. Williams, J. M. Kelly and T. Gunnlaugsson, The Development of Ruthenium(II) Polypyridyl Complexes and Conjugates for In Vitro Cellular and In Vivo Applications, Chem. Soc. Rev., 2017, 46, 7706–7756 Search PubMed.
- I. A. Bhat, R. Jain, M. M. Siddiqui, D. K. Saini and P. S. Mukherjee, Water Soluble Pd8L4 Self-assembled Molecular Barrel as an Aqueous Carrier for Hydrophobic Curcumin, Inorg. Chem., 2017, 56, 5352–5360 Search PubMed.
- S. S. Lucky, K. C. Soo and Y. Zhang, Nanoparticles in Photodynamic Therapy, Chem. Rev., 2015, 115, 1990–2042 Search PubMed.
- W. Ngo, B. Stordy, J. Lazarovits, E. K. Raja, C. L. Etienne and W. C. W. Chan, DNA-Controlled Encapsulation of Small Molecules in Protein Nanoparticles, J. Am. Chem. Soc., 2020, 142, 17938–17943 Search PubMed.
- A. Pradhan, S. Haldar, K. B. Mallik, M. Ghosh, M. Bera, N. Sepay, D. Schollmeye, S. K. Ghatake, S. Roy and S. Saha, Mixed Phenoxo and Azido Bridged Dinuclear Nickel(II) and Copper(II) Compounds with N,N,O-Donor Schiff Bases: Synthesis, Structure, DNA Binding, DFT and Molecular Docking Study, Inorg. Chim. Acta, 2019, 484, 197–205 Search PubMed.
- H. Ji, W. Wang, O. Qiao and X. Hao, Review of Carrier-Free Self-Assembly of Anticancer Nanodrugs, ACS Appl. Nano Mater., 2024, 7, 4564–4587 Search PubMed.
- B. Mansoori, A. Mohammadi, S. Davudian, S. Shirjang and B. Baradaran, The Different Mechanisms of Cancer Drug Resistance: A Brief Review, Adv. Pharm. Bull., 2017, 7, 339–348 Search PubMed.
- A. Ghosh, P. Das, M. R. Gill, P. Kar, M. G. Walker, J. A. Thomas and A. Das, Photoactive RuII-Polypyridyl Complexes that Display Sequence Selectivity and High-Affinity Binding to Duplex DNA through Groove Binding, Chem. – Eur. J., 2011, 17, 2089–2098 Search PubMed.
- E. Boisselier and D. Astruc, Gold Nanoparticles in Nanomedicine: Preparations, Imaging, Diagnostics, Therapies And Toxicity, Chem. Soc. Rev., 2009, 38, 1759–1782 Search PubMed.
- S. Hassan, G. Prakash, A. Ozturk, S. Saghazadeh, M. F. Sohail, J. Seo, M. Dockmeci, Y. S. Zhang and A. Khademhosseini, Evolution and Clinical Translation of Drug Delivery Nanomaterials, Nano Today, 2017, 15, 91–106 Search PubMed.
- P. Starha and Z. Travnicek, Non-Platinum Complexes Containing Releasable Biologically Active Ligands, Coord. Chem. Rev., 2019, 395, 130–145 Search PubMed.
- L. Zeng, P. Gupta, Y. Chen, E. Wang, L. Ji, H. Chao and Z. S. Chen, The Development of Anti-cancer Ruthenium(II) Complexes: From Single Molecule Compounds to Nanomaterials, Chem. Soc. Rev., 2017, 46, 5771–5804 Search PubMed.
- S. Wanninger, V. Lorenz, A. Subhan and F. T. Edelmann, Metal Complexes of Curcumin: Synthetic Strategies, Structures and Medicinal Applications, Chem. Soc. Rev., 2015, 44, 4986–5002 Search PubMed.
- B. Mohan, S. Estalayo-Adrian, D. Umadevi, B. la Cour Poulsen, S. Blasco, G. J. McManus, T. Gunnlaugsson and S. Shanmugaraju, Design, Synthesis, and Anticancer Studies of a p-Cymene-Ru(II)-Curcumin Organometallic Conjugate Based on a Fluorescent 4-Amino-1,8-naphthalimide Troger's Base Scaffold, Inorg. Chem., 2022, 61, 11592–11599 Search PubMed.
- Y. R. Zheng, K. Suntharalingam, T. C. Johnstone and S. J. Lippard, Encapsulation of Pt(IV) Prodrugs within a Pt(II) Cage For Drug Delivery, Chem. Sci., 2015, 6, 1189–1193 Search PubMed.
- I. Bratsos, S. Jedner, T. Gianferrara and E. Alessio, Ruthenium Anticancer Compounds: Challenges and Expectations, Chimia, 2007, 61, 692–697 Search PubMed.
- C. G. Hartinger, M. A. Jakupec, S. Zorbas-Seifried, B. Kynast, H. Zorbas and B. K. Keppler, A New Redox-Active Anticancer Agent-Preclinical Development and Results of a Clinical Phase I Study in Tumor Patients, Chem. Biodiversity, 2008, 5, 2140–2155 Search PubMed.
- U. Lodemann, R. Einspanier, F. Scharfen, H. Martens and A. Bondzio, Effects of Zinc on Epithelial Barrier Properties and Viability in a Human and a Porcine Intestinal Cell Culture Model, Toxicol. In Vitro, 2013, 27, 834–843 Search PubMed.
- P. Mahato, A. Ghosh, S. K. Mishra, A. Shrivastav, S. Mishra and A. Das, Zn(II) Based Colorimetric Sensor for ATP and Its Use as a Viable Staining Agent in Pure Aqueous Media of pH 7.2, Chem. Commun., 2010, 46, 9134–9136 Search PubMed.
- W. N. Lipscomb and N. Strater, Recent Advances in Zinc Enzymology, Chem. Rev., 1996, 96, 2375–2433 Search PubMed.
- P. Ashokkumar, A. H. Ashoka, M. Collot, A. Das and A. S. Klymchenko, A Fluorogenic BODIPY Molecular Rotor as an Apoptosis Marker, Chem. Commun., 2019, 55, 6902–6905 Search PubMed.
- P. Mahato, A. Ghosh, S. K. Mishra, A. Shrivastav, S. Mishra and A. Das, Zn(II)-Cyclam Based Chromogenic Sensors for Recognition of ATP in Aqueous Solution Under Physiological Conditions and Their Application as Viable Staining Agents for Microorganism, Inorg. Chem., 2011, 50, 4162–4170 Search PubMed.
- J. L. Qin, W. Y. Shen, Z. F. Chen, L. F. Zhao, Q. P. Qin, Y. C. Yu and H. Liang, Oxoaporphine Metal Complexes (CoII, NiII, ZnII) With High Antitumor Activity By Inducing Mitochondria-Mediated Apoptosis and S-phase Arrest in HepG2, Sci. Rep., 2017, 7, 46056 Search PubMed.
- F. X. Wang, M. H. Chen, X. Y. Hu, R. R. Ye, C. P. Tan, L. N. Ji and Z. W. Mao, Ester-Modified Cyclometalated Iridium (III) Complexes As Mitochondria-Targeting Anticancer Agents, Sci. Rep., 2016, 6, 38954 Search PubMed.
- M. Porchia, M. Pellei, F. Del Bello and C. Santini, Zinc Complexes with Nitrogen Donor Ligands as Anticancer Agents, Molecules, 2020, 25, 5814 Search PubMed.
- M. Pellei, F. Del Bello, M. Porchia and C. Santini, Zinc Coordination Complexes as Anticancer Agents, Coord. Chem. Rev., 2021, 445, 214088 Search PubMed.
- E. Alessio and L. Messori, Anticancer Drug Candidates Face-to-Face: A Case Story in Medicinal Inorganic Chemistry, Molecules, 2019, 24, 1995 Search PubMed.
- C. Sonkar, S. Sarkar and S. Mukhopadhyay, Ruthenium (II)-Arene Complexes as Anti-Metastatic Agents, and Related Techniques, RSC Med. Chem., 2022, 13, 22–38 Search PubMed.
- A. Majumder, N. Dutta, S. Dey, P. Sow, A. Samadder, G. Vijaykumar, K. Rangan and M. Bera, A Family of [Zn6] Complexes from the Carboxylate-Bridge-Supported Assembly of [Zn2] Building Units: Synthetic, Structural, Spectroscopic, and Systematic Biological Studies, Inorg. Chem., 2021, 60, 17608–17626 Search PubMed.
- S. Haldar, A. Patra and M. Bera, Exploring The Catalytic Activity of New Water Soluble Dinuclear Copper(II) Complexes Towards The Glycoside Hydrolysis, RSC Adv., 2014, 4, 62851–62861 Search PubMed.
- S. Haldar, A. Patra, G. Vijaykumar, L. Carrella and M. Bera, Dinuclear and Tetranuclear Complexes of Copper Coordinated by an Anthracene-Based New μ-Bis(tridentate) Ligand: Synthesis, Structure, Spectroscopy and Magnetic Properties, Polyhedron, 2016, 117, 542–551 Search PubMed.
- M. Bera, G. T. Musie and D. R. Powell, Synthesis and Characterization of New Mono- and Heptazinc Complexes with Unusual Amide Coordination Modes, Inorg. Chem., 2009, 48, 4625–4627 Search PubMed.
- G. C. Giri, A. Patra, G. Vijaykumar, L. Carrella and M. Bera, Hydrolytically Active Tetranuclear [NiII2]2 Complexes: Synthesis, Structure, Spectroscopy and Phosphoester Hydrolysis, RSC Adv., 2015, 5, 99270–99283 Search PubMed.
- S. Haldar, G. Vijaykumar, L. Carrella, S. Batha, G. T. Musie and M. Bera, Inorganic Phosphate and Arsenate within New Tetranuclear Copper and Zinc Complexes: Syntheses, Crystal Structures, Magnetic, Electrochemical, and Thermal Studies, ACS Omega, 2017, 2, 1535–1549 Search PubMed.
- G. C. Giri, S. Haldar, A. K. Ghsoh, P. Chowdhury, L. Carrella, U. Ghosh and M. Bera, New Cyclic Tetranuclear Copper(II) Complexes Containing Quadrilateral Cores: Synthesis, Structure, Spectroscopy and Their Interactions with DNA in Aqueous Solution, J. Mol. Struct., 2017, 1142, 175–184 Search PubMed.
- A. Majumder, C. Sarkar, I. Das, S. Sk, S. Bandyopadhyay, S. Mandal and M. Bera, Design, Synthesis and Evaluation of a Series of Zinc(II) Complexes of Anthracene-Affixed Multifunctional Organic Assembly as Potential Antibacterial and Antibiofilm Agents against Methicillin-Resistant Staphylococcus aureus, ACS Appl. Mater. Interfaces, 2023, 15, 22781–22804 Search PubMed.
- S. Y. van der Zanden, X. Qiao and J. Neefjes, New Insights Into The Activities And Toxicities of The Old Anticancer Drug Doxorubicin, FEBS J., 2021, 288, 6095–6111 Search PubMed.
- Y. Guan, S. Jiang, W. Ye, X. Ren, X. Wang, Y. Zhang, M. Yin, K. Wang, Y. Tao, J. M. Yang, D. Cao and Y. Cheng, Combined Treatment of Mitoxantrone Sensitizes Breast Cancer Cells To Rapalogs Through Blocking eEF-2K-Mediated Activation of Akt and Autophagy, Cell Death Dis., 2020, 11, 948 Search PubMed.
- M. Ghanbari-Movahed, T. Kaceli, A. Mondal, M. H. Farzaei and A. Bishayee, Recent Advances in Improved Anticancer Efficacies of Camptothecin Nano-Formulations: A Systematic Review, Biomedicines, 2021, 9, 480 Search PubMed.
- S. Sk, S. Bandyopadhyay, C. Sarkar, I. Das, A. Gupta, M. Sadangi, S. Mondal, M. Banerjee, G. Vijaykumar, J. N. Behera, S. Konar, S. Mandal and M. Bera, Unraveling Multicopper [Cu3] and [Cu6] Clusters with Rare μ3-Sulfato and Linear μ2-Oxido-Bridges as Potent Antibiofilm Agents against Multidrug-Resistant Staphylococcus aureus, ACS Appl. Bio Mater., 2024, 7, 2423–2449 Search PubMed.
- A. Patra and M. Bera, Spectroscopic Investigation of New Water Soluble MnII2 and MgII2 Complexes for the Substrate Binding Models of Xylose/Glucose Isomerases, Carbohydr. Res., 2014, 384, 87–98 Search PubMed.
- T. S. Kurtikyan, V. A. Hayrapetyan, M. M. Mehrabyan and P. C. Ford, Six-Coordinate Nitrito and Nitrato Complexes of Manganese Porphyrin, Inorg. Chem., 2014, 53, 11948–11959 Search PubMed.
-
B. J. Hathaway, G. Wilkinson, R. G. Gillard and J. A. McCleverty, Comprehensive Coordination Chemistry, ed., Pergamon, Oxford, 1987, vol. 2, p. 413 Search PubMed.
- L. Augouy, N. Huby, L. Hirsch, A. van der Lee and P. Gerbier, Molecular Engineering to Improve The Charge Carrier Balance in Single-Layer Silole-Based OLEDs, New J. Chem., 2009, 33, 1290–1300 Search PubMed.
- N. J. Rose and R. S. Drago, Molecular Addition Compounds of Iodine: An Absolute Method for the Spectroscopic Determination of Equilibrium Constants, J. Am. Chem. Soc., 1959, 81, 6138–6141 Search PubMed.
- R. Diana and B. Panunzi, The Role of Zinc(II) Ion in Fluorescence Tuning of Tridentate Pincers: A Review, Molecules, 2020, 25, 4984 Search PubMed.
- S. Haldar, N. Dutta, G. Vijaykumar, A. Das, L. Carrella, A. Oliver and M. Bera, Synthesis, Structure and Properties of New Heterometallic Octanuclear Li2Na2Cu4 and Decanuclear Li2Zn8 Complexes, Polyhedron, 2019, 172, 58–66 Search PubMed.
- P. Portius, B. Peerless, M. Davis and R. Campbell, Homoleptic Poly(nitrato) Complexes of Group 14 Stable at Ambient Conditions, Inorg. Chem., 2016, 55, 8976–8984 Search PubMed.
- R. Fouad, I. A. Shaaban, T. E. Ali, M. A. Assiri and S. S. Shenouda, Co(II), Ni(II), Cu(II) and Cd(II)-Thiocarbonohydrazone Complexes: Spectroscopic, DFT, Thermal, and Electrical Conductivity Studies, RSC Adv., 2021, 11, 37726–37743 Search PubMed.
- G. G. Marvin and L. B. Woolaver, Thermal Decomposition of Perchlorates, Ind. Eng. Chem., Anal. Ed., 1945, 17, 474–476 Search PubMed.
- C. Paquet, T. Lacelle, X. Liu, B. Deore, A. J. Kell, S. Lafreniere and P. R. L. Malenfant, The Role of Amine Ligands in Governing Film Morphology and Electrical Properties of Copper Films Derived from Copper Formate-Based Molecular Inks, Nanoscale, 2018, 10, 6911–6921 Search PubMed.
- A. Addison, T. N. Rao, J. Reedijk, J. van Rijn and G. C. Verschoor, Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen-Sulphur Donor Ligands; The Crystal and Molecular Structure of Aqua[1,7- bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane] Copper(II) Perchlorate, J. Chem. Soc., Dalton Trans., 1984, 1349–1356 Search PubMed.
- N. Dutta, S. Haldar, G. Vijaykumar, S. Paul, A. P. Chattopadhyay, L. Carrella and M. Bera, Phosphatase-Like Activity of Tetranuclear Iron(III) and Zinc(II) Complexes, Inorg. Chem., 2018, 57, 10802–10820 Search PubMed.
- B. Fernandez, I. Fernandez, J. Cepeda, M. Medina-O’Donnell, E. E. Rufino-Palomares, A. Raya-Baron, S. Gomez-Ruiz, A. Perez-Jimenez, J. A. Lupianez, F. J. Reyes-Zurita and A. Rodríguez-Dieguez, Modulating Anticancer Potential by Modifying the Structural Properties of a Family of Zinc Metal−Organic Chains Based on 4-Nitro-1H-pyrazole, Cryst. Growth Des., 2018, 18, 969–978 Search PubMed.
- A. Tamayo, L. Escriche, J. Casabo, B. Covelo and C. Lodeiro, Synthesis, Complexation and Spectrofluorometric Studies of a New NS3 Anthracene-Containing Macrocyclic Ligand, Eur. J. Inorg. Chem., 2006, 2997–3004 Search PubMed.
- X. Dai, B. Zhang, W. Zhou and Y. Liu, High-Efficiency Synergistic Effect of Supramolecular Nanoparticles Based on Cyclodextrin Prodrug on Cancer Therapy, Biomacromolecules, 2020, 21, 4998–5007 Search PubMed.
- S. A. Tysoe, R. J. Morgan, A. D. Baker and T. C. Strekas, Spectroscopic Investigation of Differential Binding Modes of DELTA- and LAMBDA-Ru(bpy)2(ppz)2+ with Calf Thymus DNA, J. Phys. Chem., 1993, 97, 1707–1711 Search PubMed.
- D. Das, A. Banaspati, N. Das, B. Bora, M. K. Raza and T. K. Goswami, Visible Light-Induced Cytotoxicity Studies on Co(II) Complexes having an Anthracene-Based Curcuminoid Ligand, Dalton Trans., 2019, 48, 12933–12942 Search PubMed.
- F. J. M. Almes and D. Porschke, Mechanism of Intercalation into the DNA Double Helix by Ethidium, Biochemistry, 1993, 32, 4246–4253 Search PubMed.
-
J. R. Lakowicz, Fluorescence Quenching: Theory and Applications. Principles of Fluorescence Spectroscopy, Kluwer Academic/Plenum Publishers, New York, 1999, pp. 53–127 Search PubMed.
- X. Z. Feng, Z. Yang, L. J. Wang and C. Bai, Investigation of the Interaction between Acridine Orange and Bovine Serum Albumin, Talanta, 1998, 47, 1223–1229 Search PubMed.
- N. Shahabadi, F. Shiri, S. Hadidi, K. Farshadfar, M. Darbemamieh and S. M. Roe, The Role of Both Intercalation and Groove Binding at AT-rich DNA Regions in the Interaction Process of a Dinuclear Cu(I) Complex Probed by Spectroscopic and Simulation Analysis, J. Mol. Liq., 2021, 335, 116290 Search PubMed.
- W. D. Wilson, F. A. Tanious, D. Ding, A. Kumar, D. W. Boykin, P. Colson, C. Houssier and C. Bailly, Nucleic Acid Interactions of Unfused Aromatic Cations: Evaluation of Proposed Minor-Groove, Major-Groove, and Intercalation Binding Modes, J. Am. Chem. Soc., 1998, 120, 10310–10321 Search PubMed.
- B. Ray, S. Agarwal, N. Lohani, M. R. Rajeswari and R. Mehrotra, Structural, Conformational and Thermodynamic Aspects of Groove-Directed-Intercalation of Flavopiridol into DNA, J. Biomol. Struct. Dyn., 2016, 34, 2518–2535 Search PubMed.
- M. K. Sivoňová, M. Vilèková, J. Kliment, S. Mahmood, J. Jureèeková, S. Dušenková, I. Waczulíková, P. Slezák and D. Dobrota, Association of p53 and p21 Polymorphisms with Prostate Cancer, Biomed. Rep., 2015, 3, 707–714 Search PubMed.
- T. Jiang, C. Zhou, J. Gu, Y. Liu, L. Zhao, W. Li, G. Wang, Y. Li and L. Cai, Enhanced Therapeutic Effect of Cisplatin on the Prostate Cancer in Tumor-Bearing Mice by Transfecting the Attenuated Salmonella Carrying a Plasmid Co-Expressing p53 Gene and mdm2 siRNA, Cancer Lett., 2013, 337, 133–142 Search PubMed.
- S. Nagata, H. Nagase, K. Kawane, N. Mukae and H. Fukuyama, Degradation of Chromosomal DNA during Apoptosis, Cell Death Differ., 2003, 10, 108–116 Search PubMed.
- P. R. Walker, L. Kokileva, J. LeBlanc and M. Sikorska, Detection of the Initial Stages of DNA Fragmentation in Apoptosis, Biotechniques, 1993, 15, 1032–1040 Search PubMed.
- A. A. Nazarov, J. Risse, W. H. Ang, F. Schmitt, O. Zava, A. Ruggi, M. Groessl, R. Scopelitti, L. Juillerat-Jeanneret, C. G. Hartinger and P. J. Dyson, Anthracene-Tethered Ruthenium(II) Arene Complexes as Tools to Visualize the Cellular Localization of Putative Organometallic Anticancer Compounds, Inorg. Chem., 2012, 51, 3633–3639 Search PubMed.
- D. Richardson, E. Tran and P. Ponka, The Potential of Iron Chelators of the Pyridoxal Isonicotinoyl Hydrazone Class as Effective Antiproliferative Agents, Blood, 1995, 86, 4295–4306 Search PubMed.
- C. M. Pfeffer and A. T. K. Singh, Apoptosis: A Target for Anticancer Therapy, Int. J. Mol. Sci., 2018, 19, 448 Search PubMed.
- H. S. Park, R. Sun, E. J. Lee, J. Kim and N. H. Hur, Triazole-Bridged Zinc Dinuclear Complexes: Mechanochemical Synthesis, Crystal Structure, and Biological Activity, ACS Omega, 2022, 7, 40860–40868 Search PubMed.
- M. Anjomshoa, S. J. Fatemi, M. Torkzadeh-Mahani and H. Hadadzadeh, DNA and BSA Binding Studies and Anticancer Activity Against Human Breast Cancer Cells (MCF-7) of the Zinc(II) Complex Coordinated by 5,6-diphenyl-3-(2-pyridyl)-1,2,4-triazine, Spectrochim. Acta, Part A, 2014, 127, 511–520 Search PubMed.
- M. Narwane, D. P. Dorairaj, Y. L. Chang, R. Karvembu, Y. H. Huang, H. W. Chang and S. C. N. Hsu, Tris-(2-pyridyl)-pyrazolyl Borate Zinc(II) Complexes: Synthesis, DNA/Protein Binding and In Vitro Cytotoxicity Studies, Molecules, 2021, 26, 7341 Search PubMed.
- M. K. Bhattacharyya, A. Gogoi, S. Chetry, D. Dutta, A. K. Verma, B. Sarma, A. Franconetti and A. Frontera, Antiproliferative Evaluation and Supramolecular Association in Mn(II) and Zn(II) Bipyridine Complexes: Combined Experimental and Theoretical Studies, J. Inorg. Biochem., 2019, 200, 110803 Search PubMed.
- J. Li, R. Liu, J. Jiang, X. Liang, L. Huang, G. Huang, H. Chen, L. Pan and Z. Ma, Zinc(II)-Terpyridine Complexes: Substituent Effect on Photoluminescence, Antiproliferative Activity, and DNA Interaction, Molecules, 2019, 24, 4519 Search PubMed.
- R. A. Khan, A. de Almeida, K. Al-Farhan, A. Alsalme, A. Casini, M. Ghazzali and J. Reedijk, Transition-Metal Norharmane Compounds as Possible Cytotoxic Agents: New Insights Based on a Coordination Chemistry Perspective, J. Inorg. Biochem., 2016, 165, 128–135 Search PubMed.
-
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 03, Revision A.1, Gaussian, Inc., Pittsburgh, PA, 2003 Search PubMed.
- A. D. Becke, Density-Functional Thermochemistry III: The Role of Exact Exchange, J. Chem. Phys., 1993, 98, 5648–5652 Search PubMed.
- R. Ditchfield, W. J. Hehre and J. A. Pople, Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules, J. Chem. Phys., 1971, 54, 724–728 Search PubMed.
- R. Ditchfield, W. J. Hehre and J. A. Pople, Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules, J. Chem. Phys., 1972, 56, 2257–2261 Search PubMed.
- S. Dey, I. Nagpal, P. Sow, R. Dey, A. Chakrovorty, B. Bhattacharjee, S. Saha, A. Majumder, M. Bera, N. Subbarao, S. Nandi, S. H. Molla, P. Guptaroy, S. K. Abraham, A. R. Khuda-Bukhsh and A. Samadder, Morroniside Interaction with Poly (ADP-ribose) Polymerase Accentuates Metabolic Mitigation of Alloxan-Induced Genotoxicity and Hyperglycaemia: A Molecular Docking Based In Vitro and In Vivo Experimental Therapeutic Insight, J. Biomol. Struct. Dyn., 2023, 2246585 Search PubMed.
- R. Dey, S. Dey, P. Sow, A. Chakrovorty, B. Bhattacharjee, S. Nandi and A. Samadder, Novel PLGA-Encapsulated-Nanopiperine Promotes Synergistic Interaction of p53/PARP-1/Hsp90 Axis to Combat ALX-Induced-Hyperglycemia, Sci. Rep., 2024, 14, 9483 Search PubMed.
- A. Paul, J. Das, S. Das, A. Samadder and A. R. Khuda-Bukhsh, Anticancer Potential of Myricanone, a Major Bioactive Component of Myrica Cerifera: Novel Signaling Cascade For Accomplishing Apoptosis, J. Acupunct. Meridian Stud., 2013, 6, 188–198 Search PubMed.
- D. Sarkar, P. Das, S. Basak and N. Chattopadhyay, Binding Interaction of Cationic Phenazinium Dyes with Calf Thymus DNA: A Comparative Study, J. Phys. Chem. B, 2008, 112, 9243–9249 Search PubMed.
- G. Cohen and H. Einsenberg, Viscosity and Sedimentation Study of Sonicated DNA-Proflavine Complexes, Biopolymers, 1969, 8, 45–55 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: FTIR, 1H & 13C NMR, and ESI-MS of Hahpa; FTIR spectra of 1–3; UV-vis absorption and fluorescence emission titration spectra, and viscosity measurement concerning the DNA binding studies of 1–3; Scatchard plots for 1–3; figures associated with % cytotoxicity of 1–3 in the normal L6 skeletal muscle cell line and % cytotoxicity of the precursor zinc salts in the HeLa cell line; and fluorescence intensity histogram profiles showing the expression of p53 protein and nuclear condensation in 1–3 treated HeLa cells. See DOI: https://doi.org/10.1039/d4ma01278j |
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article b and
Manindranath
Bera
b and
Manindranath
Bera
 *a
*a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrolyte-type.40 The FTIR spectra (Fig. S5–S7, ESI†) of 1–3 showed characteristic asymmetric (νas) and symmetric (νs) stretching bands of the carboxylates: 1570 (νas) and 1403 cm−1 (νs) for 1, 1583 (νas) and 1384 cm−1 (νs) for 2, and 1594 (νas) and 1407 cm−1 (νs) for 3. The relatively higher value of Δ (Δ = νas(COO−) − νs(COO−)) at ∼167, 199 and 187 cm−1 for 1, 2 and 3, respectively strongly suggests the monodentate terminal binding of the carboxylate functionalities.41 The bands observed between 3424 and 3453 cm−1 correspond to the ν(O–H) vibrations of the coordinated H2O molecules in 1–3. In addition, the terminal coordination of the NO3− group in compound 2 is suggested by the occurrence of sharp bands at 1789 and 1325 cm−1.42 Verification of an uncoordinated ClO4− ion outside the coordination sphere in compound 3 is indicated by a sharp unsplit band at 1090 cm−1.43
1 electrolyte-type.40 The FTIR spectra (Fig. S5–S7, ESI†) of 1–3 showed characteristic asymmetric (νas) and symmetric (νs) stretching bands of the carboxylates: 1570 (νas) and 1403 cm−1 (νs) for 1, 1583 (νas) and 1384 cm−1 (νs) for 2, and 1594 (νas) and 1407 cm−1 (νs) for 3. The relatively higher value of Δ (Δ = νas(COO−) − νs(COO−)) at ∼167, 199 and 187 cm−1 for 1, 2 and 3, respectively strongly suggests the monodentate terminal binding of the carboxylate functionalities.41 The bands observed between 3424 and 3453 cm−1 correspond to the ν(O–H) vibrations of the coordinated H2O molecules in 1–3. In addition, the terminal coordination of the NO3− group in compound 2 is suggested by the occurrence of sharp bands at 1789 and 1325 cm−1.42 Verification of an uncoordinated ClO4− ion outside the coordination sphere in compound 3 is indicated by a sharp unsplit band at 1090 cm−1.43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for (Zn2+)/(ahpa)−. Fig. 1 insets show the titration profiles for ZnCl2, Zn(NO3)2·6H2O and Zn(ClO4)2·6H2O confirming the formation of the 1
1 for (Zn2+)/(ahpa)−. Fig. 1 insets show the titration profiles for ZnCl2, Zn(NO3)2·6H2O and Zn(ClO4)2·6H2O confirming the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Zn2+)/(ahpa)− bound assemblies in solution. The spectral changes observed upon binding of Zn(II) with the (ahpa)− scaffold are very similar to the results reported by our research group for the interaction of Zn(II) with anthracene-incorporated μ-bis(tridentate) organic assembly.36 Addition of increasing concentrations of Zn(II) ions to the (ahpa)− scaffold induced the red-shift of the absorption bands, which clearly explained the high stability of the as-synthesized Zn(II) compounds (1–3). Accordingly, the binding constant (Kb) values were calculated using the UV-vis titration data by applying the method of Rose and Drago.45 The calculated Kb values of the organic scaffold-bound Zn(II) compounds are (2.31 ± 0.14) × 104 M−1, (2.10 ± 0.17) × 104 M−1 and (3.66 ± 0.10) × 104 M−1 for 1, 2 and 3, respectively.
1 (Zn2+)/(ahpa)− bound assemblies in solution. The spectral changes observed upon binding of Zn(II) with the (ahpa)− scaffold are very similar to the results reported by our research group for the interaction of Zn(II) with anthracene-incorporated μ-bis(tridentate) organic assembly.36 Addition of increasing concentrations of Zn(II) ions to the (ahpa)− scaffold induced the red-shift of the absorption bands, which clearly explained the high stability of the as-synthesized Zn(II) compounds (1–3). Accordingly, the binding constant (Kb) values were calculated using the UV-vis titration data by applying the method of Rose and Drago.45 The calculated Kb values of the organic scaffold-bound Zn(II) compounds are (2.31 ± 0.14) × 104 M−1, (2.10 ± 0.17) × 104 M−1 and (3.66 ± 0.10) × 104 M−1 for 1, 2 and 3, respectively.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Zn2+)/(ahpa)− bound assemblies (1–3) are formed in solution (Fig. 2 insets). The chelation promoted fluorescence-on can be justified by considering that the Zn(II) ions with completely filled d-orbitals are known to increase the fluorescence intensity via either the photo-induced electron transfer or energy transfer mechanism.46 The binding constant (Kb) values of (Zn2+)/(ahpa)− assemblies calculated from the fluorescence titration data are found to be (5.27 ± 0.20) × 103 M−1, (4.86 ± 0.28) × 103 M−1 and (6.38 ± 0.16) × 103 M−1 for 1, 2 and 3, respectively. The fluorescence titration outcomes suggest that the multifunctional organic scaffold Hahpa forms 1
1 (Zn2+)/(ahpa)− bound assemblies (1–3) are formed in solution (Fig. 2 insets). The chelation promoted fluorescence-on can be justified by considering that the Zn(II) ions with completely filled d-orbitals are known to increase the fluorescence intensity via either the photo-induced electron transfer or energy transfer mechanism.46 The binding constant (Kb) values of (Zn2+)/(ahpa)− assemblies calculated from the fluorescence titration data are found to be (5.27 ± 0.20) × 103 M−1, (4.86 ± 0.28) × 103 M−1 and (6.38 ± 0.16) × 103 M−1 for 1, 2 and 3, respectively. The fluorescence titration outcomes suggest that the multifunctional organic scaffold Hahpa forms 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 compounds with Zn(II) ions in solution.
1 compounds with Zn(II) ions in solution.




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) log[Q]
log[Q]

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model between the (ahpa)− scaffold and Zn(II) ion in solution. The structures of 1–3 were optimized and ascertained by density functional theory (DFT) computation. On the other hand, 1–3 showed an excellent anticancer efficacy with significant inhibition of % cell viability by inducing apoptotic cell death in HeLa cells; they remained inefficient in inducing cytotoxicity and apoptosis when administered to normal cells. Furthermore, 1 displayed the highest ROS generation and DNA binding ability and maximum p53 protein expression and nuclear condensation, thus showing a much better anticancer activity in comparison to 2 and 3. Therefore, from our present exploration, it can be suggested that these novel Zn(II) compounds can be utilized for target-specific chemo-therapeutic drug design for interrupting the molecular mechanism involved in the advancement and propagation of the frightful ailment cancer.
1 binding model between the (ahpa)− scaffold and Zn(II) ion in solution. The structures of 1–3 were optimized and ascertained by density functional theory (DFT) computation. On the other hand, 1–3 showed an excellent anticancer efficacy with significant inhibition of % cell viability by inducing apoptotic cell death in HeLa cells; they remained inefficient in inducing cytotoxicity and apoptosis when administered to normal cells. Furthermore, 1 displayed the highest ROS generation and DNA binding ability and maximum p53 protein expression and nuclear condensation, thus showing a much better anticancer activity in comparison to 2 and 3. Therefore, from our present exploration, it can be suggested that these novel Zn(II) compounds can be utilized for target-specific chemo-therapeutic drug design for interrupting the molecular mechanism involved in the advancement and propagation of the frightful ailment cancer.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; v/v) and dried in a vacuum desiccator containing anhydrous CaCl2. Yield: 2.165 g (80%). Anal. calc. for C18H17NO2: C, 77.40%; H, 6.13%; N, 5.01%. Found: C, 77.27%; H, 5.99%; N, 5.09%. FTIR (KBr, cm−1): ν = 3386(b), 1624(s), 1449(s), 1385(s), 1344(s), 1288(s), 1262(s), 1218(s), 1160(s), 1053(s), 886(s), 844(s), 784(s), 772(s), 737(s), 702(s), 598(s). 1H NMR (400 MHz, DMSO-d6, room temperature, δ): 8.80 (s, 1H), 8.52 (d, 2H), 8.20 (d, 2H), 7.71 (t, 2H), 7.62 (t, 2H), 5.27 (s, 2H), 3.65 (s, 2H), 2.86 (t, 1H), 2.76 (t, 1H).
4; v/v) and dried in a vacuum desiccator containing anhydrous CaCl2. Yield: 2.165 g (80%). Anal. calc. for C18H17NO2: C, 77.40%; H, 6.13%; N, 5.01%. Found: C, 77.27%; H, 5.99%; N, 5.09%. FTIR (KBr, cm−1): ν = 3386(b), 1624(s), 1449(s), 1385(s), 1344(s), 1288(s), 1262(s), 1218(s), 1160(s), 1053(s), 886(s), 844(s), 784(s), 772(s), 737(s), 702(s), 598(s). 1H NMR (400 MHz, DMSO-d6, room temperature, δ): 8.80 (s, 1H), 8.52 (d, 2H), 8.20 (d, 2H), 7.71 (t, 2H), 7.62 (t, 2H), 5.27 (s, 2H), 3.65 (s, 2H), 2.86 (t, 1H), 2.76 (t, 1H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9; v/v) to make the working solution. The cells were administered with 1 μL per 1 mL of the working solution in fresh cell culture media and incubated for 30 min, after which they were fixed and analyzed for appropriate data. Independent experiments with precursor zinc salts used in the present study were carried out by employing the same method as specified above. The L6 cells (normal cell line), which were used only to check the % of cell viability, were also maintained applying the above-mentioned protocol.
9; v/v) to make the working solution. The cells were administered with 1 μL per 1 mL of the working solution in fresh cell culture media and incubated for 30 min, after which they were fixed and analyzed for appropriate data. Independent experiments with precursor zinc salts used in the present study were carried out by employing the same method as specified above. The L6 cells (normal cell line), which were used only to check the % of cell viability, were also maintained applying the above-mentioned protocol.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 M−1 cm−1 at 260 nm.85 The CT-DNA was examined for its purity by monitoring the ratio of the absorbance at 260 nm to that at 280 nm having the value of 1.8–1.9. By adding an equal amount of DNA to both compound and reference solutions, the absorbance of the DNA itself was subtracted.
600 M−1 cm−1 at 260 nm.85 The CT-DNA was examined for its purity by monitoring the ratio of the absorbance at 260 nm to that at 280 nm having the value of 1.8–1.9. By adding an equal amount of DNA to both compound and reference solutions, the absorbance of the DNA itself was subtracted.







